Optimizing Anticoagulation in Valvular Heart Disease: Navigating NOACs and VKAs
Abstract
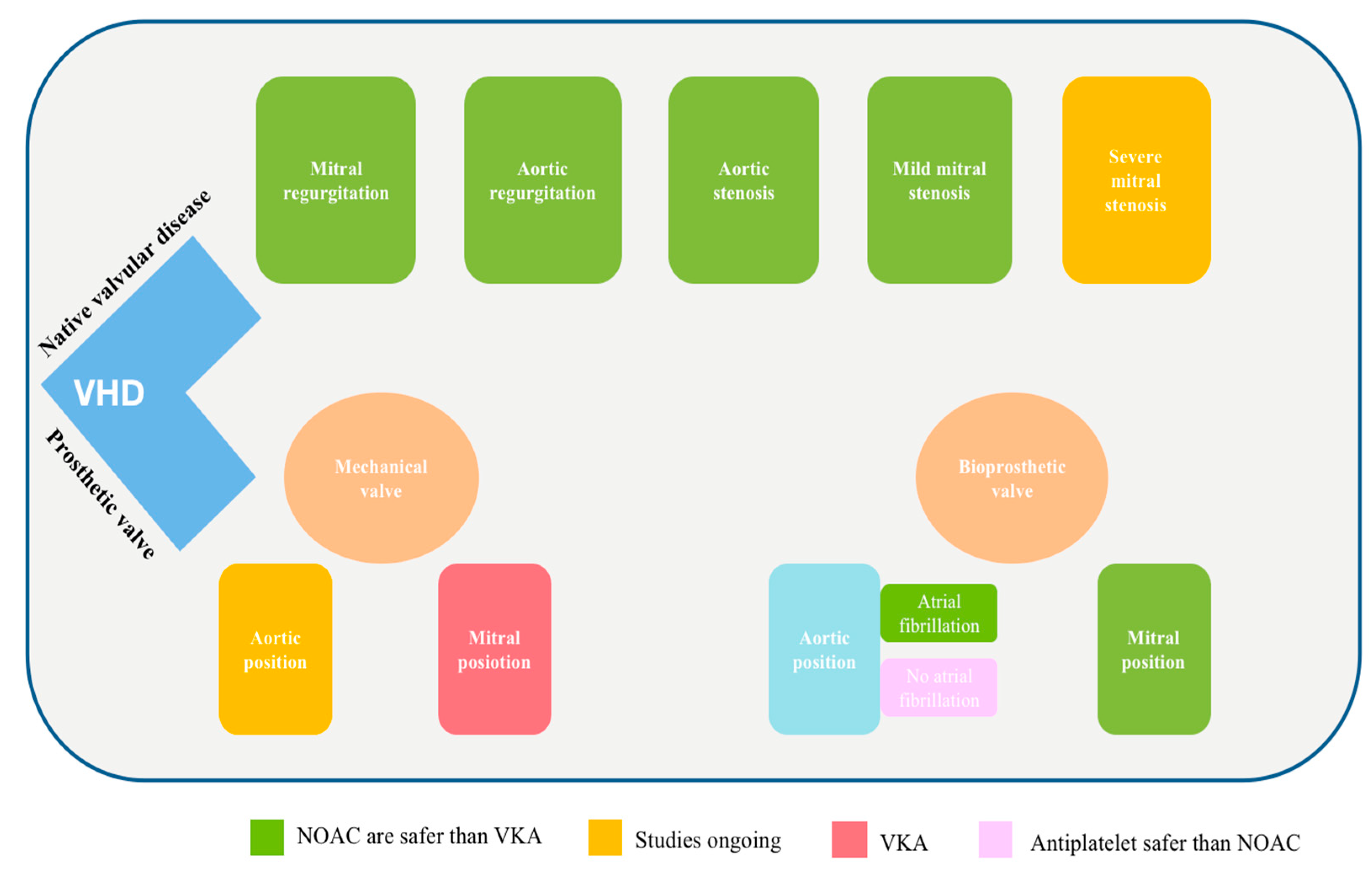
:1. Introduction
2. NOAC in Native Valvular Disease
2.1. NOAC in Native Mitral Valvular Disease
2.2. NOAC in Native Aortic Valvular Disease
3. NOAC in Mitral Valve Repair
4. NOAC in Bioprosthetic Mitral Valves
5. NOAC in Mechanical Mitral Prosthetic Valves
6. NOAC in Transcatheter Aortic Valve Implantation
7. NOAC and Mechanical Aortic Valve
8. NOAC in Patients with VHD and Other Comorbidities
8.1. Acute Thrombosis
8.2. Coronary Heart Disease
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rostagno, C. Heart Valve Disease in Elderly. World J Cardiol. 2019, 11, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.L.; Singer, D.E.; Ovbiagele, B.; Wu, Y.-L.; Ahmed, M.A.; Lee, M. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: A systematic review and meta-analysis. J. Am. Heart Assoc. 2017, 6, e005835. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Mocumbi, A.; Narayanan, K.; Jouven, X.; Celermajer, D.S. Persisting burden and challenges of rheumatic heart disease. Eur. Heart. J. 2021, 42, 3338–3348. [Google Scholar] [CrossRef]
- Watkins, D.A.; Beaton, A.Z.; Carapetis, J.R.; Karthikeyan, G.; Mayosi, B.M.; Wyber, R.; Yacoub, M.H.; Zühlke, L.J. Rheumatic heart disease worldwide: JACC scientific expert panel. J. Am. Coll. Cardiol. 2018, 72, 1397–1416. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nyaga, U.F.; Ndoadoumgue, A.L.; Nkeck, J.R.; Ngouo, A.; Bigna, J.J. Meta-analysis of the incidence, prevalence, and correlates of atrial fibrillation in rheumatic heartdisease. Glob. Heart 2020, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Allan, V.; Denaxas, S.; Shah, A.; Kotecha, D.; Lambiase, P.D.; Joseph, J.; Lund, L.H.; Hemingway, H. Subtypes of atrial fibrillation with concomitant valvular heart disease derived from electronic health records: Phenotypes, population prevalence, trends and prognosis Europace. Clin. Res. 2019, 21, 1776–1784. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. ESC Scientific Document Group, ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the Efficacy and Safety of New Oral Anticoagulants with Warfarin in Patients with Atrial Fibrillation: A Meta-Analysis of Randomised Trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Yao, X.; Gersh, B.J.; Noseworthy, P.A. Direct oral anticoagulants in patients with atrial fibrillation and valvular heart disease other than significant mitral stenosis and mechanical valves: A meta-analysis. Circulation 2017, 135, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Collet, J.P.; de Caterina, R.; Fauchier, L.; Lane, D.A.; Larsen, T.B.; Marin, F.; Morais, J.; Narasimhan, C.; Olshansky, B.; et al. Antithrombotic therapy in atrial fbrillation associated with valvular heart disease: A joint consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology working group on thrombosis, endorsed by the ESC working group on valvular heart disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacifc Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofsiología (SOLEACE). Europace 2017, 19, 1757–1758. [Google Scholar]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ ACCP/HRS Guideline for the diagnosis and management of atrial fibrillation: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Fleming, H.A.; Bailey, S.M. Mitral valve disease, systemic embolism and anticoagulants. Postgrad. Med. J. 1971, 47, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Giancaterino, S.; Hsu, J.C. Valvular atrial fibrillation. J. Am. Coll. Cardiol. 2019, 73, 3360–3361. [Google Scholar] [CrossRef] [PubMed]
- Molteni, M.; Polo Friz, H.; Primitz, L.; Marano, G.; Boracchi, P.; Cimminiello, C. The definition of valvular and non-valvular atrial fibrillation: Results of a physicians’ survey. Europace 2014, 16, 1720–1725. [Google Scholar] [CrossRef]
- Petty, G.W.; Khandheria, B.K.; Whisnant, J.P.; Sicks, J.D.; M O, W.; Wiebers, D.O. Predictors of cerebrovascular events and death among patients with valvular heart disease: A population-based study. Stroke 2000, 31, 2628–2635. [Google Scholar] [CrossRef]
- Wolf, P.A.; Dawber, T.R.; Thomas, E., Jr.; Kannel, W.B. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham study. Neurology 1978, 28, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Developed with the Special Contribution of the European Heart Rhythm Association (EHRA); Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS); Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Brueckmann, M.; Granger, C.B.; Kappetein, A.P.; Mack, M.J.; Blatchford, J.; Devenny, K.; Friedman, J.; Guiver, K.; et al. RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N. Engl. J. Med. 2013, 369, 1206–1214. [Google Scholar] [CrossRef]
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef]
- Lee, W.-C.; Shih, J.-Y.; Fang, H.-Y.; Wu, P.-J.; Fang, C.-Y.; Chen, H.-C.; Fang, Y.-N.; Chang, W.-T.; Chen, M.-C. Comparing Vitamin K Antagonists and Direct Oral Anticoagulants in Patients With Atrial Fibrillation Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. Clin. Appl. Thromb./Hemost. 2023, 29, 10760296231158585. [Google Scholar] [CrossRef]
- Li, D.; Ma, X.; Zhou, X.; Qian, Y. Non-Vitamin K Oral Anticoagulant After Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 755009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lip, G.Y.H.; Jensen, M.; Melgaard, L.; Skjøth, F.; Nielsen, P.B.; Larsen, T.B. Stroke and bleeding risk scores in patients with atrial fibrillation and valvular heart disease: Evaluating ‘valvular heart disease’ in a nationwide cohort study. Europace 2019, 21, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chan, E.W.; Hai, J.J.; Wong, C.K.; Lau, Y.M.; Huang, D.; Lam, C.C.; Tam, C.C.F.; Wong, Y.T.A.; Yung, S.Y.A.; et al. Protocol, rationale and design of DAbigatran for Stroke PreVention In Atrial Fibrillation in MoDerate or Severe Mitral Stenosis (DAVID-MS): A randomised, open-label study. BMJ Open 2020, 10, e038194. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Connolly, S.J.; Ntsekhe, M.; Benz, A.; Rangarajan, S.; Lewis, G.; Yun, Y.; Sharma, S.K.; Maklady, F.; Elghamrawy, A.E.; et al. INVICTUS Investigators. The INVICTUS rheumatic heart disease research program: Rationale, design and baseline characteristics of a randomized trial of rivaroxaban compared to vitamin K antagonists in rheumatic valvular disease and atrial fibrillation. Am. Heart J. 2020, 225, 69–77. [Google Scholar] [CrossRef]
- Zuhlke, L.; Engel, M.E.; Karthikeyan, G.; Rangarajan, S.; Mackie, P.; Cupido, B.; Mauff, K.; Islam, S.; Joachim, A.; Daniels, R.; et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: The Global Rheumatic Heart Disease Registry (the REMEDY study). Eur. Heart J. 2015, 36, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, M.D.; Parise, H.; Nagarakanti, R.; Noack, H.; Brueckmann, M.; Clemens, A.; Reilly, P.; Connolly, S.; Yusuf, S.; Wallentin, L. Comparison of dabigatran versus warfarin in patients with atrial fibrillation and valvular heart disease: The RE-LY trial. Circulation 2016, 134, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Breithardt, G.; Baumgartner, H.; Berkowitz, S.D.; Hellkamp, A.S.; Piccini, J.P.; Stevens, S.R.; Lokhnygina, Y.; Patel, M.R.; Halperin, J.L.; Singer, D.E.; et al. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur. Heart J. 2014, 35, 3377–3385. [Google Scholar] [CrossRef] [PubMed]
- Avezum, A.; Lopes, R.D.; Schulte, P.J.; Lanas, F.; Gersh, B.J.; Hanna, M.; Pais, P.; Erol, C.; Diaz, R.; Bahit, M.C.; et al. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease. Circulation 2015, 132, 624–632. [Google Scholar] [CrossRef]
- De Caterina, R.; Renda, G.; Carnicelli, A.P.; Nordio, F.; Trevisan, M.; Mercuri, M.F.; Ruff, C.T.; Antman, E.M.; Braunwald, E.; Giugliano, R.P. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. J. Am. Coll. Cardiol. 2017, 69, 1372–1382. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Milojevic, M.; Praz, F.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2021, 395, ezac209. [Google Scholar]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circ. Am. Heart Assoc. 2021, 143, e72–e227. [Google Scholar]
- Kim, J.Y.; Kim, S.-H.; Myong, J.-P.; Kim, Y.R.; Kim, T.-S.; Kim, J.-H.; Jang, S.-W.; Oh, Y.-S.; Lee, M.Y.; Rho, T.-H. Outcomes of direct oral anticoagulants inpatients with mitral stenosis. J. Am. Coll. Cardiol. 2019, 73, 1123–1131. [Google Scholar] [CrossRef]
- Tse, H.-F.; Wang, Y.-J.; Ai-Abdullah, M.A.; Pizarro-Borromeo, A.B.; Chiang, C.-E.; Krittayaphong, R.; Singh, B.; Vora, A.; Wang, C.-X.; Zubaid, M.; et al. Stroke prevention in atrial fibrillation--an Asian stroke perspective. Heart Rhythm. 2013, 10, 1082–1088. [Google Scholar] [CrossRef]
- Chong, B.-H.; Chan, K.-H.; Pong, V.; Lau, K.-K.; Chan, Y.-H.; Zuo, M.-L.; Lui, W.-M.; Leung, G.K.-K.; Lau, C.-P.; Tse, H.-F.; et al. Use of aspirin in Chinese after recovery from primary intracranial haemorrhage. Thromb. Haemost. 2012, 107, 241–247. [Google Scholar] [CrossRef]
- Friberg, L.; Rosenqvist, M.; Lip, G.Y. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish atrial fibrillation cohort study. Eur. Heart J. 2012, 33, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.; Lau, W.C.Y.; Siu, C.W.; Lip, G.Y.; Leung, W.K.; Anand, S.; Man, K.K.; Wong, I.C. Effect of suboptimalanticoagulation treatment with antiplatelet therapy and warfarin on clinical outcomes in patients with nonvalvular atrial fibrillation: A population-wide cohort study. Heart Rhythm. 2016, 13, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.C.; Mahboobani, N.R.; Lee, R.; Siu, C.W.; Cheung, R.T.F.; Ho, S.L.; Lau, K.K.; Chan, K.H. Warfarin associated intracerebral hemorrhage in Hong Kong Chinese. Neurol. Res. 2014, 36, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sadeghipour, P.; Pouraliakbar, H.; Parsaee, M.; Shojaeifard, M.; Farrashi, M.; JamalKhani, S.; Beheshti, A.T.; Rostambeigi, S.; Meimand, S.E.; Firouzi, A.; et al. RIvaroxaban in mitral stenosis (RISE MS): A pilot randomized clinical trial. Int. J. Cardiol. 2022, 356, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Connolly, S.J.; Yusuf, S. Rivaroxaban in Rheumatic Heart Disease–Associated Atrial Fibrilation. N. Engl. J. Med. 2022, 387, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Teppo, K.; Airaksinen, K.E.J.; Biancari, F.; Jaakkola, J.; Halminen, O.; Linna, M.; Haukka, J.; Putaala, J.; Mustonen, P.; Kinnunen, J.; et al. Aortic Stenosis and Outcomes in Patients With Atrial Fibrillation: A Nationwide Cohort Study. J. Am. Heart Assoc. 2023, 12, e029337. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.; Gislason, G.H.; Køber, L.; Abdulla, J.; Martinsson, A.; Smith, J.G.; Torp-Pedersen, C.; Andersson, C. Incidence of ischemic stroke in individuals with and without aortic valve stenosis: A Danish retrospective cohort study. Stroke 2020, 51, 1364–1371. [Google Scholar] [CrossRef]
- Andell, P.; Li, X.; Martinsson, A.; Andersson, C.; Stagmo, M.; Zöller, B.; Sundquist, K.; Smith, J.G. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart 2017, 103, 1696–1703. [Google Scholar] [CrossRef]
- Loscalzo, J. From clinical observation to mechanism—Heyde’s syndrome. N. Engl. J. Med. 2012, 367, 1954–1956. [Google Scholar] [CrossRef]
- Abuelazm, M.; Abdelazeem, B.; Katamesh, B.E.; Gamal, M.; Simhachalam Kutikuppala, L.V.; Kheiri, B.; Brašić, J.R.; Paul, T.K. The Effificacy and Safety of Direct Oral Anticoagulants versus Standard of Care in Patients without an Indication of Anti-Coagulants after Transcatheter Aortic Valve Replacement: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2022, 11, 6781. [Google Scholar] [CrossRef]
- Chugh, Y.; Patel, K.; Gonzalez, C.A.M.; Li, D.; Gössl, M. Anticoagulation in Patients with Aortic Stenosis and Atrial Fibrillation. Struct. Hear. 2020, 4, 360–368. [Google Scholar] [CrossRef]
- Dawwas, G.K.; Cuker, A. Apixaban versus Rivaroxaban in Patients with Atrial Fibrillation and Valvular Heart Disease: A Population-Based Study. Ann. Intern. Med. 2022, 175, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Nathan, A.S.; Yang, L.; Geng, Z.; Dayoub, E.J.; Khatana, S.A.M.; Fiorilli, P.N.; Herrmann, H.C.; Szeto, W.Y.; Atluri, P.; Acker, M.A.; et al. Oral anticoagulant use in patients with atrial fibrillation and mitral valve repair. Am. Heart J. 2021, 232, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Butany, J.; Fayet, C.; Ahluwalia, M.S.; Blit, P.; Ahn, C.; Munroe, C.; Israel, N.; Cusimano, R.J.; Leask, R.L. Biological replacement heart valves. Identif. Eval. Cardiovasc. Pathol. 2003, 12, 119–139. [Google Scholar] [CrossRef]
- David, T.E.; Ho, W.I.; Christakis, G.T. Thromboembolism in patients with aortic porcine bioprostheses. Ann. Thorac. Surg. 1985, 40, 229–233. [Google Scholar] [CrossRef]
- Asopa, S.; Patel, A.; Dunning, J. Is short-term anticoagulation necessary after mitral valve repair? Interact. Cardiovasc. Thorac. Surg. 2006, 5, 761–765. [Google Scholar] [CrossRef]
- Guimaraes, H.P.; Lopes, R.D.; Silva, P.G.D.B.E.; Liporace, I.L.; Sampaio, R.O.; Tarasoutchi, F.; Hoffmann-Filho, C.R.; Patriota, R.D.L.S.; Leiria, T.L.; Lamprea, D.; et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N. Engl. J. Med. 2020, 383, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Shim, C.Y.; Seo, J.; Kim, Y.J.; Lee, S.H.; De Caterina, R.; Lee, S.; Hong, G.R. Explore the Efficacy and Safety of Edoxaban in Patients after Heart Valve Repair or Bioprosthetic Valve Replacement (ENAVLE) study group. Efficacy and safety of edoxaban in patients early after surgical bioprosthetic valve implantation or valve repair: A randomized clinical trial. J. Thorac. Cardiovasc. Surg. 2021, 202. [Google Scholar]
- Strange, J.E.; Sindet-Pedersen, C.; Staerk, L.; Grove, E.L.; Gerds, T.A.; Torp-Pedersen, C.; Gislason, G.H.; Olesen, J.B. All-cause mortality, stroke, and bleeding in patients with atrial fibrillation and valvular heart disease. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, f93–f100. [Google Scholar] [CrossRef]
- Carnicelli, A.P.; De Caterina, R.; Halperin, J.L.; Renda, G.; Ruff, C.T.; Trevisan, M.; Nordio, F.; Mercuri, M.F.; Antman, E.; Giugliano, R.P.; et al. Edoxaban for the Prevention of Thromboembolism in Patients with Atrial Fibrillation and Bioprosthetic Valves. Circulation. 2017, 135, 1273–1275. [Google Scholar] [CrossRef]
- Guimarães, P.O.; Pokorney, S.D.; Lopes, R.D.; Wojdyla, D.M.; Gersh, B.J.; Giczewska, A.; Carnicelli, A.; Lewis, B.S.; Hanna, M.; Wallentin, L.; et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair: Insights from the ARISTOTLE trial. Clin. Cardiol. 2019, 42, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; Gott, V.L.; Harker, L.A.; Miller, J.; Naftel, D.C.; Turpie, A.G.G.; Akins, C.W. Mechanical valves. AnnThorac Surg. 1996, 62, 1567–1570. [Google Scholar]
- Sun, J.C.J.; Davidson, M.J.; Lamy, A.; Eikelboom, J.W. Antithrombotic management of patients with prosthetic heart valves: Current evidence and future trends. Lancet. 2009, 374, 565–576. [Google Scholar] [CrossRef]
- Fanaroff, A.C.; Vora, A.N.; Lopes, R.D. Non-vitamin K antagonist oral anticoagulants in patients with valvular heart disease. Eur. Heart J. Suppl. 2022, 24, A19–A31. [Google Scholar] [CrossRef]
- Al Rawahi, M.N.; Al-Maqbali, J.S.; Al Noumani, J.; Al Alawi, A.M.; Essebag, V. Novel Oral Anticoagulants in Patients With Atrial Fibrillation and Moderate to Severe Mitral Stenosis: A Systematic Review. Cureus 2023, 15, e33222. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Stafford, A.R. Dabigatran is less effective than warfarin at attenuating mechanical heart valve-induced thrombin generation. J. Am. Heart Assoc. 2015, 4, e002322. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.; Asgar, A.; Cartier, R.; Virmani, R.; Bonan, R. Anatomo-pathological analysis after CoreValve Revalving system implantation. Eurointervention 2009, 5, 78–85. [Google Scholar] [CrossRef]
- Durães, A.R.; de S.L. Bitar, Y.; Lima, M.L.G.; Santos, C.C.; Schonhofen, I.S.; Filho, J.A.L.; Roever, L. Usefulness and Safety of Rivaroxaban i Patients Following Isolated Mitral Valve Replacement With a Mechanical Prosthesis. Am. J. Cardiol. 2018, 122, 10471050. [Google Scholar] [CrossRef]
- Chu, M.W.A.; Ruel, M.; Graeve, A.; Gerdisch, M.W.; Damiano, R.J.; Smith, R.L.; Keeling, W.B.; Wait, M.A.; Hagberg, R.C.; Quinn, R.D.; et al. PROACT Mitral Investigators. Low-Dose vs Standard W arfarin After Mechanical Mitral Valve Replacement: A Randomized Trial. Ann. Thorac. Surg. 2023, 115, 929–938. [Google Scholar] [CrossRef]
- Greco, A.; Capodanno, D. Anticoagulation after transcatheter aortic valve implantation: Current status. Interv Cardiol. 2020, 15, e02. [Google Scholar] [CrossRef] [PubMed]
- Al-Azizi, K.; Hamandi, M.; Mack, M. Clinical trials of transcatheter aortic valve replacement. Heart 2019, 105, s6–s9. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.; Prendergast, B.; Hildick-Smith, D.; Blackman, D.; Anderson, R.; Spence, M.S.; Mylotte, D.; Smith, D.; Wilding, B.; Chapman, C.; et al. An Update on Anti-thrombotic Therapy Following Transcatheter Aortic Valve Implantation: Expert Cardiologist Opinion from a UK and Ireland Delphi Group. Interv. Cardiol. 2023, 18, e13. [Google Scholar] [CrossRef]
- Tanawuttiwat, T.; Stebbins, A.; Marquis-Gravel, G.; Vemulapalli, S.; Kosinski, A.S.; Cheng, A. Use of Direct Oral Anticoagulant and Outcomes in Patients With Atrial Fibrillation after Transcatheter Aortic Valve Replacement: Insights From the STS/ACC TVT Registry. J. Am. Heart Assoc. 2022, 11, e023561. [Google Scholar] [CrossRef]
- Gatto, L.; Scalia, L. The temptation of anticoagulant therapy after transcatheter aortic valve implantation. Eur. Heart J. Suppl. 2023, 25, B95–B98. [Google Scholar] [CrossRef]
- Geis, N.A.; Kiriakou, C.; Chorianopoulos, E.; Uhlmann, L.; Katus, H.A.; Bekeredjian, R. NOAC monotherapy in patients with concomitant indications for oral anticoagulation undergoing transcatheter aortic valve implantation. Clin. Res. Cardiol. 2018, 107, 799–806. [Google Scholar] [CrossRef]
- Seeger, J.; Gonska, B.; Rodewald, C.; Rottbauer, W.; Wöhrle, J. Apixaban in patients with atrial fibrillation after transfemoral aortic valve replacement. JACC Cardiovasc. Interv. 2017, 10, 66–74. [Google Scholar] [CrossRef]
- Didier, R.; Lhermusier, T.; Auffret, V.; Eltchaninoff, H.; Le Breton, H.; Cayla, G.; Commeau, P.; Collet, J.P.; Cuisset, T.; Dumonteil, N.; et al. TAVR patients requiring anticoagulation: Direct oral anticoagulant or vitamin K antagonist? JACC Cardiovasc. Interv. 2021, 14, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.H.; Backer, O.; Olesen, J.B.; Gerds, T.A.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Søndergaard, L.; Køber, L.; Fosbøl, E.L. Vitamin K antagonists versus direct oral anticoagulants after transcatheter aortic valve implantation in atrial fibrillation. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 11–19. [Google Scholar] [CrossRef]
- Jochheim, D.; Barbanti, M.; Capretti, G.; Stefanini, G.G.; Hapfelmeier, A.; Zadrozny, M.; Baquet, M.; Fischer, J.; Theiss, H.; Todaro, D.; et al. Oral anticoagulant type and outcomes after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2019, 12, 1566–1576. [Google Scholar] [CrossRef]
- Ten Berg, J.; Sibbing, D.; Rocca, B.; Van Belle, E.; Chevalier, B.; Collet, J.-P.; Dudek, D.; Gilard, M.; Gorog, D.A.; Grapsa, J.; et al. Management of antithrombotic therapy in patients undergoing transcatheter aortic valve implantation: A consensus document of the ESC working group on thrombosis and the European association of percutaneous cardiovascular interventions (EAPCI), in collaboration with the ESC council on valvular heart disease. Eur. Heart J. 2021, 42, 2265–2269. [Google Scholar] [PubMed]
- Ryu, R.; Tran, R. DOACs in Mechanical and Bioprosthetic Heart Valves: A Narrative Review of Emerging Data and Future Directions. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221103578. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Unverdorben, M.; Hengstenberg, C.; Möllmann, H.; Mehran, R.; López-Otero, D.; Nombela-Franco, L.; Moreno, R.; Nordbeck, P.; Thiele, H.; et al. ENVISAGE-TAVI AF Investigators. Edoxaban versus vitamin K antagonist for atrial fibrillation after TAVR. N. Engl. J. Med. 2021, 385, 2150–2160. [Google Scholar] [CrossRef]
- Pokorney, S.D.; Rao, M.P.; Wojdyla, D.M.; Gersh, B.J.; Lopes, R.D.; Lewis, B.S.; Hanna, M.; Avezum, A.; Wallentin, L.; Alexander, J.H.; et al. Apixaban use in patients with atrial fibrillation with bioprosthetic valves: Insights from ARISTOTLE. Circulation 2015, 132, A17277. [Google Scholar] [CrossRef]
- Collet, J.P. Anti-Thrombotic strategy to lower all cardiovascular and neurologic ischemic and hemorrhagic events after trans-aortic valve implantation for aortic stenosis—ATLANTIS. In Proceedings of the American College of Cardiology Virtual Annual Scientific Session (ACC), Virtual Meeting, 15–17 May 2021. [Google Scholar]
- Wang, T.Y.; Svensson, L.G.; Wen, J.; Vekstein, A.; Gerdisch, M.; Rao, V.U.; Moront, M.; Johnston, D.; Lopes, R.D.; Chavez, A.; et al. Apixaban or warfarin in patients with an On-X mechanical aortic valve. NEJM Evid. 2023, 2, EVIDoa2300067. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.S.; Teixeira, A.; Henriques, T.S.; Monteiro, H.; Martins, C. AF-React study: Prevalence of thrombotic events in patients with atrial fibrillation receiving NOACs—Real-world data analysis from northern Portugal primary healthcare. Front. Med. 2024, 11, 1273304. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef]
- Pastori, D.; Menichelli, D.; Gingis, R.; Pignatelli, P.; Violi, F. Tailored Practical Management of Patients With Atrial Fibrillation: A Risk Factor-Based Approach. Front. Cardiovasc. Med. 2019, 6, 17. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef]
- Connolly, S.J.; Eikelboom, J.; Joyner, C.; Diener, H.-C.; Hart, R.; Golitsyn, S.; Flaker, G.; Avezum, A.; Hohnloser, S.H.; Diaz, R.; et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 2011, 364, 806–817. [Google Scholar] [CrossRef] [PubMed]

| Study | Comparison | Major Outcomes | CHA2DS2-VASc Scores | Age | Comorbidities | Percent of Patients with Particularly Aetiology of Valvopathy | Reference |
|---|---|---|---|---|---|---|---|
| ARISTOTLE | Apixaban vs. VKA | When it came to avoiding stroke or systemic embolism, apixaban outperformed warfarin, produced less bleeding, and decreased mortality. | the mean CHADS2 score was 2.1 | median age was 70 years | 19% previously experienced a transient ischaemic attack or stroke, or systemic embolis | 19.4% Mitral regurgitation 0.7% Mitral stenosis (mild) 2.1% Aortic stenosis | [10] |
| ROCKET-AF | Rivaroxaban vs. VKA | Warfarin and rivaroxaban emerged to b[e equally efficient in averting strokes and systemic embolism. | Mean score 3–3.5 | Median age 73 | 90.5% with hypertension, 62.5% with heart failure, and 40.0% with diabetes. A history of stroke, systemic embolism, or transient ischaemic attack was present in 54.8% of the patients. | 12.4% mitral regurgitation 3.4% aortic regurgitation 1.5% aortic stenosis | [9] |
| RE-LY | Dabigatran vs. VKA | Compared to warfarin, dabigatran 150 mg was linked to equal rates of serious bleeding but lower incidence of stroke and systemic embolism. Dabigatran 110 mg showed comparable statistics for systemic embolism and stroke, along with decreased rates of severe bleeding. | mean CHADS2 score was 2.1 | 65 to 74 years | diabetes mellitus, hypertension, or coronary artery disease | 17.1% Mitral regurgitation 1.1% Mitral stenosis (mild) 2.6% Aortic stenosis | [8] |
| ENGAGE AF | Edoxaban vs. VKA | When it came to preventing stroke or systemic embolism, both once-daily edoxaban regimens were comparable to warfarin. | Mean score 2.8 | Median age 72 | Diabetes mellitus, hypertension, congestive heart failure, prior stroke | 10.7% mitral regurgitation 1.7% aortic regurgitation 0.8% aortic stenosis | [11] |
| Study | Population | Comparison | Major Outcomes | CHA2DS2-VASc Scores | Age | Comorbidities | Percent of Patients with Particularly Aetiology of Valvopath | Reference |
|---|---|---|---|---|---|---|---|---|
| DAVID-MS | Moderate or severe rheumatic mitral stenosis | Dabigatran vs.VKA | not yet conducted | - | >18 years | moderate or severe mitral stenosis | on-going | [29] |
| RISE MS | Atrial fibrillation with mitral stenosis | Rivaroxaban vs. warfarin | Over the course of a one-year follow-up, there were no differences in ischaemic stroke, systemic embolic events, or severe bleeding between the rivaroxaban and warfarin groups. | was not defined | Mean age 56–60 | Hypertension, diabetes, heart failure, ischemic cerebrovascular accidents, coronary artery disease | All patients included had moderate to severe mitral stenosis | [44] |
| INVICTUS-VK | atrial fibrillation and moderate or severe rheumatic mitral stenosis | Rivaroxaban vs.VKA | Compared to rivaroxaban medication, vitamin K antagonist therapy resulted in a decreased composite rate of cardiovascular events or mortality without a greater bleeding rate. | CHA2DS2VASc score of at least 2 | average age of the patients was 50.5 years | Rheumatic heart disease and atrial fibrillation, hypertension, coronary artery disease, diabetes mellitus, congestive heart failure | Moderate-to-severe mitral stenosis was present in 81.9% of the patients | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouatu, A.; Buliga-Finiș, O.N.; Tanase, D.M.; Badescu, M.C.; Dima, N.; Floria, M.; Popescu, D.; Richter, P.; Rezus, C. Optimizing Anticoagulation in Valvular Heart Disease: Navigating NOACs and VKAs. J. Pers. Med. 2024, 14, 1002. https://doi.org/10.3390/jpm14091002
Ouatu A, Buliga-Finiș ON, Tanase DM, Badescu MC, Dima N, Floria M, Popescu D, Richter P, Rezus C. Optimizing Anticoagulation in Valvular Heart Disease: Navigating NOACs and VKAs. Journal of Personalized Medicine. 2024; 14(9):1002. https://doi.org/10.3390/jpm14091002
Chicago/Turabian StyleOuatu, Anca, Oana Nicoleta Buliga-Finiș, Daniela Maria Tanase, Minerva Codruta Badescu, Nicoleta Dima, Mariana Floria, Diana Popescu, Patricia Richter, and Ciprian Rezus. 2024. "Optimizing Anticoagulation in Valvular Heart Disease: Navigating NOACs and VKAs" Journal of Personalized Medicine 14, no. 9: 1002. https://doi.org/10.3390/jpm14091002









