Differences between Kidney Transplant Recipients from Deceased Donors with Diabetes Mellitus as Identified by Machine Learning Consensus Clustering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Data Collection
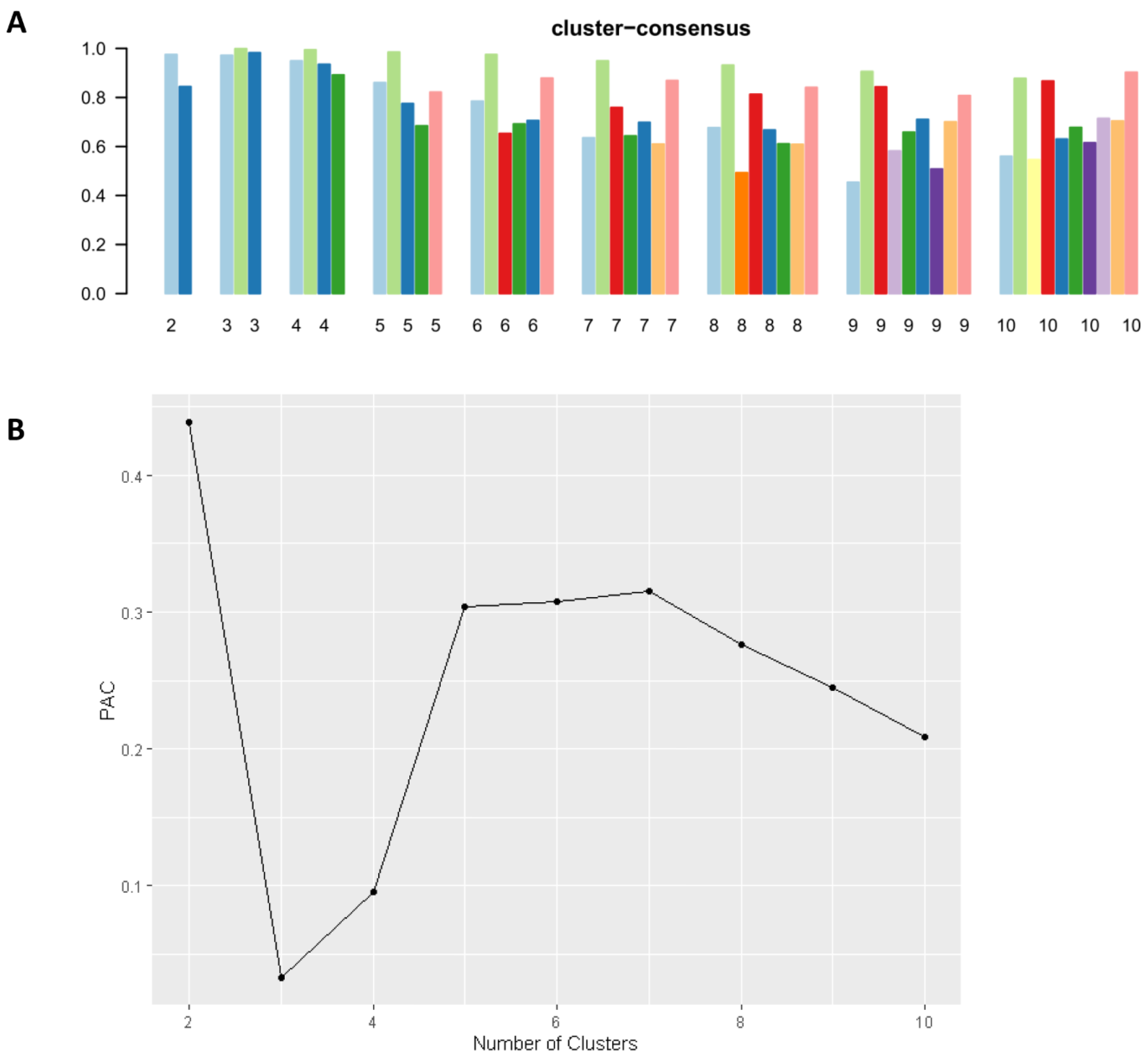
2.3. Clustering Analysis
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Each Cluster of Kidney Transplant Recipients from Diabetic Donors
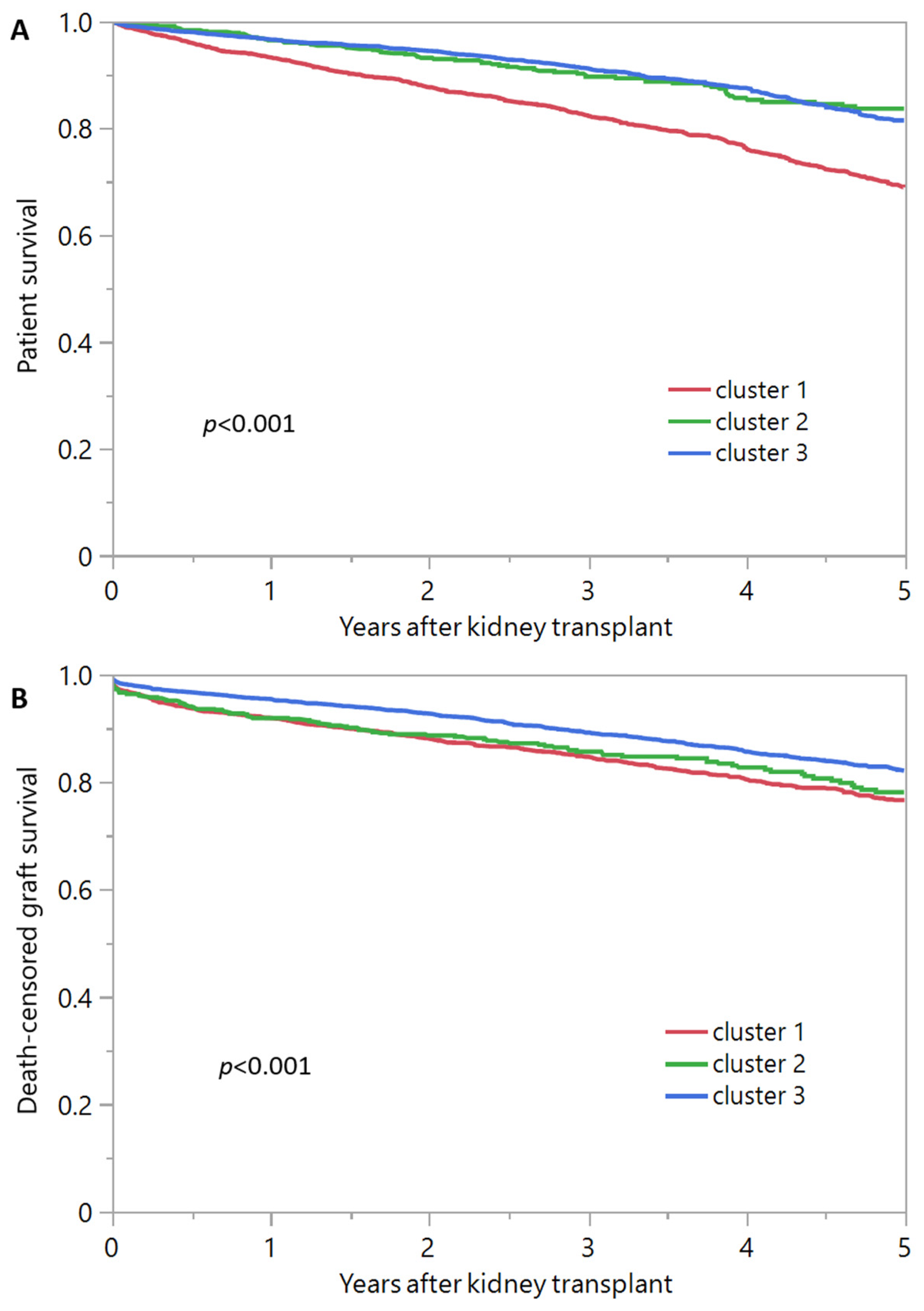
3.2. Post-Transplant Outcomes of Each Cluster of Kidney Transplant Recipients from Diabetic Donors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B.; et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Woo, K.; Yi, J.A. Epidemiology of end-stage kidney disease. Semin. Vasc. Surg. 2021, 34, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Lentine, K.L.; Smith, J.M.; Miller, J.M.; Skeans, M.A.; Prentice, M.; Robinson, A.; Foutz, J.; Booker, S.E.; Israni, A.K.; et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am. J. Transplant. 2021, 21 (Suppl. S2), 21–137. [Google Scholar] [CrossRef]
- Merion, R.M.; Ashby, V.B.; Wolfe, R.A.; Distant, D.A.; Hulbert-Shearon, T.E.; Metzger, R.A.; Ojo, A.O.; Port, F.K. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 2005, 294, 2726–2733. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.B.; Bloom, R.D.; Reese, P.P.; Porrett, P.M.; Forde, K.A.; Sawinski, D.L. National outcomes of kidney transplantation from deceased diabetic donors. Kidney Int. 2016, 89, 636–647. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.B.; Eddinger, K.C.; Locke, J.E.; Forde, K.A.; Reese, P.P.; Sawinski, D.L. Survival Benefit of Transplantation with a Deceased Diabetic Donor Kidney Compared with Remaining on the Waitlist. Clin. J. Am. Soc. Nephrol. 2017, 12, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Waikar, S.S.; Schmidt, I.M.; Landis, J.R.; Hsu, C.Y.; Shafi, T.; Feldman, H.I.; Anderson, A.H.; Wilson, F.P.; Chen, J.; et al. Subtyping CKD Patients by Consensus Clustering: The Chronic Renal Insufficiency Cohort (CRIC) Study. J. Am. Soc. Nephrol. 2021, 32, 639–653. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Kaewput, W.; Kovvuru, K.; Hansrivijit, P.; Kanduri, S.R.; Bathini, T.; Chewcharat, A.; Leeaphorn, N.; Gonzalez-Suarez, M.L.; Cheungpasitporn, W. Promises of Big Data and Artificial Intelligence in Nephrology and Transplantation. J. Clin. Med. 2020, 9, 1107. [Google Scholar] [CrossRef] [Green Version]
- Schwantes, I.R.; Axelrod, D.A. Technology-Enabled Care and Artificial Intelligence in Kidney Transplantation. Curr. Transplant. Rep. 2021, 8, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Vaitla, P.; Jadlowiec, C.C.; Leeaphorn, N.; Mao, S.A.; Mao, M.A.; Pattharanitima, P.; Bruminhent, J.; Khoury, N.J.; Garovic, V.D.; et al. Use of Machine Learning Consensus Clustering to Identify Distinct Subtypes of Black Kidney Transplant Recipients and Associated Outcomes. JAMA Surg. 2022, 157, e221286. [Google Scholar] [CrossRef]
- MacEachern, S.J.; Forkert, N.D. Machine learning for precision medicine. Genome 2021, 64, 416–425. [Google Scholar] [CrossRef]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Monti, S.; Tamayo, P.; Mesirov, J.; Golub, T. Consensus clustering: A resampling-based method for class discovery and visualization of gene expression microarray data. Mach. Learn. 2003, 52, 91–118. [Google Scholar] [CrossRef]
- Șenbabaoğlu, Y.; Michailidis, G.; Li, J.Z. Critical limitations of consensus clustering in class discovery. Sci. Rep. 2014, 4, 6207. [Google Scholar] [CrossRef] [Green Version]
- Stolyar, A.G.; Budkar, L.N.; Solodushkin, S.I.; Iumanova, I.F. Influence of Factors Associated with the Deceased-Donor on Kidney Transplant Outcomes. Exp. Clin. Transplant. 2015, 13, 394–401. [Google Scholar]
- Lasserre, J.; Arnold, S.; Vingron, M.; Reinke, P.; Hinrichs, C. Predicting the outcome of renal transplantation. J. Am. Med. Inform. Assoc. 2012, 19, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef]
- Singh, N.; Washburn, K.; Schenk, A.; El-Hinnawi, A.; Bumgardner, G.; Logan, A.; Rajab, A. The impact of donor and recipient diabetes on renal transplant outcomes. Clin. Transplant. 2020, 34, e14115. [Google Scholar] [CrossRef]
- Hsu, C.T.; Wen, M.C.; Chiu, H.F.; Tsai, S.F.; Yu, T.M.; Yang, C.K.; Wu, M.J.; Chen, C.H. Ongoing donor-transmitted diabetic kidney disease in kidney transplant recipients with fair sugar control: A single center retrospective study. BMC Nephrol. 2020, 21, 458. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Cole, E.H.; Cardella, C.J.; Cattran, D.C.; Schiff, J.; Tinckam, K.J.; Kim, S.J. Impact of deceased donor diabetes mellitus on kidney transplant outcomes: A propensity score-matched study. Transplantation 2009, 88, 251–260. [Google Scholar] [CrossRef]
- Mohan, S.; Tanriover, B.; Ali, N.; Crew, R.J.; Dube, G.K.; Radhakrishnan, J.; Hardy, M.A.; Ratner, L.E.; McClellan, W.; Cohen, D. Availability, utilization and outcomes of deceased diabetic donor kidneys; analysis based on the UNOS registry. Am. J. Transplant. 2012, 12, 2098–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilman, R.L.; Mathur, A.; Smith, M.L.; Kaplan, B.; Reddy, K.S. Increasing the Use of Kidneys from Unconventional and High-Risk Deceased Donors. Am. J. Transplant. 2016, 16, 3086–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Stewart, D.E.; Cherikh, W.S.; Wainright, J.L.; Kucheryavaya, A.; Woodbury, M.; Snyder, J.J.; et al. OPTN/SRTR 2015 Annual Data Report: Kidney. Am. J. Transplant. 2017, 17 (Suppl. S1), 21–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diena, D.; Allesina, A.; Fop, F.; Mella, A.; Cavallo, R.; Costa, C.; Dolla, C.; Gallo, E.; De Rosa, F.G.; Lavacca, A.; et al. Relationship between Cytomegalovirus Viremia and Long-Term Outcomes in Kidney Transplant Recipients with Different Donor Ages. Microorganisms 2023, 11, 458. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Miao, J.; Jadlowiec, C.C.; Mao, S.A.; Mao, M.A.; Vaitla, P.; Leeaphorn, N.; Kaewput, W.; Pattharanitima, P.; Tangpanithandee, S.; et al. Differences between Very Highly Sensitized Kidney Transplant Recipients as Identified by Machine Learning Consensus Clustering. Medicina 2023, 59, 977. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Vaitla, P.; Jadlowiec, C.C.; Leeaphorn, N.; Mao, S.A.; Mao, M.A.; Qureshi, F.; Kaewput, W.; Qureshi, F.; Tangpanithandee, S.; et al. Distinct Phenotypes of Non-Citizen Kidney Transplant Recipients in the United States by Machine Learning Consensus Clustering. Medicines 2023, 10, 25. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Jadlowiec, C.C.; Mao, S.A.; Mao, M.A.; Leeaphorn, N.; Kaewput, W.; Pattharanitima, P.; Nissaisorakarn, P.; Cooper, M.; Cheungpasitporn, W. Distinct phenotypes of kidney transplant recipients aged 80 years or older in the USA by machine learning consensus clustering. BMJ Surg. Interv. Health Technol. 2023, 5, e000137. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Vaitla, P.; Jadlowiec, C.C.; Mao, S.A.; Mao, M.A.; Acharya, P.C.; Leeaphorn, N.; Kaewput, W.; Pattharanitima, P.; Tangpanithandee, S.; et al. Differences between kidney retransplant recipients as identified by machine learning consensus clustering. Clin. Transplant. 2023, 37, e14943. [Google Scholar] [CrossRef]
- Mella, A.; Torazza, M.C.; Finocchietti, D.; Fop, F.; Allesina, A.; Dolla, C.; Giraudi, R.; Biancone, L. Non-adherence assessment to immunosuppressant therapy with a self-report questionnaire and intra-patient variability in renal transplantation: Risk factors and clinical correlations. Minerva Urol. Nephrol. 2023, 75, 92–98. [Google Scholar] [CrossRef]
- Rose, C.; Gill, J.; Gill, J.S. Association of Kidney Transplantation with Survival in Patients with Long Dialysis Exposure. Clin. J. Am. Soc. Nephrol. 2017, 12, 2024–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolla, C.; Naso, E.; Mella, A.; Allesina, A.; Giraudi, R.; Torazza, M.C.; Vanzino, S.B.; Gallo, E.; Lavacca, A.; Fop, F.; et al. Impact of type 2 diabetes mellitus on kidney transplant rates and clinical outcomes among waitlisted candidates in a single center European experience. Sci. Rep. 2020, 10, 22000. [Google Scholar] [CrossRef] [PubMed]
- Jannat-Khah, D.P.; Unterbrink, M.; McNairy, M.; Pierre, S.; Fitzgerald, D.W.; Pape, J.; Evans, A. Treating loss-to-follow-up as a missing data problem: A case study using a longitudinal cohort of HIV-infected patients in Haiti. BMC Public Health 2018, 18, 1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knol, M.J.; Janssen, K.J.; Donders, A.R.; Egberts, A.C.; Heerdink, E.R.; Grobbee, D.E.; Moons, K.G.; Geerlings, M.I. Unpredictable bias when using the missing indicator method or complete case analysis for missing confounder values: An empirical example. J. Clin. Epidemiol. 2010, 63, 728–736. [Google Scholar] [CrossRef]
- White, I.R.; Carlin, J.B. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Stat. Med. 2010, 29, 2920–2931. [Google Scholar] [CrossRef]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Hedden, S.L.; Woolson, R.F.; Carter, R.E.; Palesch, Y.; Upadhyaya, H.P.; Malcolm, R.J. The impact of loss to follow-up on hypothesis tests of the treatment effect for several statistical methods in substance abuse clinical trials. J. Subst. Abuse Treat. 2009, 37, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Donders, A.R.T.; Van Der Heijden, G.J.; Stijnen, T.; Moons, K.G. A gentle introduction to imputation of missing values. J. Clin. Epidemiol. 2006, 59, 1087–1091. [Google Scholar] [CrossRef]

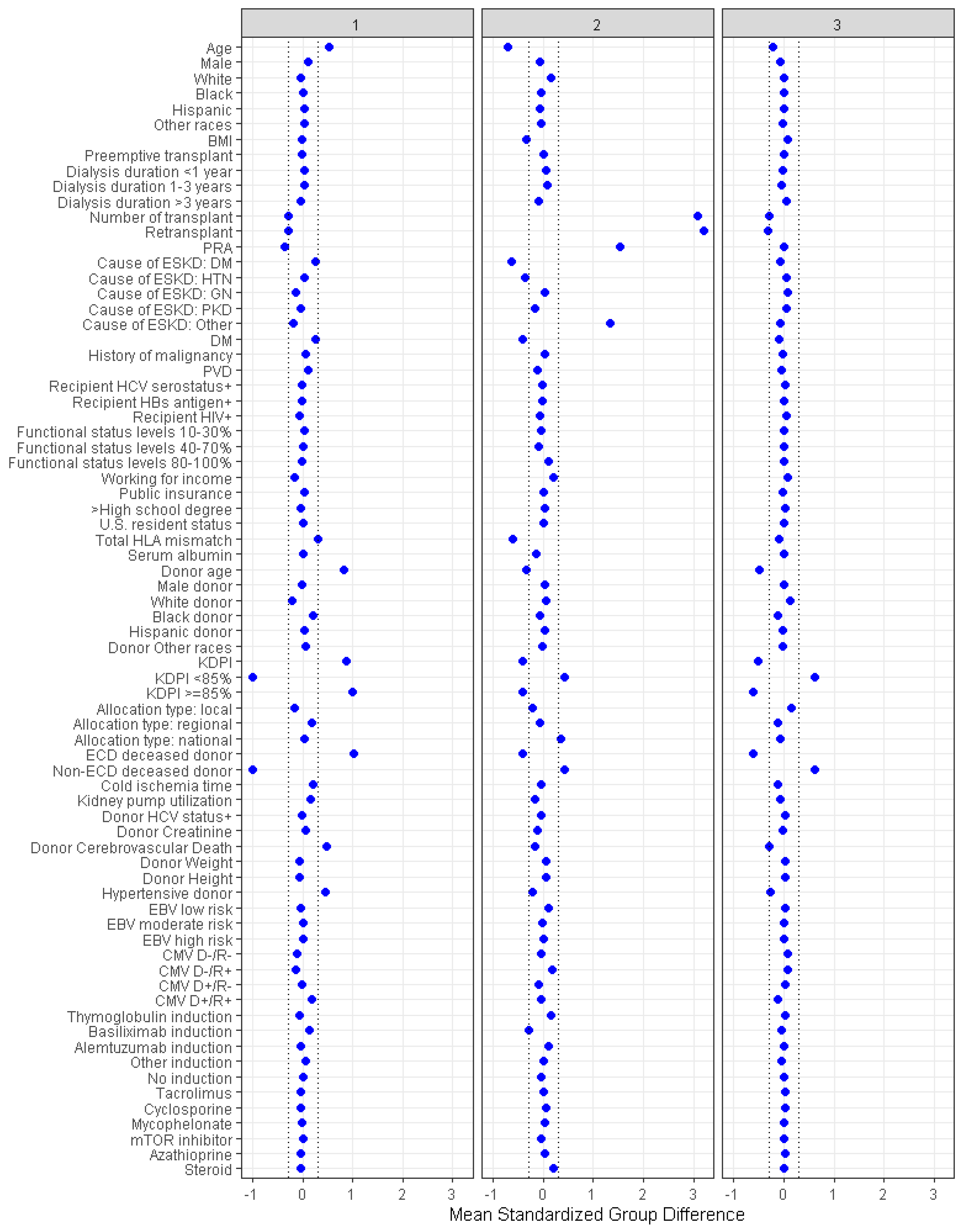
| All (n = 7876) | Cluster 1 (n = 2903) | Cluster 2 (n = 687) | Cluster 3 (n = 4286) | p-Value | |
|---|---|---|---|---|---|
| Recipient Age (year) | 58 ± 12 | 64 ± 8 | 49 ± 13 | 55 ± 12 | <0.001 |
| Recipient male sex | 4955 (63) | 1992 (69) | 408 (59) | 2555 (60) | <0.001 |
Recipient race
| 0.001 | ||||
| 3187 (40) | 1114 (38) | 325 (47) | 1748 (41) | ||
| 2579 (33) | 952 (33) | 210 (31) | 1417 (33) | ||
| 1395 (18) | 547 (19) | 100 (15) | 748 (17) | ||
| 715 (9) | 290 (10) | 52 (8) | 373 (9) | ||
| Body mass index (kg/m2) | 28.7 ± 5.2 | 28.6 ± 4.9 | 26.9 ± 5.1 | 29.0 ± 5.4 | <0.001 |
| Kidney re-transplant | 702 (9) | 15 (1) | 687 (100) | 0 (0) | <0.001 |
Dialysis duration
| <0.001 | ||||
| 744 (9) | 257 (9) | 68 (10) | 419 (10) | ||
| 633 (8) | 263 (9) | 65 (9) | 305 (7) | ||
| 1800 (23) | 717 (25) | 178 (26) | 905 (21) | ||
| 4699 (60) | 1666 (57) | 376 (55) | 2657 (62) | ||
Cause of end-stage kidney disease
| <0.001 | ||||
| 2656 (34) | 1319 (45) | 27 (4) | 1310 (31) | ||
| 2121 (27) | 816 (28) | 72 (10) | 1233 (29) | ||
| 1208 (15) | 305 (11) | 114 (17) | 789 (18) | ||
| 6626 (8) | 201 (7) | 24 (3) | 401 (9) | ||
| 1265 (16) | 262 (9) | 450 (66) | 553 (13) | ||
Comorbidity
| |||||
| 3348 (43) | 1586 (55) | 147 (21) | 1615 (38) | <0.001 | |
| 718 (9) | 306 (11) | 66 (10) | 346 (8) | 0.002 | |
| 790 (10) | 379 (13) | 45 (7) | 366 (9) | <0.001 | |
| PRA, median (IQR) | 0 (0, 24) | 0 (0, 0) | 88 (46, 98) | 0 (0, 25) | <0.001 |
| Positive HCV serostatus | 341 (4) | 110 (4) | 27 (4) | 204 (5) | 0.12 |
| Positive HBs antigen | 147 (2) | 51 (2) | 11 (2) | 85 (2) | 0.68 |
| Positive HIV serostatus | 69 (1) | 8 (0.3) | 1 (0.2) | 60 (1) | <0.001 |
Functional status
| 0.03 | ||||
| 21 (0.3) | 11 (0.4) | 0 (0) | 10 (0.2) | ||
| 3326 (42) | 1249 (43) | 257 (37) | 1820 (42) | ||
| 4529 (58) | 1643 (57) | 430 (63) | 2456 (57) | ||
| Working income | 1821 (23) | 477 (16) | 220 (32) | 1124 (26) | <0.001 |
| Public insurance | 6272 (80) | 2353 (81) | 547 (80) | 3372 (79) | 0.049 |
| US resident | 7834 (99) | 2890 (100) | 684 (100) | 4260 (99) | 0.62 |
| Undergraduate education or above | 3888 (49) | 1375 (47) | 351 (51) | 2162 (50) | 0.02 |
| Serum albumin (g/dL) | 4.0 ± 0.6 | 4.0 ± 0.6 | 3.9 ± 0.6 | 4.0 ± 0.6 | 0.001 |
Kidney donor status
| <0.001 | ||||
| 5413 (69) | 630 (22) | 603 (88) | 4180 (98) | ||
| 2463 (31) | 2273 (78) | 84 (12) | 106 (2) | ||
| Donor age | 48 ± 12 | 58 ± 6.3 | 44 ± 11 | 42 ± 11 | <0.001 |
| Donor male sex | 4296 (55) | 1553 (54) | 382 (56) | 2361 (55) | 0.35 |
Donor race
| <0.001 | ||||
| 4846 (62) | 1493 (51) | 438 (64) | 2915 (68) | ||
| 1246 (16) | 686 (24) | 88 (13) | 472 (11) | ||
| 1373 (17) | 542 (19) | 130 (19) | 701 (16) | ||
| 411 (5) | 182 (6) | 31 (5) | 198 (5) | ||
| Donor weight (kg) | 94 ± 26 | 92 ± 23 | 95 ± 28 | 95 ± 27 | <0.001 |
| Donor height (cm) | 170 ± 11 | 169 ± 11 | 170 ± 11 | 170 ± 12 | <0.001 |
| Donor hypertension | 5414 (69) | 2590 (89) | 406 (59) | 2418 (56) | <0.001 |
| Donor positive HCV serostatus | 147 (2) | 46 (2) | 8 (1) | 93 (2) | 0.07 |
| Donor cerebrovascular death | 3100 (39) | 1828 (63) | 210 (31) | 1062 (25) | <0.001 |
| Donor creatinine (mg/dL) | 1.2 ± 0.9 | 1.3 ± 0.8 | 1.1 ± 0.7 | 1.2 ± 0.9 | <0.001 |
| KDPI ≥ 85 | 2415 (31) | 2226 (77) | 79 (12) | 110 (3) | <0.001 |
| HLA mismatch, median (IQR) | 4 (4, 5) | 5 (4, 5) | 4 (2, 5) | 4 (3, 5) | <0.001 |
| Cold ischemia time (hours) | 19.3 ± 9.2 | 21.2 ± 9.5 | 18.9 ± 8.7 | 18.1 ± 8.8 | <0.001 |
| Kidney on pump | 4546 (58) | 1888 (65) | 341 (50) | 2317 (54) | <0.001 |
Allocation type
| <0.001 | ||||
| 5374 (68) | 1754 (60) | 401 (58) | 3219 (75) | ||
| 1313 (17) | 690 (24) | 95 (14) | 528 (12) | ||
| 1189 (15) | 459 (16) | 191 (28) | 539 (13) | ||
EBV status
| 0.004 | ||||
| 34 (0.4) | 4 (0.1) | 8 (1) | 22 (1) | ||
| 7199 (91) | 2660 (92) | 622 (91) | 3917 (91) | ||
| 643 (8) | 239 (8) | 57 (8) | 347 (8) | ||
CMV status
| <0.001 | ||||
| 865 (11) | 224 (8) | 67 (10) | 574 (13) | ||
| 1850 (23) | 508 (18) | 214 (31) | 1128 (26) | ||
| 3681 (47) | 1633 (56) | 302 (44) | 1746 (41) | ||
| 1480 (19) | 538 (19) | 104 (15) | 838 (20) | ||
Induction immunosuppression
| |||||
| 4550 (58) | 1583 (55) | 448 (65) | 2519 (59) | <0.001 | |
| 1226 (16) | 420 (14) | 132 (19) | 674 (16) | 0.008 | |
| 1593 (20) | 745 (26) | 61 (9) | 787 (18) | <0.001 | |
| 178 (2) | 90 (3) | 16 (2) | 72 (2) | <0.001 | |
| 691 (9) | 254 (9) | 52 (8) | 385 (9) | 0.48 | |
Maintenance Immunosuppression
| |||||
| 7081 (90) | 2576 (89) | 617 (90) | 3888 (91) | 0.02 | |
| 147 (2) | 34 (1) | 17 (2) | 96 (2) | 0.002 | |
| 7194 (91) | 2634 (91) | 634 (92) | 3926 (92) | 0.29 | |
| 23 (0.3) | 3 (0.10 | 3 (0.4) | 17 (0.4) | 0.06 | |
| 96 (1) | 36 (1) | 4 (1) | 56 (1) | 0.27 | |
| 5328 (68) | 1900 (65) | 527 (77) | 2901 (68) | <0.001 |
| Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|
| Primary non-function | 39 (1.3) | 4 (0.6) | 35 (0.8) |
| OR for primary non-function | 1.65 (1.05–2.62) | 0.71 (0.25–2.01) | 1 (ref) |
| Delayed graft function | 991 (34) | 241 (35) | 1282 (30) |
| OR for delayed graft function | 1.21 (1.10–1.34) | 1.27 (1.07–1.50) | 1 (ref) |
| 1-year survival | 93.2% | 96.4% | 96.5% |
| HR for 1-year death | 2.01 (1.60–2.51) | 1.02 (0.65–1.60) | 1 (ref) |
| 5-year survival | 68.8% | 83.6% | 81.4% |
| HR for 5-year death | 1.92 (1.69–2.17) | 0.97 (0.75–1.24) | 1 (ref) |
| 1-year death-censored graft survival | 91.9% | 91.8% | 95.4% |
| HR for 1-year death-censored graft failure | 1.81 (1.48–2.20) | 1.80 (1.32–2.45) | 1 (ref) |
| 5-year death-censored graft survival | 76.6% | 78.1% | 82.1% |
| HR for 5-year death-censored graft failure | 1.46 (1.28–1.66) | 1.36 (1.09–1.67) | 1 (ref) |
| 1-year acute rejection | 183 (6.3) | 53 (7.7) | 253 (5.9) |
| OR for 1-year acute rejection | 1.07 (0.88–1.31) | 1.33 (0.98–1.81) | 1 (ref) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongprayoon, C.; Miao, J.; Jadlowiec, C.C.; Mao, S.A.; Mao, M.A.; Leeaphorn, N.; Kaewput, W.; Pattharanitima, P.; Tangpanithandee, S.; Krisanapan, P.; et al. Differences between Kidney Transplant Recipients from Deceased Donors with Diabetes Mellitus as Identified by Machine Learning Consensus Clustering. J. Pers. Med. 2023, 13, 1094. https://doi.org/10.3390/jpm13071094
Thongprayoon C, Miao J, Jadlowiec CC, Mao SA, Mao MA, Leeaphorn N, Kaewput W, Pattharanitima P, Tangpanithandee S, Krisanapan P, et al. Differences between Kidney Transplant Recipients from Deceased Donors with Diabetes Mellitus as Identified by Machine Learning Consensus Clustering. Journal of Personalized Medicine. 2023; 13(7):1094. https://doi.org/10.3390/jpm13071094
Chicago/Turabian StyleThongprayoon, Charat, Jing Miao, Caroline C. Jadlowiec, Shennen A. Mao, Michael A. Mao, Napat Leeaphorn, Wisit Kaewput, Pattharawin Pattharanitima, Supawit Tangpanithandee, Pajaree Krisanapan, and et al. 2023. "Differences between Kidney Transplant Recipients from Deceased Donors with Diabetes Mellitus as Identified by Machine Learning Consensus Clustering" Journal of Personalized Medicine 13, no. 7: 1094. https://doi.org/10.3390/jpm13071094












