Surface Integrity of Austenitic Manganese Alloys Hard Layers after Cavitation Erosion
Abstract
:1. Introduction
2. Materials and Experimental Details
- -
- Basic coated electrode: CITOMANGAN
- -
- Electrode diameter: 3.25 mm
- -
- Polarity: DC+
- -
- Pulse current, Ip: 180 A
- -
- Base current, Ib: 90 A (50% of Ip)
- -
- Average current, Im: 135 A
- -
- Arc voltage, U: 22 V
- -
- Pulse frequency, f = 1/tc = 5 Hz
- -
- Cycle time, tc = 1/f = 1/5 = 0.2 s
- -
- Pulse time, tp = 0.5 tc = 0.1 s
- -
- Base time, tb = 0.5 tc = 0.1 s (tp = tb)
- -
- Arc length, la = 3.0 mm
- -
- Welding rate, v = 27–28 cm/min.
- Installed power: 500 W
- Vibration frequency: 20 kHz
- Vibration amplitude: 50 μm ± 5%
- Supply voltage: 220 V/50 Hz
- Working fluid: tap water
3. Discussion of the Experimental Results
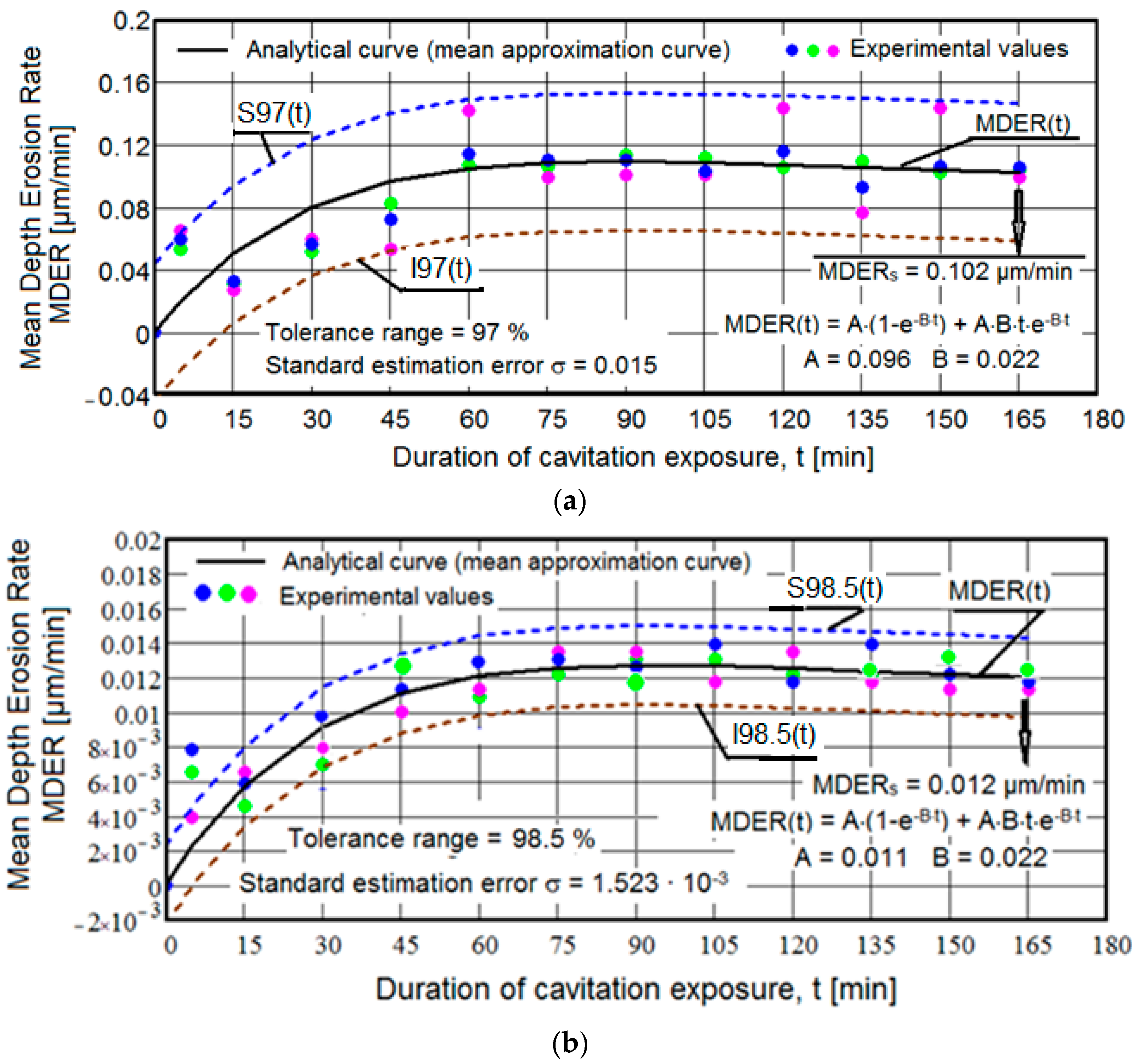
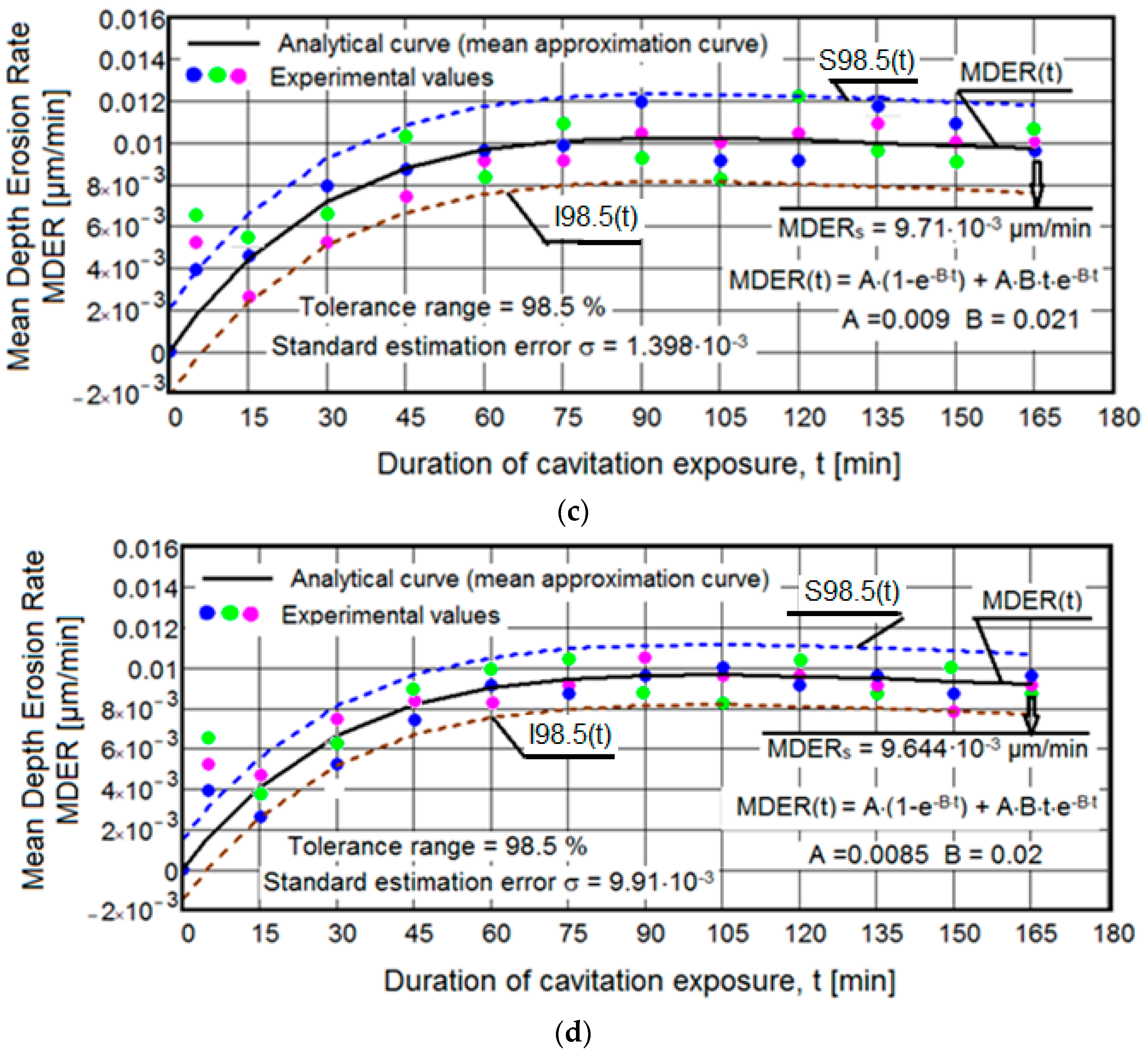
3.1. Cavitation Curves
- -
- For the mean depth erosion rate during an intermediate period,
- -
- -
- For the mean standard deviation,
- -
- For the 99%tolerance interval,
- -
- For the 90%tolerance interval,
- -
- There are very small values of the mean standard deviation (maximum of 0.015) and a high tolerance interval (minimum of 97%), indicating the accuracy of the experimental program conducted on sets of three samples from each structural state. Based on our experience, we believe that these statistical parameter values are due not only to the rigorous control of the functional parameters of the vibrating apparatus but also to the heat treatment technologies and pulsed current welding deposition, which have led to high hardness values on the cavitated surface. The fact that, for samples with deposited layers, the values of the mean standard deviation are of the same order, below 0.01, and the tolerance intervals are identical (98.5%), is a confirmation of the much higher hardness of these structures.
- -
- Regardless of the structural state, there is interference and random arrangement of the experimental values of the three samples relative to the mediating curve MDER(t). This behavior is natural and shows that at the same cavitation duration, the sizes of the fractured and expelled grains differ, but overall, the behavior of the structures under the cyclic demands of cavitation microjets is identical.
- -
- For all samples, the MDER(t) mediating curve tends to decrease slightly towards the final value MDERs (9.664 × 10−3 µm/min. for samples with three deposited layers, 9.71 × 10−3 µm/min. for samples with two deposited layers, 0.012 µm/min for samples with one deposited layer, and 0.102 µm/min for samples with heat treatment). This behavior, according to our experience and the literature data, is characteristic of structures with very high resistance to the pressure forces created upon impact with microjets and shock waves generated during cavitation [1,5,6]. Also, this evolution is caused by the fact that after reaching maximum values, with the increase in cavitation duration until the end of the test, under the impact of pressure forces of microjets and shock waves, the cavities created expand in the plane, their increase in depth being much smaller, because of the damping effect of air and water penetrated into them. It should be emphasized that the cyclic cavitation load generates a significant effect of hardening by mechanical work on the layer under stress, which contributes most to the reduction in mass losses and, consequently, to the reduction in the erosion penetration rate.
- -
- For samples with deposited layers, the experimental values recorded after 5 min of cavitation attack are located outside the dispersion band. These high values are the effects of losses generated by the removal of abrasive dust and the tips of roughness, and do not express actual material losses.
- -
- Based on the mean depth erosion rate values, MDERs, the cavitation erosion resistance of the structure with deposited layers is significantly superior to the structure obtained after solution treatment. By comparison, the increase in cavitation resistance, compared to the structure obtained by the applied heat treatment, is approximately 8.5 times for samples with one deposited layer, about 10.5 times for samples with two deposited layers, and about 10.55 times for samples with three deposited layers.
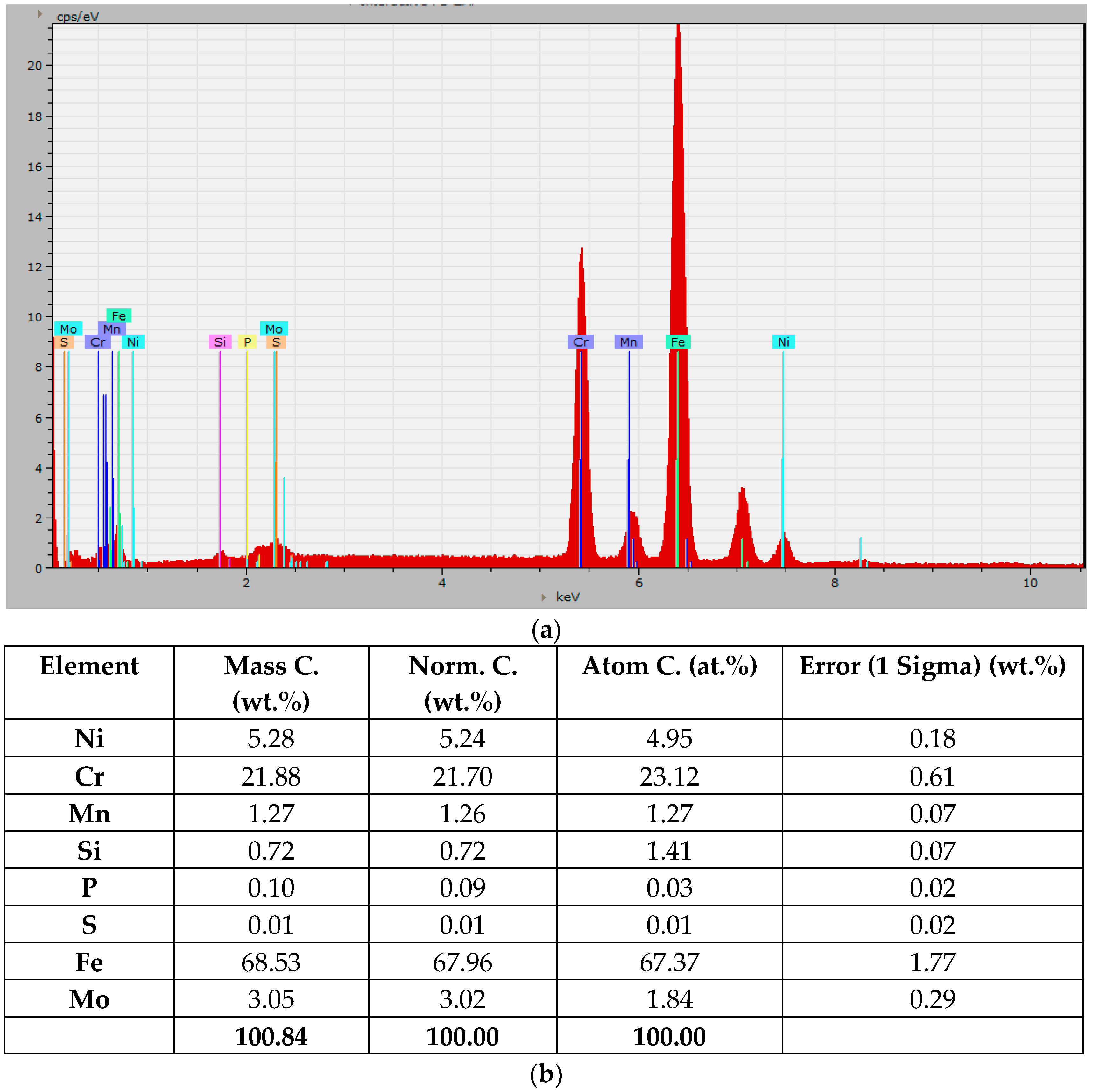
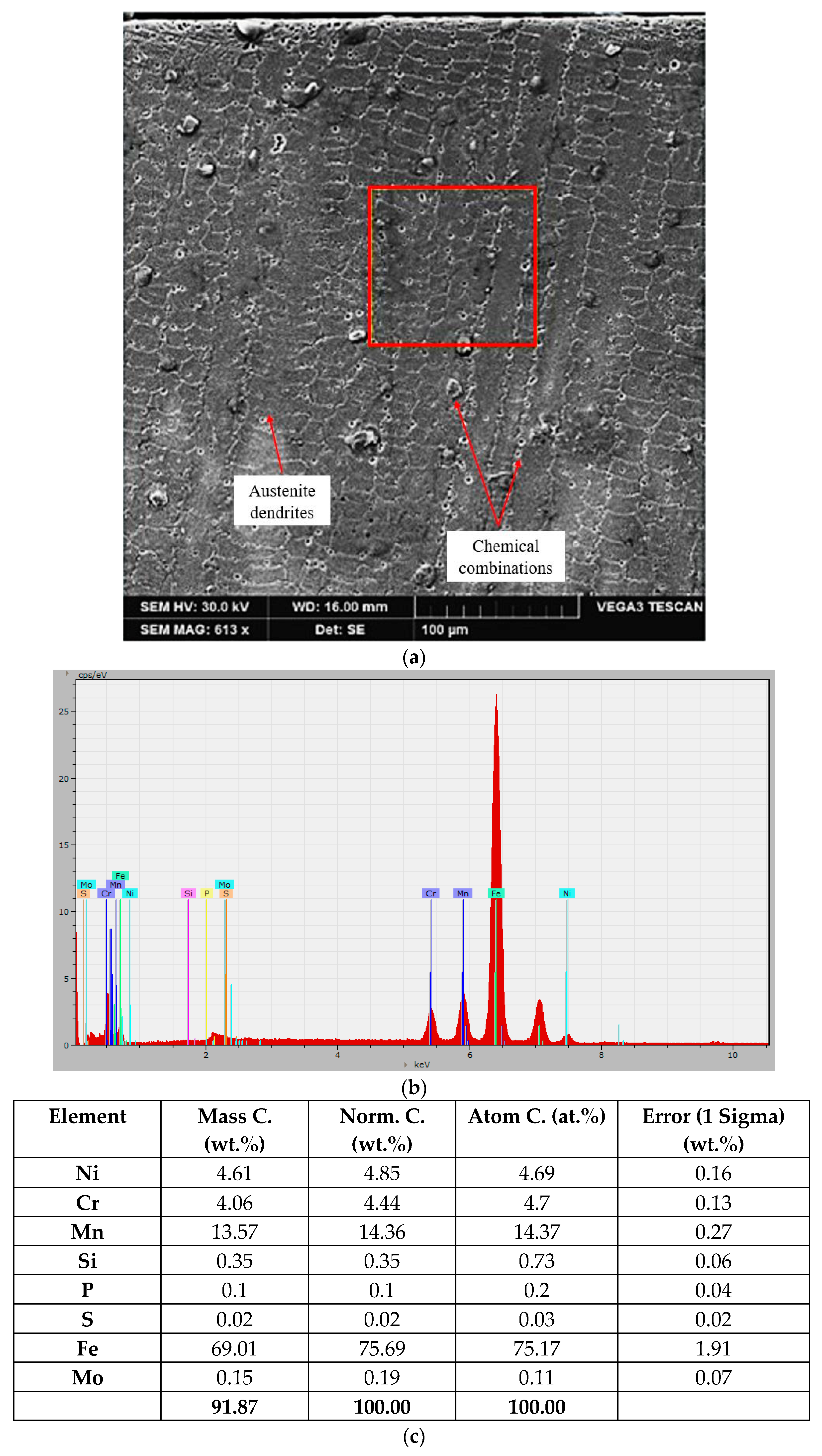
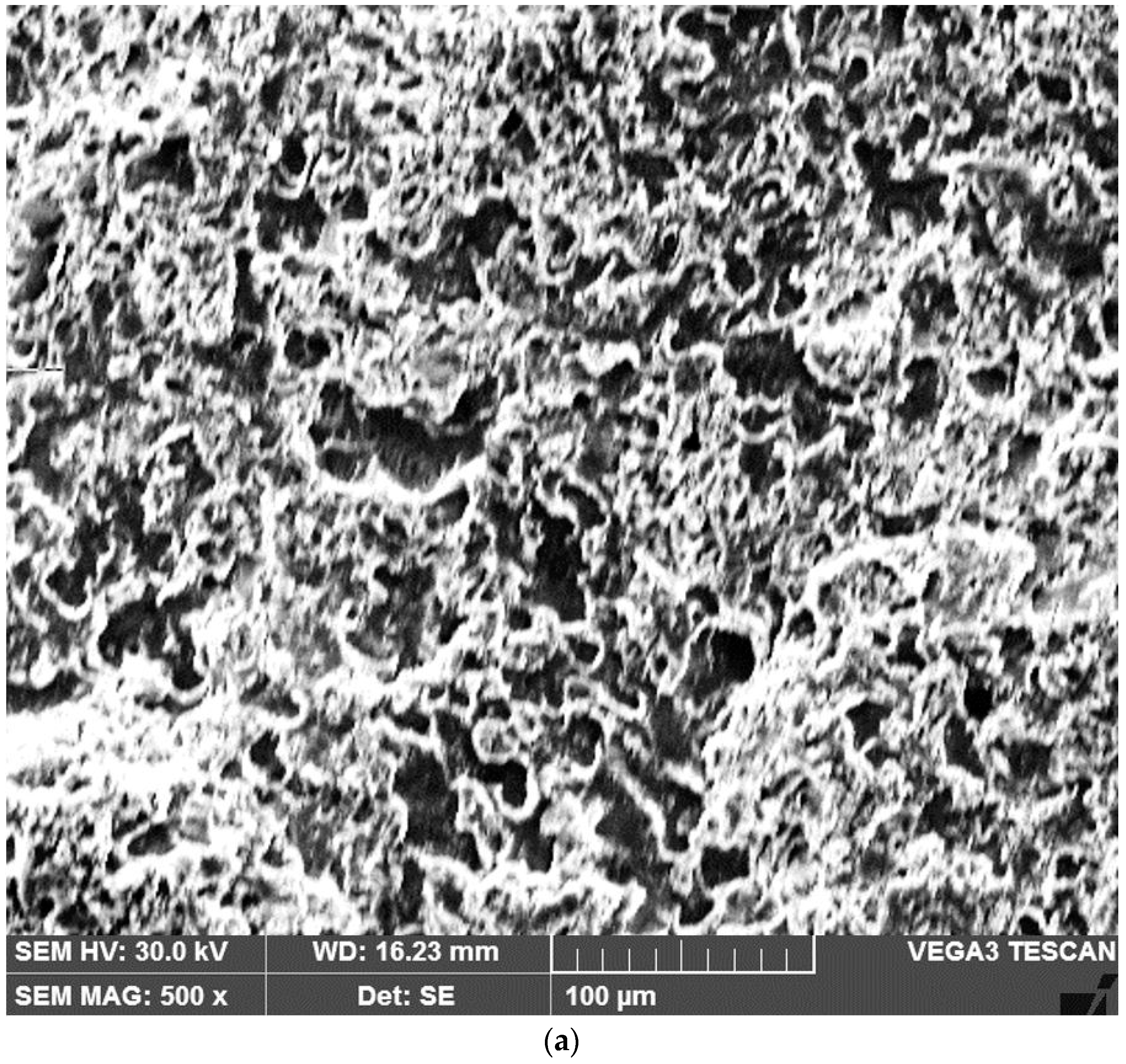
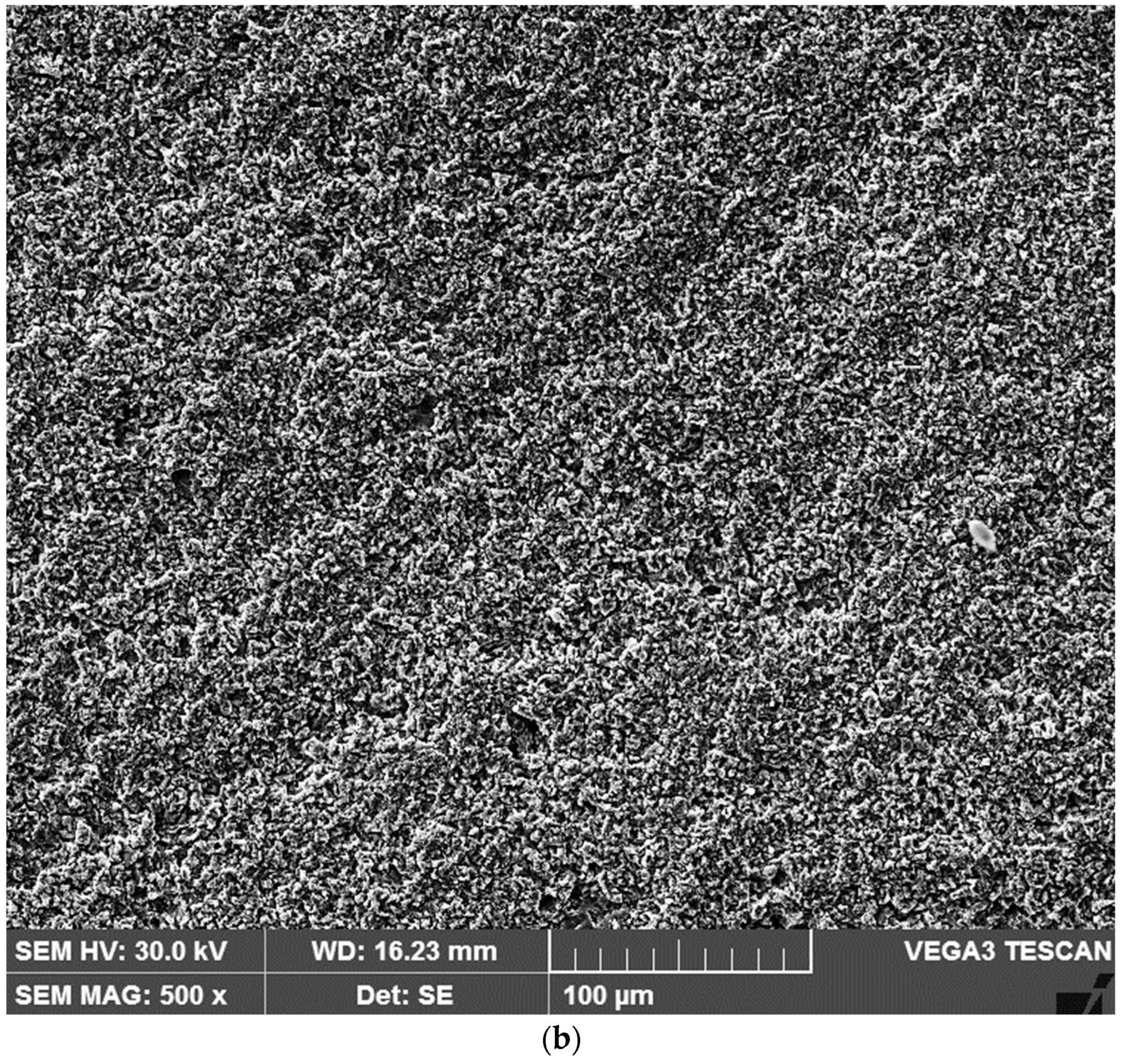
3.2. Microstructural Observations. Degree of Dilution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carlton, J. Cavitation, in Marine Propellers and Propulsion, 2nd ed.; Carlton, J., Ed.; Butterworth-Heinemann: Oxford, UK, 2012; pp. 209–250. [Google Scholar]
- Lloyd’s Register Propeller root cavitation erosion. Tech. Matters 2006, 1, 4–5.
- Ge, M.; Sun, C.; Zhang, G.; Coutier-Delgosha, O.; Fan, D. Combined suppression effects on hydrodynamic cavitation performance in Venturi-type reactor for process intensification. Ultrason. Sonochem. 2022, 86, 106035. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Petkovšek, M.; Zhang, G.; Jacobs, D.; Coutier-Delgosha, O. Cavitation dynamics and thermodynamic effects at elevated temperatures in a small Venturi channel. Int. J. Heat Mass Transfer 2021, 170, 120970. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Li, B.; Zhai, J.; Song, J. Cavitation Erosion Characteristics for Different Metal Surface and Influencing Factors in Water Flowing System. Appl. Sci. 2022, 12, 5840. [Google Scholar] [CrossRef]
- Ghose, J.P.; Gokarn, R.P. Propeller Materials. Basic Ship Propulsion; Allied Publishers: Kharagpur, India, 2004; pp. 166–173. [Google Scholar]
- Fitch, E.C. Cavitation Wear in Hydraulic Systems. Machinery Lubrication. 2011. Available online: http://www.machinerylubrication.com/Articles/Print/380 (accessed on 20 June 2024).
- Shinde, P.; Satam, A. Cavitation Effect in Centrifugal Pump. International Journal of Researchers. Sci. Dev. 2014, 2, 20–23. [Google Scholar]
- Kurosawa, Y.; Kato, K.; Saito, S.; Kubo, M.; Uzuka, T.; Fujii, Y.; Takahashi, H. Basic study of brain injury mechanism caused by cavitation. Engineering in Medicine and Biology Society 2009. EMBC 2009. In Proceedings of the Annual International Conference of the IEEE, Minneapolis, MN, USA, 3–6 September 2009; pp. 7224–7227. [Google Scholar]
- Brennen, C.E. Hydrodynamics of Pumps—Bubble Dynamics, Damage and Noise; NREC: Pittsburgh, PA, USA, 1994; Available online: http://brennen.caltech.edu/HTMPUM/chap6.htm (accessed on 11 July 2024).
- Carlton, J.S. Cavitation. Marine Propellers and Propulsion; Elsevier: Amsterdam, The Netherlands, 2012; pp. 431–458. [Google Scholar]
- Materials for Propeller Fabrication. Rules for Classification and Construction Materials and Welding, 2nd ed.; Germanischer Lloyd Aktiengesellschaft: Hamburg, Germany, 2009; pp. 1–11. Available online: https://www.dnv.com/rules-standards/gl-rules-guidelines/ (accessed on 25 June 2024).
- Practical Guidelines for the Fabrication of Duplex Stainless Steels; International Molybdenum Association (IMOA): London, UK, 2014; Available online: https://www.imoa.info/download_files/stainless-steel/Duplex_Stainless_Steel_3rd_Edition.pdf (accessed on 15 June 2024).
- Duplex Stainless Steel: IMOA. Available online: https://www.imoa.info/molybdenum-uses/molybdenum-grade-stainless-steels/duplex-stainless-steel.php (accessed on 10 May 2024).
- Abreu, C.M.; Cristóbal, M.J.; Losada, R.; Nóvoa, X.R.; Pena, G.; Pérez, M.C. Comparative study of passive films of different stainless steels developed on alkaline medium. Electrochim. Acta 2004, 49, 3049–3056. [Google Scholar] [CrossRef]
- Wallén, B. Corrosion of Duplex Stainless Steels in Seawater. In Duplex Stainless Steels, World Conference; KCI Publishing: Zutphen, The Netherlands, 1997; Volume 5, pp. 59–71. [Google Scholar]
- Francis, R. Bimetallic Corrosion, Guides to Good Practice in Corrosion Control; 1982; Available online: https://www.npl.co.uk/getattachment/research/electrochemistry/corrosion-guides/Corrosion-Guide-No-5.pdf.aspx?lang=en-GB (accessed on 25 June 2024).
- Aribo, S. Corrosion and Erosion-Corrosion. Behaviour of Lean Duplex Stainless Steels in Marine and Oilfield Environments. Ph.D. Thesis, University of Leeds, Leeds, UK, 2014. Available online: https://etheses.whiterose.ac.uk/7170/1/PhD%20corrections-%20Thesis%20-Sunday%20Aribo.pdf (accessed on 10 July 2024).
- Aalco. Stainless Steel—Duplex 2205. Available online: http://www.aalco.co.uk/datasheets/Stainless-Steel_1.4462-2205_102.ashx (accessed on 24 March 2024).
- Stainless Steel Alloy Duplex 2205, UNS S32205. Available online: https://continentalsteel.com/stainless-steel/grades/alloy-duplex-2205-uns-s32205/ (accessed on 14 June 2024).
- Ai, W.; Lo, K.H.; Kwok, C.T. Cavitation erosion of a spinodally decomposed wrought duplex stainless steel in a benign environment. Wear 2019, 424–425, 111–121. [Google Scholar] [CrossRef]
- Escobar, J.D.; Velásquez, E.; Santos, T.F.A.; Ramirez, A.J.; Lópezp, D. Improvement of cavitation erosion resistance of a duplex stainless steel through friction stir processing (FSP). Wear 2013, 297, 998–1005. [Google Scholar] [CrossRef]
- Mesa, D.H.; Pinedo, C.E.; Tschiptschin, A.P. Improvement of the cavitation erosion resistance of UNS S31803 stainless steel by duplex treatment. Surf. Coat. Technol. 2010, 205, 1552–1556. [Google Scholar] [CrossRef]
- Hattori, S.; Ishikura, R. Revision of cavitation erosion database and analysis of stainless steel data. Wear 2010, 268, 109–116. [Google Scholar] [CrossRef]
- Standard G32; Standard Method of Vibratory Cavitation Erosion Test. ASTM: West Conshohocken, PA, USA, 2016.
- Mitelea, I.; Mutascu, D.; Karancsi, O.; Crăciunescu, C.M.; Buzdugan, D.; Utu, I.D. Microstructural Investigations of Weld Deposits from Manganese Austenitic Alloy on X2CrNiMoN22-5-3 Duplex Stainless Steel. Appl. Sci. 2024, 14, 3751. [Google Scholar] [CrossRef]
- Bordeasu, I. Monografia Laboratorului de Cercetare a Eroziunii prin Cavitatie al Universitatii Politehnica Timisoara, 1960–2020; Editura Politehnica: Timişoara, Romania, 2020. [Google Scholar]
- Bordeaşu, I.; Patrascoiu, C.; Badarau, R.; Sucitu, L.; Popoviciu, M.; Balasoiu, V. New contributions in cavitation erosion curves modeling, FME Transactions Faculty of Mechanical Engineering; University of Belgrade: Belgrade, Serbia, 2006; New Series, Volume 34, Nr.1/2006, YU ISSN 1451-2092; pp. 39–44. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitelea, I.; Bordeașu, I.; Mutașcu, D.; Crăciunescu, C.M.; Uțu, I.D. Surface Integrity of Austenitic Manganese Alloys Hard Layers after Cavitation Erosion. Lubricants 2024, 12, 330. https://doi.org/10.3390/lubricants12100330
Mitelea I, Bordeașu I, Mutașcu D, Crăciunescu CM, Uțu ID. Surface Integrity of Austenitic Manganese Alloys Hard Layers after Cavitation Erosion. Lubricants. 2024; 12(10):330. https://doi.org/10.3390/lubricants12100330
Chicago/Turabian StyleMitelea, Ion, Ilare Bordeașu, Daniel Mutașcu, Corneliu Marius Crăciunescu, and Ion Dragoș Uțu. 2024. "Surface Integrity of Austenitic Manganese Alloys Hard Layers after Cavitation Erosion" Lubricants 12, no. 10: 330. https://doi.org/10.3390/lubricants12100330





