The Relationship between Seropositive Rheumatoid Arthritis and Congestive Heart Failure: A Nationwide Longitudinal Cohort Study in Korea
Abstract
:1. Introduction
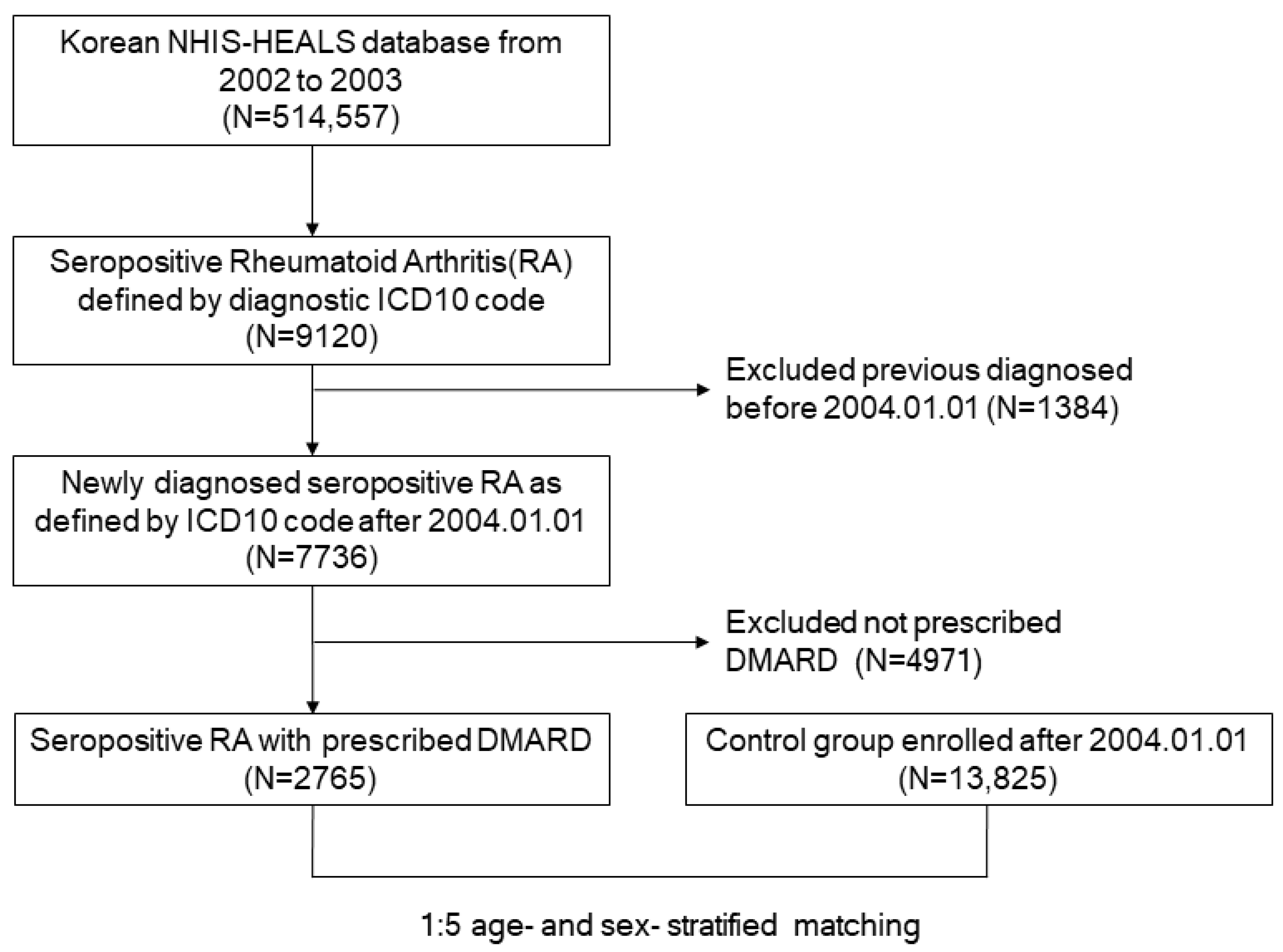
2. Materials and Methods
2.1. Data Sources
2.2. Patient Population
2.3. Definitions of CHF and Comorbidities
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Subjects
3.2. CHF in the Seropositive RA and Control Groups
3.3. Subgroup Analysis of CHF Incidence Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van den Hoek, J.; Boshuizen, H.C.; Roorda, L.D.; Tijhuis, G.J.; Nurmohamed, M.T.; van den Bos, G.A.; Dekker, J. Mortality in patients with rheumatoid arthritis: A 15-year prospective cohort study. Rheumatol. Int. 2017, 37, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Houge, I.S.; Hoff, M.; Thomas, R.; Videm, V. Mortality is increased in patients with rheumatoid arthritis or diabetes compared to the general population—The Nord-Trøndelag Health Study. Sci. Rep. 2020, 10, 3593. [Google Scholar] [CrossRef] [PubMed]
- Black, R.J.; Lester, S.; Tieu, J.; Sinnathurai, P.; Barrett, C.; Buchbinder, R.; Lassere, M.; March, L.; Proudman, S.M.; Hill, C.L. Mortality estimates and excess mortality in rheumatoid arthritis. Rheumatology 2023, 62, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sung, Y.K. Epidemiology of Rheumatoid Arthritis in Korea. J. Rheum. Dis. 2021, 28, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Maradit-Kremers, H.; Nicola, P.J.; Crowson, C.S.; Ballman, K.V.; Gabriel, S.E. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2005, 52, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Karlson, E.W.; Rimm, E.B.; Cannuscio, C.C.; Mandl, L.A.; Manson, J.E.; Stampfer, M.J.; Curhan, G.C. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003, 107, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Avina-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.S.; Quyyumi, A.A. Rheumatoid arthritis and cardiovascular disease. Curr. Atheroscler. Rep. 2008, 10, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Y.; Dasu, N.; Shah, A.; Brown, K.; Kaell, A.; Levine, A.; Dasu, K.; Raminfard, A. Incidence of congestive heart failure in rheumatoid arthritis: A review of literature and meta-regression analysis. ESC Heart Fail. 2020, 7, 3745–3753. [Google Scholar] [CrossRef]
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef]
- Nicola, P.J.; Crowson, C.S.; Maradit-Kremers, H.; Ballman, K.V.; Roger, V.L.; Jacobsen, S.J.; Gabriel, S.E. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.; Zhao, L. Association of congestive heart failure with mortality in individuals with rheumatoid arthritis: A cohort study. Clin. Rheumatol. 2024, 43, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Nicola, P.J.; Maradit-Kremers, H.; Roger, V.L.; Jacobsen, S.J.; Crowson, C.S.; Ballman, K.V.; Gabriel, S.E. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005, 52, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Khalid, U.; Egeberg, A.; Ahlehoff, O.; Lane, D.; Gislason, G.H.; Lip, G.Y.H.; Hansen, P.R. Incident Heart Failure in Patients with Rheumatoid Arthritis: A Nationwide Cohort Study. J. Am. Heart Assoc. 2018, 7, e007227. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Banerjee, U.; Hajra, A.; Chakraborty, S.; Amgai, B.; Ghosh, R.K.; Haddadin, F.I.; Modi, V.A.; Sinha, K.; Aronow, W.S.; et al. Trends of Cardiac Complications in Patients with Rheumatoid Arthritis: Analysis of the United States National Inpatient Sample; 2005–2014. Curr. Probl. Cardiol. 2019, 46, 100455. [Google Scholar] [CrossRef] [PubMed]
- Mantel, A.; Holmqvist, M.; Andersson, D.C.; Lund, L.H.; Askling, J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.G.; Yi, J.; Choi, J.M.; Chung, C.K.; Choi, U.Y.; Han, I.B.; Sohn, S. Changes in the medical burden of pyogenic and tuberculous spondylitis between 2007 and 2016: A nationwide cohort study. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2020, 73, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Choi, Y.J.; Kim, J.G.; Han, I.B.; Do Han, K.; Choi, J.M.; Sohn, S. Association of Acute Myocardial Infarction with ankylosing Spondylitis: A nationwide longitudinal cohort study. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2018, 56, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Hong, J.B.; Choi, Y.J.; Jung, J.H.; Han, I.B.; Choi, J.M.; Sohn, S. Association of Congestive Heart Failure and Death with Ankylosing Spondylitis: A Nationwide Longitudinal Cohort Study in Korea. J. Korean Neurosurg. Soc. 2019, 62, 217–224. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Cho, S.-K.; Sung, Y.-K.; Choi, C.-B.; Kwon, J.-M.; Lee, E.-K.; Bae, S.-C. Development of an algorithm for identifying rheumatoid arthritis in the Korean National Health Insurance claims database. Rheumatol. Int. 2013, 33, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Imai, K.; King, G.; Stuart, E. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Anal. 2007, 15, 199–236. Available online: http://gking.harvard.edu/files/abs/matchp-abs.shtml (accessed on 4 May 2024). [CrossRef]
- Ho, D.; Imai, K.; King, G.; Stuart, E. Matchit: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. Available online: http://gking.harvard.edu/matchit/ (accessed on 14 April 2024).
- Lee, D.H.; Choi, Y.J.; Han, I.B.; Hong, J.B.; Do Han, K.; Choi, J.M.; Sohn, S. Association of ischemic stroke with ankylosing spondylitis: A nationwide longitudinal cohort study. Acta Neurochir. 2018, 160, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Choi, E.K.; Han, K.D.; Lee, H.J.; Rhee, T.M.; Lee, S.R.; Cha, M.J.; Lim, W.H.; Kang, S.H.; Oh, S. Association between adult height, myocardial infarction, heart failure, stroke and death: A Korean nationwide population-based study. Int. J. Epidemiol. 2018, 47, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Rhee, T.M.; Lee, J.H.; Choi, E.K.; Han, K.D.; Lee, H.; Park, C.S.; Hwang, D.; Lee, S.R.; Lim, W.H.; Kang, S.H.; et al. Increased Risk of Atrial Fibrillation and Thromboembolism in Patients with Severe Psoriasis: A Nationwide Population-based Study. Sci. Rep. 2017, 7, 9973. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Cha, M.J.; Oh, S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int. J. Cardiol. 2017, 236, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Cha, M.J.; Oh, S.; Lip, G.Y.H. Temporal trends of antithrombotic therapy for stroke prevention in Korean patients with non-valvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants: A nationwide population-based study. PLoS ONE 2017, 12, e0189495. [Google Scholar] [CrossRef] [PubMed]
- Baniaamam, M.; Paulus, W.J.; Blanken, A.B.; Nurmohamed, M.T. The effect of biological DMARDs on the risk of congestive heart failure in rheumatoid arthritis: A systematic review. Expert. Opin. Biol. Ther. 2018, 18, 585–594. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu. Rev. Med. 2013, 64, 249–263. [Google Scholar] [CrossRef]
- Liuzzo, G.; Biasucci, L.M.; Trotta, G.; Brugaletta, S.; Pinnelli, M.; Digianuario, G.; Rizzello, V.; Rebuzzi, A.G.; Rumi, C.; Maseri, A.; et al. Unusual CD4+CD28nullT Lymphocytes and Recurrence of Acute Coronary Events. J. Am. Coll. Cardiol. 2007, 50, 1450–1458. [Google Scholar] [CrossRef]
- Sanghavi, N.; Ingrassia, J.P.; Korem, S.; Ash, J.; Pan, S.; Wasserman, A. Cardiovascular Manifestations in Rheumatoid Arthritis. Cardiol. Rev. 2024, 32, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory cytokines in heart failure: Mediators and markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B.; et al. Inflammatory markers and onset of cardiovascular events: Results from the Health ABC study. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Farragher, T.M.; Goodson, N.J.; Naseem, H.; Silman, A.J.; Thomson, W.; Symmons, D.; Barton, A. Association of the HLA–DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2008, 58, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Page, J.; Henry, D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: An underrecognized public health problem. Arch. Intern. Med. 2000, 160, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Garcia Rodriguez, L.A.; Hernandez-Diaz, S. Nonsteroidal antiinflammatory drugs as a trigger of clinical heart failure. Epidemiology 2003, 14, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ocon, A.J.; Reed, G.; Pappas, D.A.; Curtis, J.R.; Kremer, J.M. Short-term dose and duration-dependent glucocorticoid risk for cardiovascular events in glucocorticoid-naive patients with rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Boers, M.; Hartman, L.; Opris-Belinski, D.; Bos, R.; Kok, M.; da Silva, J.P.; Griep, E.; Klaasen, R.; Allaart, C.F.; Baudoin, P. Favorable balance of benefit and harm of long-term, low dose prednisolone added to standard treatment in rheumatoid arthritis patients aged 65+: The pragmatic, multicenter, placebo-controlled GLORIA trial. In Arthritis & Rheumatology; Wiley: Hoboken, NJ, USA, 2021; pp. 3491–3494. [Google Scholar]
- Kumar, V.; Singh, A.P.; Wheeler, N.; Galindo, C.L.; Kim, J.J. Safety profile of D-penicillamine: A comprehensive pharmacovigilance analysis by FDA adverse event reporting system. Expert. Opin. Drug Saf. 2021, 20, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- So, H.; Tam, L.-S. Nonsteroidal anti-inflammatory drugs and cardiovascular disease risk in spondyloarthritis-spectrum diseases. Curr. Opin. Rheumatol. 2022, 34, 203–208. [Google Scholar] [CrossRef]
- Nissen, S.E.; Yeomans, N.D.; Solomon, D.H.; Lüscher, T.F.; Libby, P.; Husni, M.E.; Graham, D.Y.; Borer, J.S.; Wisniewski, L.M.; Wolski, K.E. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N. Engl. J. Med. 2016, 375, 2519–2529. [Google Scholar] [CrossRef]

| Disease (ICD-10 Code) | Additional Conditions | |
|---|---|---|
| Past medical history | ||
| Hypertension | I10-I13, I15 | Claims for antihypertensive agents or SBP ≥ 140 mmHg or DBP ≥ 90 mmHg |
| Type 2 DM | E11–E14 | Claims for oral antidiabetic agents or insulin or fasting glucose ≥ 126 |
| Dyslipidemia | E78 | Claims for agents for dyslipidemia or total cholesterol ≥ 240 |
| Socioeconomic status | ||
| Low income | Composite of lowest quartile of yearly income in addition to medicare beneficiaries | |
| Outcomes | ||
| Congestive heart failure | I50 | Hospitalization ≥ 1 day |
| Variable | Seropositive RA (n = 2765) | Control (n = 13,825) | p-Value |
|---|---|---|---|
| Male, n (%) | 735 (26.6) | 3675 (26.6) | 1 |
| Age, n | 53.5 ± 8.7 | 53.5 ± 8.7 | 1 |
| AGE ≥ 65, n (%) | 352 (12.73) | 1760 (12.73) | 1 |
| Low Income, n (%) | 703 (25.42) | 3713 (26.86) | 0.126 |
| Diabetes Mellitus, n (%) | 173 (6.26) | 1396 (10.10) | <0.0001 |
| Hypertension, n (%) | 833 (30.13) | 5038 (26.44) | <0.0001 |
| Dyslipidemia, n (%) | 430 (15.55) | 2466 (17.84) | 0.004 |
| Group | No. | Event | Duration (Days) | IR (1000 Person Years) | HR (95% CI) | |
|---|---|---|---|---|---|---|
| MODEL 1 | MODEL 2 | |||||
| Control | 13,825 | 91 | 56,009,292 | 0.593 | 1 | 1 |
| RA | 2765 | 17 | 5,629,860 | 1.102 | 2.41 (1.40, 4.14) | 2.50 (1.45, 4.30) |
| Variables | Seropositive RA | Control | HR (95% CI) | |||
|---|---|---|---|---|---|---|
| N | IR (1000 Person Years) | N | IR (1000 Person Years) | |||
| Sex | Male | 4 | 1.057 | 23 | 0.591 | 2.49 (0.82, 7.55) |
| Female | 13 | 1.117 | 68 | 0.594 | 2.39 (1.29, 4.44) | |
| Age | <65 | 9 | 0.664 | 45 | 0.332 | 2.60 (1.23, 5.50) |
| ≥65 | 8 | 4.313 | 46 | 2.552 | 2.25 (1.03, 4.93) | |
| Diabetes Mellitus | No | 17 | 1.178 | 78 | 0.564 | 2.68 (1.54, 4.64) |
| Yes | 0 | - | 13 | 0.862 | - | |
| Hypertension | No | 10 | 0.932 | 38 | 0.388 | 2.99 (1.44, 6.22) |
| Yes | 7 | 1.492 | 53 | 0.955 | 2.08 (0.92, 4.71) | |
| Dyslipidemia | No | 16 | 1.234 | 74 | 0.588 | 2.71 (1.54, 4.77) |
| Yes | 1 | 0.408 | 17 | 0.615 | 0.89 (0.11, 6.97) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.S.; Hong, J.B.; Kim, H.; Sheen, S.H.; Han, I.-b.; Kim, J.G.; Jeun, S.S.; Sohn, S. The Relationship between Seropositive Rheumatoid Arthritis and Congestive Heart Failure: A Nationwide Longitudinal Cohort Study in Korea. J. Pers. Med. 2024, 14, 615. https://doi.org/10.3390/jpm14060615
Kim YS, Hong JB, Kim H, Sheen SH, Han I-b, Kim JG, Jeun SS, Sohn S. The Relationship between Seropositive Rheumatoid Arthritis and Congestive Heart Failure: A Nationwide Longitudinal Cohort Study in Korea. Journal of Personalized Medicine. 2024; 14(6):615. https://doi.org/10.3390/jpm14060615
Chicago/Turabian StyleKim, Yeo Song, Je Beom Hong, Hakyung Kim, Seung Hun Sheen, In-bo Han, Jeong Gyun Kim, Sin Soo Jeun, and Seil Sohn. 2024. "The Relationship between Seropositive Rheumatoid Arthritis and Congestive Heart Failure: A Nationwide Longitudinal Cohort Study in Korea" Journal of Personalized Medicine 14, no. 6: 615. https://doi.org/10.3390/jpm14060615







