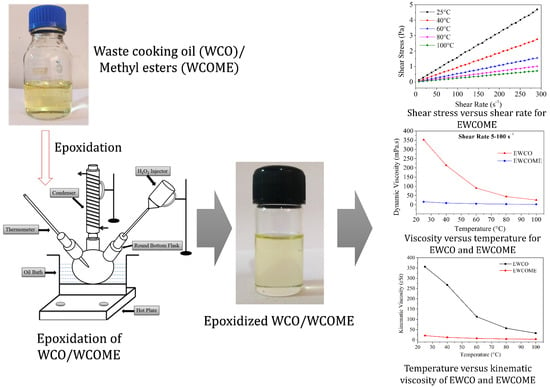
In-Situ Epoxidation of Waste Cooking Oil and Its Methyl Esters for Lubricant Applications: Characterization and Rheology
Abstract
:1. Introduction
2. Materials and Experimental Methods
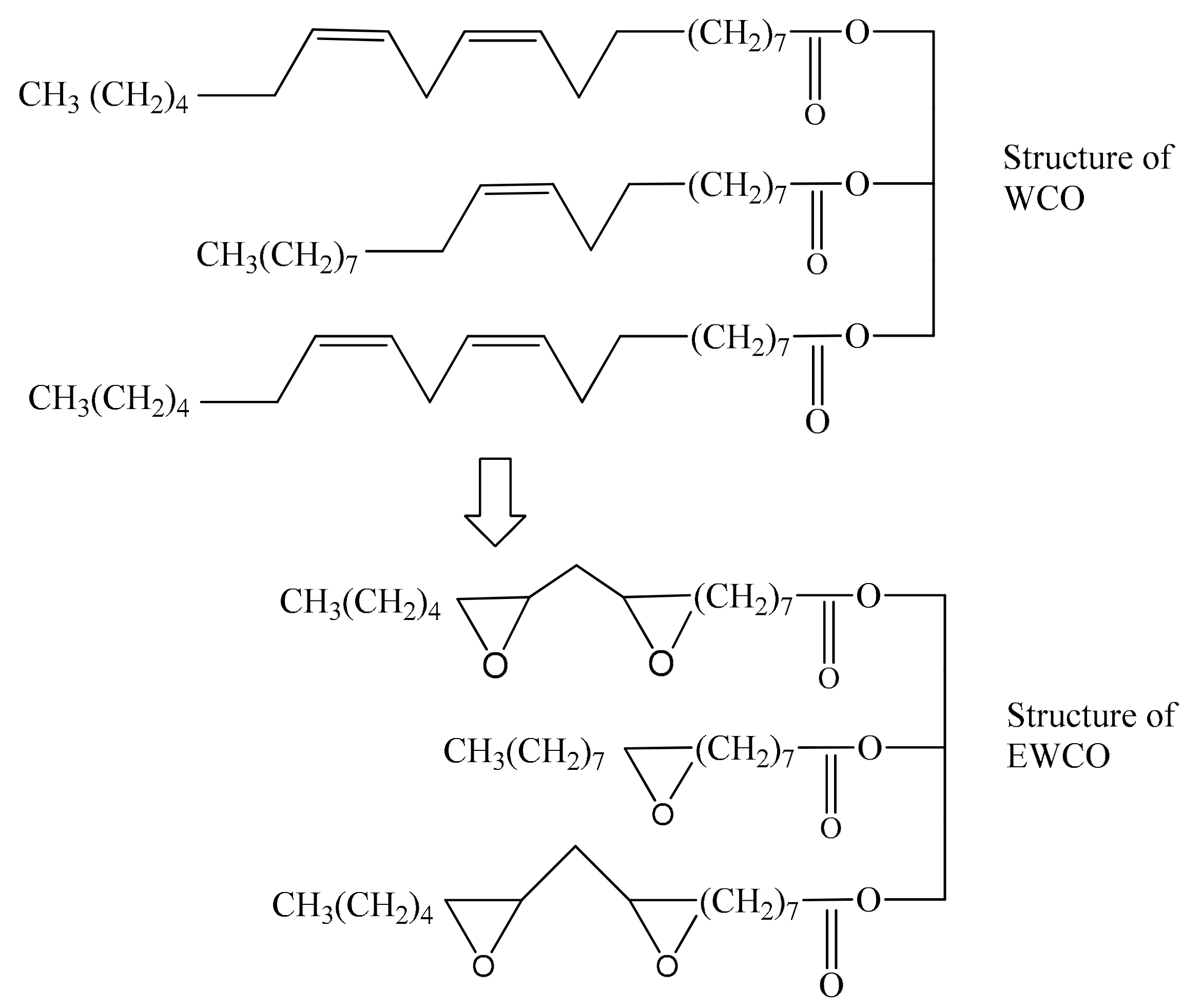
2.1. Waste Cooking Oil Methyl Esters and Epoxide Synthesis
2.2. Physical and Chemical Properties of EWCO and EWCOME
2.3. Rheological Measurements
2.4. Iodine Value (IV, gI2/100 g)
2.5. Oxirane Value (% by Mass)
2.6. Chemical Composition (GC)
2.7. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
3.1. Physicochemical Properties of WCO and WCOME Epoxides
| Properties | Unit | EWCO | EWCOME | WCO | Epoxidized Waste Cooking Oil Fatty Acid Methyl Esters [12] | Methyl Ester of 9,10-Palmitoyloxy-Acetoxy Stearic Acid (MEPASA) [33] | Epoxidized Soybean Oil (ESBO) [34] | ISO VG 32 [12] |
|---|---|---|---|---|---|---|---|---|
| Specific gravity | ‒ | 0.80 | 0.77 | 0.79 | - | - | - | - |
| Kinematic viscosity a | mm2/s | 278.9 ± 3.56 | 12.15 ± 0.87 | 49.84 ± 1.39 | 15.9 | 44.58 | 170.85 | 28.8 |
| Density | kg/m3 | 802.1 ± 4.89 | 773.8 ± 3.71 | 792.05 ± 4.58 | - | 942 | - | - |
| Iodine value | g I2/100 g | 1.74 ± 0.14 | 2.41 ± 0.17 | 132.94 ± 2.94 | - | 5.05 | 9.11 | - |
| Acid value | Mg KOH/g | 0.30 ± 0.07 | 0.20 ± 0.04 | 3.0 ± 0.12 | - | - | 0.09 | - |
| Free fatty acid | Mg KOH/g | 0.15 ± 0.04 | 0.10 ± 0.02 | 1.5 ± 0.05 | - | - | 0.045 | - |
| Refractive index at 24 °C | ‒ | 1.47 ± 0.1 | 1.48 ± 0.08 | 1.47 ± 0.07 | - | - | - | - |
| Pour point | °C | −6.2 ± 0.17 | 2.40 ± 0.03 | −7.8 ± 0.13 | −15 | −20 | - | −6 |
| Moisture content | % | 0.25 ± 0.02 | 0.18 ±0.03 | 0.27 ± 0.02 | - | - | - | - |
| Oxirane content (experimental) | ‒ | 4.69 ± 0.4 | 4.81 ± 0.5 | ‒ | - | - | - | - |
| Oxirane content (theoretical) | ‒ | 7.72 ± 0.2 | 7.94 ± 0.3 | ‒ | - | - | - | - |
| Relative percentage Conversion of oxirane (%) | ‒ | 60.75 | 60.57 | ‒ | - | - | - | - |
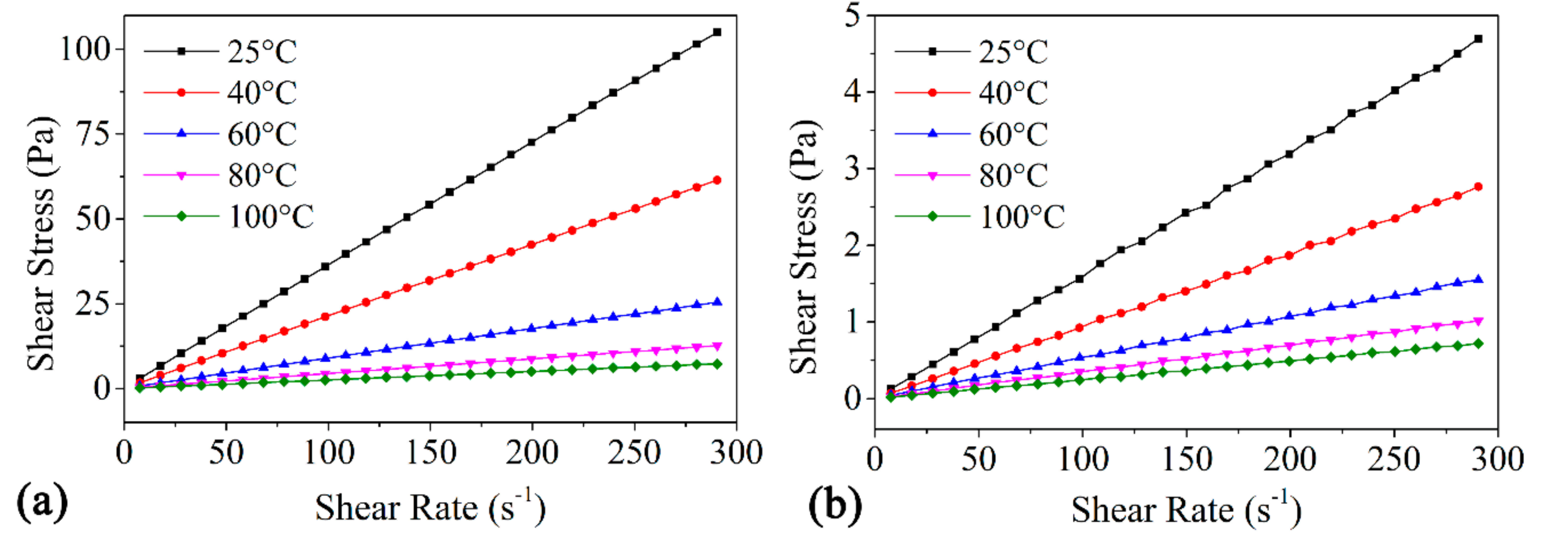
3.2. Power-Law Model Fitting
3.3. Applications of EWCO and EWCOME
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. Renewable lubricants. Biolubricants 2013, 1–9. [Google Scholar] [CrossRef]
- Lea, C.W. European development of lubricants derived from renewable resources. Ind. Lubr. Tribol. 2002, 54, 268–274. [Google Scholar] [CrossRef]
- Octave, S.; Thomas, D. Biorefinery: Toward an industrial metabolism. Biochimie 2009, 91, 659–664. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Somidi, A.K.R.; Dalai, A.K.; Borugadda, V.B.; Somidi, A.K.R.; Dalai, A.K. Chemical/Structural Modification of Canola Oil and Canola Biodiesel: Kinetic Studies and Biodegradability of the Alkoxides. Lubricants 2017, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Rani, S.; Joy, M.L.; Nair, K.P. A comparative study of polymeric additives as biodegradable viscosity boosters for biolubricant formulations. Proc. Inst. Mech. Eng. Part J. J. Eng. Tribol. 2015, 229, 1079–1085. [Google Scholar] [CrossRef]
- Hinman, M.; Rhee, I.S. Chapter 45|Environmental Characteristics of Fuels and Lubricants. In Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing, 2nd ed.; Totten, G., Shah, R., Forester, D., Eds.; ASTM International: West Conshohocken, PA, USA, 2004; ISBN 978-0-8031-7090-2. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Improved thermo-oxidative stability of structurally modified waste cooking oil methyl esters for bio-lubricant application. J. Clean. Prod. 2016, 112, 4515–4524. [Google Scholar] [CrossRef]
- India Industrial Lubricant Market by Lubricant Type (Metal Working Fluid, Industrial Engine Oil, Hydraulic Oil, Grease, Gear Oil and Others), By Application, By End Use, By Sales Channel, Competition, Forecast & Opportunities, 2013–2023. 2019. Available online: https://www.techsciresearch.com/report/india-industrial-lubricant-market/3750.html (accessed on 12 January 2021).
- Carole, T.M.; Pellegrino, J.; Paster, M.D. Opportunities in the industrial biobased products industry. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2004, 115, 871–885. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Qu, J.P. Mechanical and rheological properties of epoxidized soybean oil plasticized poly(lactic acid). J. Appl. Polym. Sci. 2009, 112, 3185–3191. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Khot, S.N. Development and application of triglyceride-based polymers and composites. J. Appl. Polym. Sci. 2001, 82, 703–723. [Google Scholar] [CrossRef]
- Guo, A.; Demydov, D.; Zhang, W.; Petrović, Z.S. Structure and Properties of Halogenated and Non-halogenated Soy-Based Polyols. J. Polym. Environ. 2002, 10, 49–52. [Google Scholar] [CrossRef]
- Berry, G.C.; Fox, T. The viscosity of polymers and their concentrated solutions. Fortschr. der Hochpolym. 2006, 261–357. [Google Scholar] [CrossRef]
- García-Zapateiro, L.A.; Franco, J.M.; Valencia, C.; Delgado, M.A.; Gallegos, C. Viscous, thermal and tribological characterization of oleic and ricinoleic acids-derived estolides and their blends with vegetable oils. J. Ind. Eng. Chem. 2013, 19, 1289–1298. [Google Scholar] [CrossRef]
- Campanella, A.; Rustoy, E.; Baldessari, A.; Baltanás, M.A. Lubricants from chemically modified vegetable oils. Bioresour. Technol. 2010, 101, 245–254. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Perez, J.M. Biobased industrial fluids and lubricants. J. Am. Oil Chem. Soc. 2002, 136. [Google Scholar]
- Wang, E.; Xiang Ma, X.; Tang, S.; Yan, R.; Wang, Y.; Riley, W.W.; Reaney, J.T.M. Synthesis and oxidative stability of trimethylolpropane fatty acid triester as a biolubricant base oil from waste cooking oil. Biomass Bioenergy 2014, 66, 371–378. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Ruseckaite, R.A. Pressure sensitive adhesives based on epoxidized soybean oil: Correlation between curing conditions and rheological properties. J. Am. Oil Chem. Soc. 2018, 95, 4. [Google Scholar] [CrossRef]
- Hernández-Sierra, M.T.; Bravo-Sánchez, M.G.; José, E.; Báez, J.E.; Luis, D.; Aguilera-Camacho, L.D.; García-Miranda, J.S.; Moreno, K.J. Improvement Effect of Green Lubricants on the Tribological and Mechanical Performance of 4140 Steel. Appl. Sci. 2019, 9, 4896. [Google Scholar] [CrossRef] [Green Version]
- Libor, M. Rheology of epoxy networks near the gel point. Polym. Bull. 1991, 116, 109–116. [Google Scholar]
- Sun, S.; Yang, G.; Bi, Y.; Liang, H. Lubricants from chemically modified vegetable oils. J. Am. Oil Chem. Soc. 2011, 88, 245–254. [Google Scholar] [CrossRef]
- Frenkel, J. The Viscosity of Liquids. Nature 1930, 125, 581–582. [Google Scholar] [CrossRef] [Green Version]
- Borugadda, V.B.; Goud, V.V. Synthesis of Waste Cooking Oil Epoxide as a Bio-Lubricant Base Stock: Characterization and Optimization Study. J. Bioprocess Eng. Biorefinery 2014, 3, 57–72. [Google Scholar] [CrossRef]
- Enweremadu, C.C.; Mbarawa, M.M. Technical aspects of production and analysis of biodiesel from used cooking oil-A review. Renew. Sustain. Energy Rev. 2009, 13, 2205–2224. [Google Scholar] [CrossRef]
- Dinda, S.; Patwardhan, A.V.; Goud, V.V.; Pradhan, N.C. Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour. Technol. 2008, 99, 3737–3744. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Thermal, oxidative and low temperature properties of methyl esters prepared from oils of different fatty acids composition: A comparative study. Thermochim. Acta 2014, 577, 33–40. [Google Scholar] [CrossRef]
- La Scala, J.; Wool, R.P. Rheology of chemically modified triglycerides. J. Appl. Polym. Sci. 2005, 95, 774–783. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Yang, S.; Chen, H.; Wang, D. The study of epoxidized rapeseed oil used as a potential biodegradable lubricant. J. Am. Oil Chem. Soc. 2000, 77, 561–563. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Upadhyay, S.N. Advancements in development and characterization of biodiesel: A review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Synthesis of novel alkoxylated triacylglycerols and their lubricant base oil properties. Ind. Crops Prod. 2005, 21, 13–119. [Google Scholar] [CrossRef]
- Kleinová, A.; Fodran, P.; Brnčalová, L.; Cvengroš, J. Substituted esters of stearic acid as potential lubricants. Biomass Bioenergy 2008, 32, 366–371. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z. Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind. Crop. Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Pang, B.; Wang, S.; Chen, W.; Hassan, M.; Lu, H. Effects of flow behavior index and consistency coefficient on hydrodynamics of power-law fluids and particles in fluidized beds. Powder Technol. 2020, 366, 249–260. [Google Scholar] [CrossRef]
- Yen, H.Y.; Yang, M.H. The effect of metal ions additives on the rheological behavior of polyacrylamide solution. Polym. Test. 2003, 22, 389–393. [Google Scholar] [CrossRef]
- Gorla, G.; Kour, S.M.; Padmaja, K.V.; Karuna, M.S.L.; Prasad, R.B.N. Preparation and properties of lubricant base stocks from epoxidized karanja oil and its alkyl esters. Ind. Eng. Chem. Res. 2013, 52, 16598–16605. [Google Scholar] [CrossRef]
- Hwang, H.; Erhan, S.Z. Synthetic lubricant basestocks from epoxidized soybean oil and Guerbet alcohols. Ind. Crop. Prod. 2006, 23, 311–317. [Google Scholar] [CrossRef]
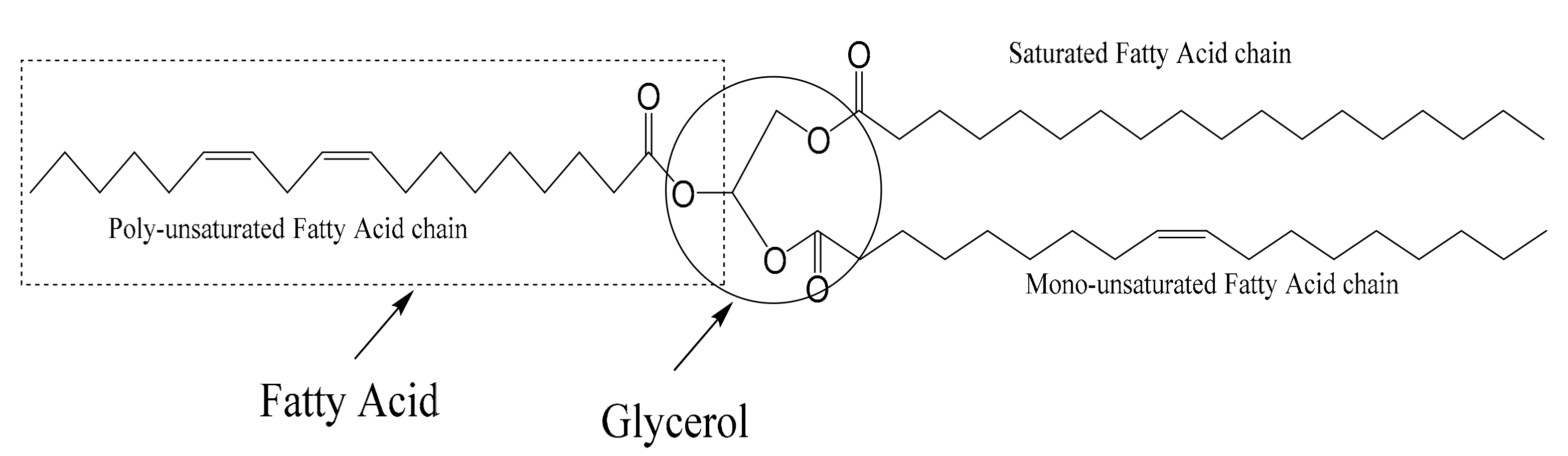

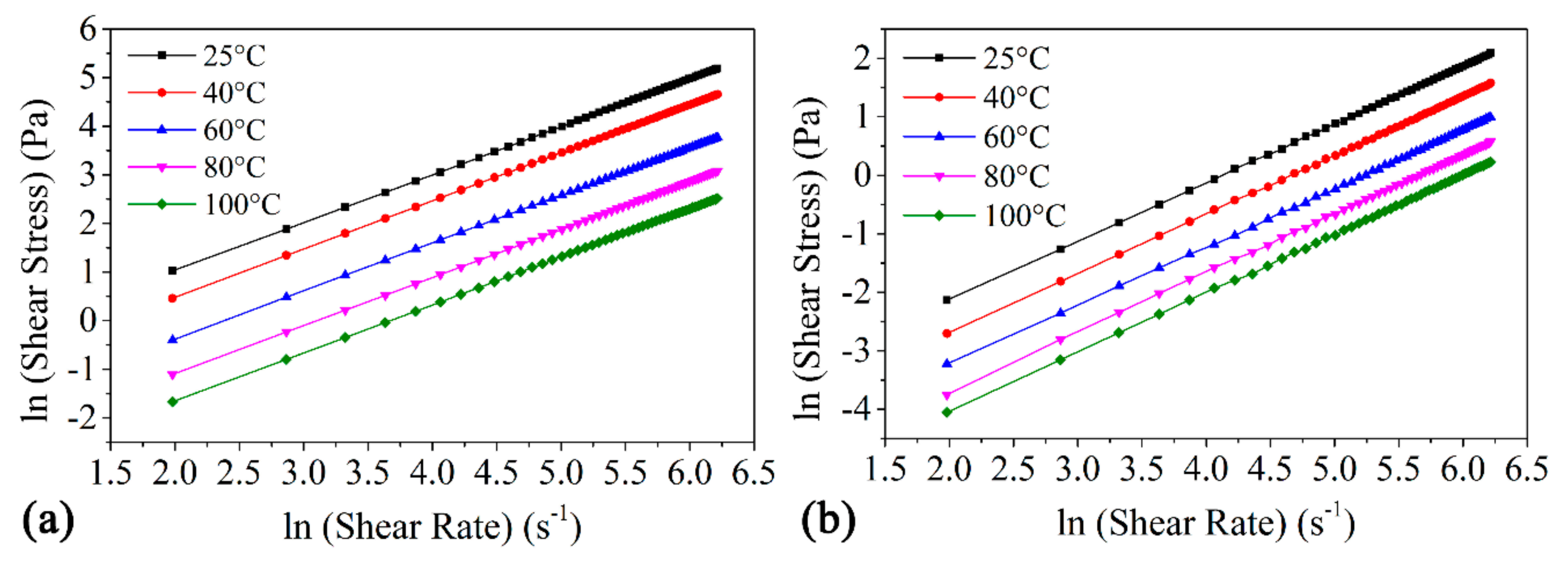
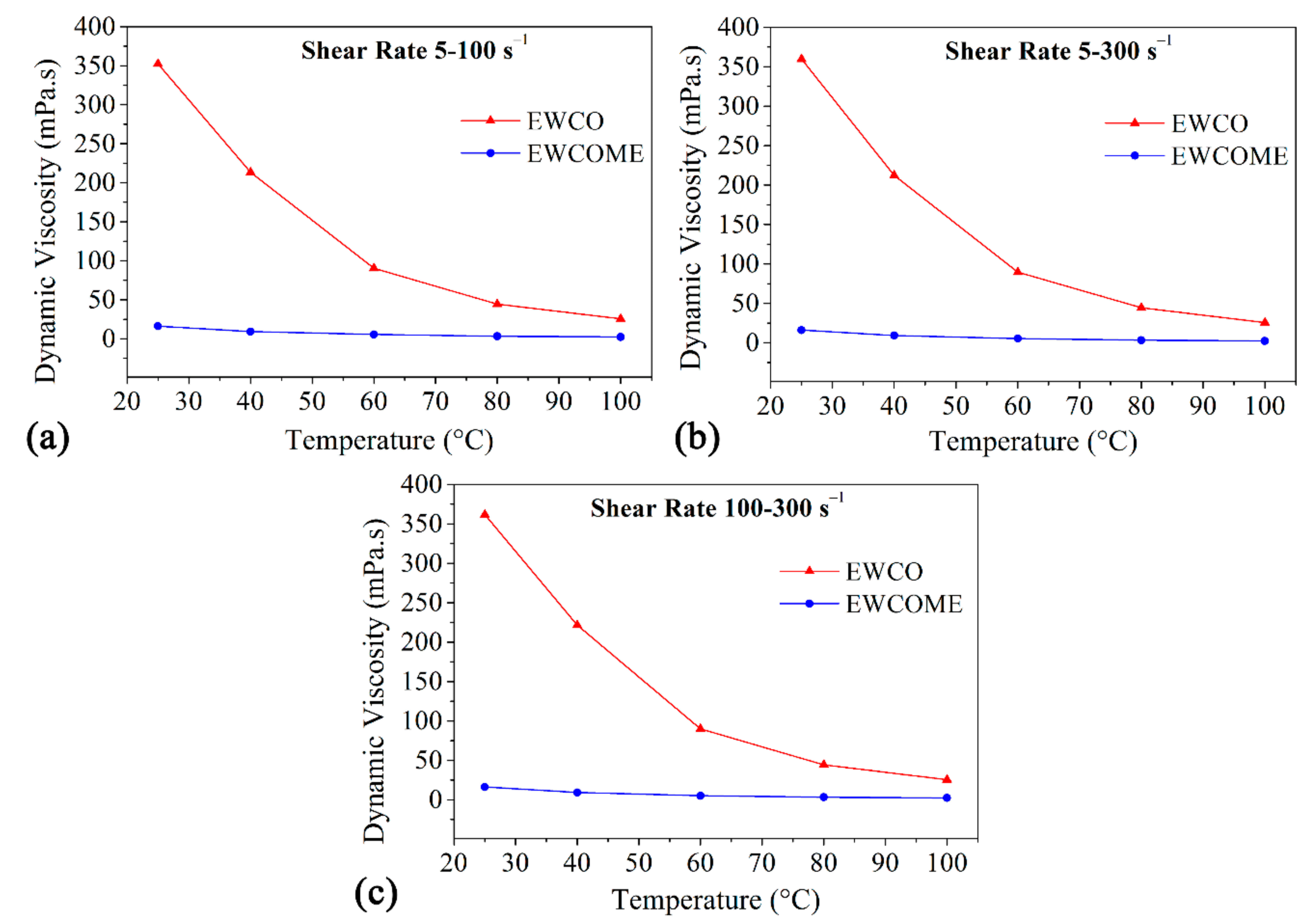
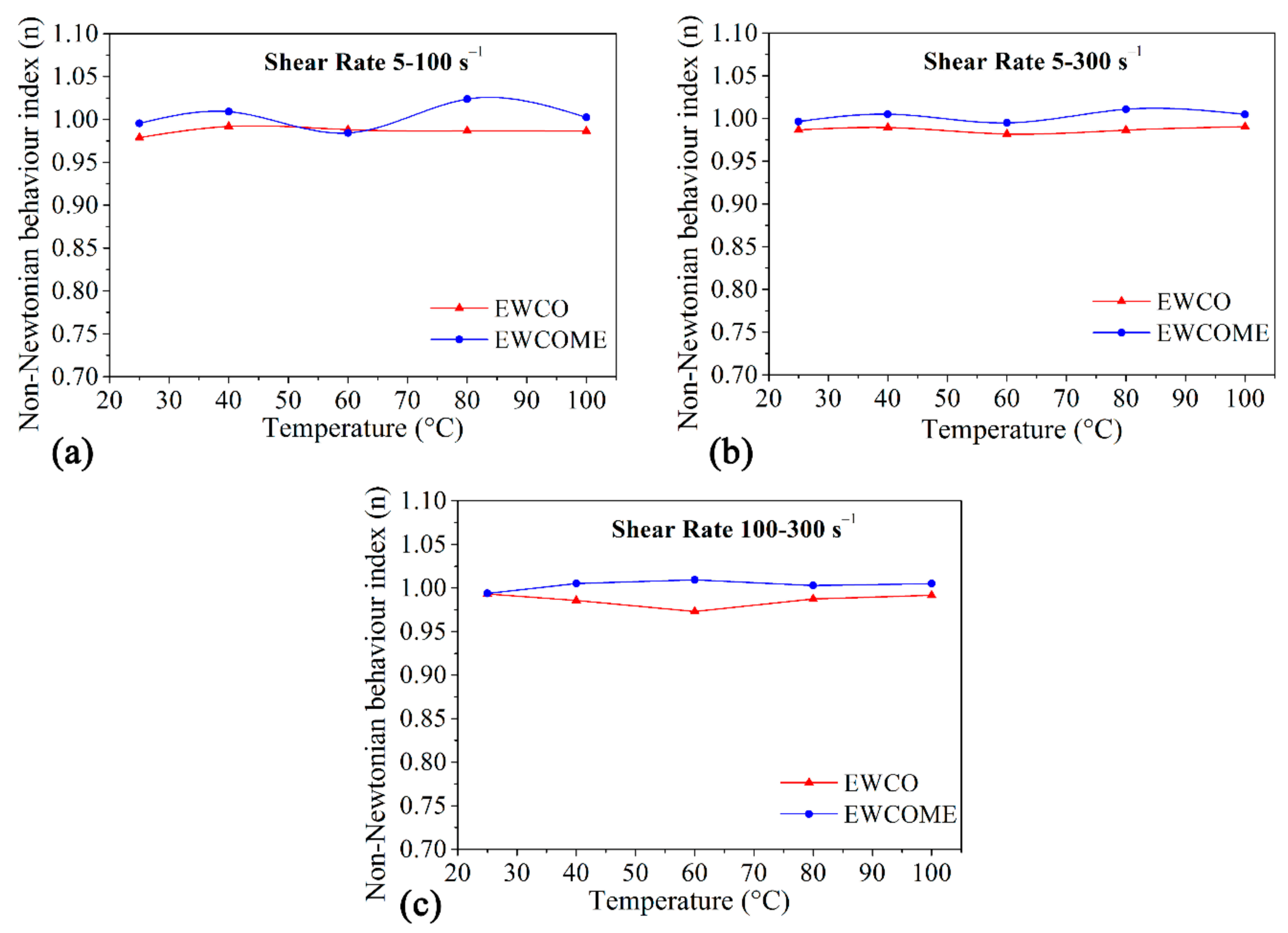
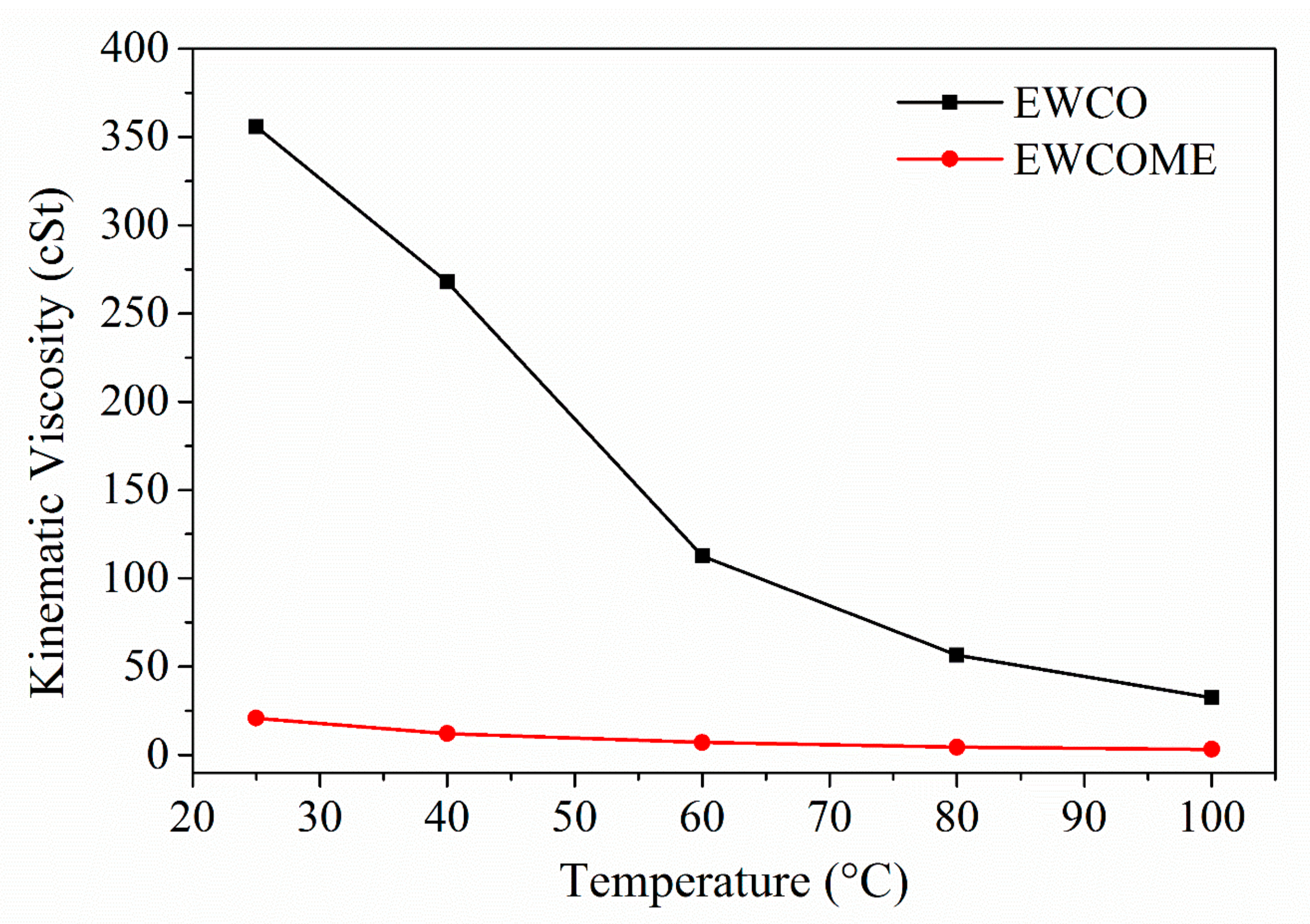
| Fatty Acid | Carbon Number | Fatty Acid Composition (%) of WCO, WCOME | The Chemical Name of the Fatty Acid |
|---|---|---|---|
| Oleic | (C18:1) | 23.96 ± 1.6 | 9-octadecenoic |
| Linoleic | (C18:2) | 39.16 ± 1.7 | 9,12-octadecadienoic |
| Linolenic | (C18:3) | 5.25 ± 0.6 | 9,12,15-octadecadienoic |
| Saturated | (C16:0) | 18.27 ± 1.2 | - |
| EWCO | EWCOME | |||||
|---|---|---|---|---|---|---|
| Shear rate (s−1) | 5–100 | 5–300 | 100–300 | 5–100 | 5–300 | 100–300 |
| Temperature 40 °C | ||||||
| k (mPa.s) | 213.30 | 212.35 | 221.68 | 9.25 | 9.30 | 9.29 |
| kexp (mPa.s) | 222.97 | 224.90 | 239.88 | 9.07 | 9.19 | 9.18 |
| n | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 |
| Temperature 100 °C | ||||||
| k (mPa.s) | 25.56 | 25.56 | 25.53 | 2.42 | 2.40 | 2.40 |
| kexp (mPa.s) | 26.72 | 26.38 | 26.24 | 2.41 | 2.39 | 2.39 |
| n | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 |
| ISO Viscosity | Viscosity at 40 °C (mm2/s) | Viscosity Limits (mm2/s) | Viscosity Index [12] | |
|---|---|---|---|---|
| Mid-Range | Minimum | Maximum | ||
| Epoxidized waste cooking oil (present study) | 267.92 | 248.65 | 281.45 | 164.94 |
| Epoxidized waste cooking oil methyl esters (present study) | 12.15 | 10.47 | 13.84 | 151.97 |
| ISO VG 2 | 2.2 | 1.98 | 2.42 | - |
| ISO VG 3 | 3.2 | 2.88 | 3.52 | - |
| ISO VG 5 | 4.6 | 4.14 | 4.06 | - |
| ISO VG 7 | 6.8 | 6.12 | 7.48 | - |
| ISO VG 10 | 10 | 9.00 | 11 | - |
| ISO VG 15 | 15 | 13.50 | 16.5 | - |
| ISO VG 22 | 22 | 19.80 | 24.2 | - |
| ISO VG 32 | 32 | 28.80 | 35.2 | >90 |
| ISO VG 46 | 46 | 41.40 | 50.6 | >90 |
| ISO VG 68 | 68 | 61.20 | 74.8 | >198 |
| ISO VG 100 | 100 | 90 | 110 | >216 |
| ISO VG 150 | 150 | 135 | 165 | - |
| ISO VG 220 | 220 | 198 | 242 | - |
| ISO VG 320 | 320 | 288 | 352 | - |
| ISO VG 460 | 460 | 414 | 506 | - |
| ISO VG 680 | 680 | 612 | 748 | - |
| ISO VG 1000 | 1000 | 900 | 1100 | - |
| ISO VG 2200 | 2200 | 1980 | 2420 | - |
| ISO VG 3200 | 3200 | 2880 | 3520 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, A.K.; Borugadda, V.B.; Goud, V.V. In-Situ Epoxidation of Waste Cooking Oil and Its Methyl Esters for Lubricant Applications: Characterization and Rheology. Lubricants 2021, 9, 27. https://doi.org/10.3390/lubricants9030027
Paul AK, Borugadda VB, Goud VV. In-Situ Epoxidation of Waste Cooking Oil and Its Methyl Esters for Lubricant Applications: Characterization and Rheology. Lubricants. 2021; 9(3):27. https://doi.org/10.3390/lubricants9030027
Chicago/Turabian StylePaul, Atanu Kumar, Venu Babu Borugadda, and Vaibhav V. Goud. 2021. "In-Situ Epoxidation of Waste Cooking Oil and Its Methyl Esters for Lubricant Applications: Characterization and Rheology" Lubricants 9, no. 3: 27. https://doi.org/10.3390/lubricants9030027
APA StylePaul, A. K., Borugadda, V. B., & Goud, V. V. (2021). In-Situ Epoxidation of Waste Cooking Oil and Its Methyl Esters for Lubricant Applications: Characterization and Rheology. Lubricants, 9(3), 27. https://doi.org/10.3390/lubricants9030027