Challenges in Assessing Repellency via the Behavioral Response by the Global Pest Tribolium castaneum to Protect Stored Grains
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Essential Oils and Other Chemicals
2.3. Essential Oil Screening Experiments
2.3.1. Small Wind Tunnel
Individual Exposure
Group Exposure
2.3.2. Large Wind Tunnel
2.3.3. Individual Behavioral Tracking with Ethovision
2.3.4. Taxis Release–Recapture Experiments
3. Results
3.1. Small Wind Tunnel
3.1.1. Individual Exposure
3.1.2. Group Exposure
3.2. Large Wind Tunnel
3.3. Ethovision
3.4. Taxis Release–Recapture Experiments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Ed.) The State of Food Security and Nutrition in the World; FAO: Rome, Italy, 2019; ISBN 978-92-5-131570-5. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The State of Food Security and Nutrition in the World. Available online: https://www.fao.org/3/cc0639en/online/sofi-2022/key-messages.html (accessed on 4 November 2022).
- Kendall, H.W.; Pimentel, D. Constraints on the Expansion of the Global Food Supply. Ambio 1994, 23, 198–205. [Google Scholar]
- Pimentel, D.; Huang, X.; Cordova, A.; Pimentel, M. Impact of Population Growth on Food Supplies and Environment. Popul. Environ. 1997, 19, 9–14. [Google Scholar] [CrossRef]
- World Food Programme. A Global Food Crisis. Available online: https://www.wfp.org/global-hunger-crisis (accessed on 4 November 2022).
- FAO. Global Food Losses and Food Waste. Available online: https://www.fao.org/3/mb060e/mb060e00.htm (accessed on 7 November 2022).
- Sawicka, B. Post-Harvest Losses of Agricultural Produce. In Zero Hunger; Leal Filho, W., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Encyclopedia of the UN Sustainable Development Goals; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–16. ISBN 978-3-319-69626-3. [Google Scholar]
- Pimentel, D. Handbook of Pest Management in Agriculture: Volume I; CRC Press, Inc.: Boca Raton, FL, USA, 1991. [Google Scholar]
- FAO. (Ed.) The state of food security and nutrition in the world. In Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018; ISBN 978-92-5-130571-3. [Google Scholar]
- Kenkel, P. Chapter 25—Economics of Grain Storage. In Storage of Cereal Grains and Their Products, 5th ed.; Rosentrater, K.A., Ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 687–696. ISBN 978-0-12-812758-2. [Google Scholar]
- Buzby, J.C.; Farah-Wells, H.; Hyman, J. The Estimated Amount, Value, and Calories of Postharvest Food Losses at the Retail and Consumer Levels in the United States; Bulletin No. 121; USDA, Economic Research Service: Washington, DC, USA, 2014. [Google Scholar]
- FAO (Ed.) The State of Food Insecurity in the World 2013: The Multiple Dimensions of Food Security; FAO: Rome, Italy, 2013; ISBN 978-92-5-107916-4. [Google Scholar]
- Harein, P.; Meronuck, R. Stored Grain Losses Due to Insects and Molds and the Importance of Proper Grain Management. In Stored Product Management, E-912; Oklahoma Cooperative Extension, Oklahoma State University: Stillwater, OK, USA, 1990; pp. 29–31. [Google Scholar]
- Hodges, R.J.; Buzby, J.C.; Bennett, B. Postharvest Losses and Waste in Developed and Less Developed Countries: Opportunities to Improve Resource Use. J. Agric. Sci. 2011, 149, 37–45. [Google Scholar] [CrossRef]
- Channaiah, L.H.; Subramanyam, B.; McKinney, L.J.; Zurek, L. Stored-Product Insects Carry Antibiotic-Resistant and Potentially Virulent Enterococci. FEMS Microbiol. Ecol. 2010, 74, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Beti, J.A.; Phillips, T.W.; Smalley, E.B. Effects of Maize Weevils (Coleoptera: Curculionidae) on Production of Aflatoxin B1 by Aspergillus flavus in Stored Corn. J. Econ. Entomol. 1995, 88, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, A.; Sbar, E. Aflatoxin Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Klich, M.A. Aspergillus flavus: The Major Producer of Aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Ponce, M.A.; Maille, J.M.; Stoll, I.; James, A.; Bruce, A.; Kim, T.N.; Scully, E.D.; Morrison, W.R., III. Microbial Vectoring Capacity by Internal- and External-Infesting Stored Product Insects after Varying Dispersal Periods between Novel Food Patches: An Underestimated Risk. Ecol. Evol. 2023, in press. [CrossRef] [PubMed]
- Wilson, D.M.; Mubatanhema, W.; Jurjevic, Z. Biology and Ecology of Mycotoxigenic Aspergillus Species as Related to Economic and Health Concerns. In Mycotoxins and Food Safety; DeVries, J.W., Trucksess, M.W., Jackson, L.S., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2002; pp. 3–17. ISBN 978-1-4615-0629-4. [Google Scholar]
- CDC Health Studies Program: Chemical Exposures—Aflatoxin. Available online: http://medbox.iiab.me/modules/en-cdc/www.cdc.gov/nceh/hsb/chemicals/aflatoxin.htm (accessed on 17 August 2024).
- Liu, Y.; Wu, F. Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Arthur, F.H. Grain Protectants: Current Status and Prospects for the Future. J. Stored Prod. Res. 1996, 32, 293–302. [Google Scholar] [CrossRef]
- Fields, P.G. Effect of Pisum sativum Fractions on the Mortality and Progeny Production of Nine Stored-Grain Beetles. J. Stored Prod. Res. 2006, 42, 86–96. [Google Scholar] [CrossRef]
- Morrison, W.R.; Larson, N.L.; Brabec, D.; Zhang, A. Methyl Benzoate as a Putative Alternative, Environmentally Friendly Fumigant for the Control of Stored Product Insects. J. Econ. Entomol. 2019, 112, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.K. Stored Product Protection: A Period of Transition; Insects Limited: Indianapolis, IN, USA, 1998; ISBN 978-0-9634373-4-1. [Google Scholar]
- Rees, D. Insects of Stored Products; CSIRO Publishing: Collingwood, Australia, 2004; ISBN 978-0-643-10263-7. [Google Scholar]
- Hamed, M.; Khattak, S. Red Flour Beetle: Development and Losses in Various Stored Food Stuffs [Wheat Shorts, Starch, Poprice, Wheat Flour, Wheat Bran, Gram Flour, Biscuits and Dry Milk]. Sarhad J. Agric. 1985, 1, 97–101. [Google Scholar]
- Naseri, B.; Majd-Marani, S. Assessment of Eight Rice Cultivars Flour for Feeding Resistance to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2020, 88, 101650. [Google Scholar] [CrossRef]
- Richards, S.; Gibbs, R.A.; Weinstock, G.M.; Brown, S.J.; Denell, R.; Beeman, R.W.; Gibbs, R.; Beeman, R.W.; Brown, S.J.; Bucher, G.; et al. The Genome of the Model Beetle and Pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.F.; Athanassiou, C.G.; Hagstrum, D.W.; Zhu, K.Y. Tribolium castaneum: A Model Insect for Fundamental and Applied Research. Annu. Rev. Entomol. 2022, 67, 347–365. [Google Scholar] [CrossRef]
- Arthur, F.H.; Fontenot, E.A. Residual Activity of Methoprene and Novaluron as Surface Treatments to Manage the Flour Beetles, Tribolium castaneum and Tribolium confusum. J. Insect Sci. 2012, 12, 95. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Arthur, F.H. Effectiveness of Insecticide-Incorporated Bags to Control Stored-Product Beetles. J. Stored Prod. Res. 2017, 70, 18–24. [Google Scholar] [CrossRef]
- Kikuta, S. Deployment of an Attractive Toxic Sugar Bait System (ATSB) with Insecticide, for Adult Tribolium castaneum (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2019, 83, 97–102. [Google Scholar] [CrossRef]
- Morrison, W.R.; Wilkins, R.V.; Gerken, A.R.; Scheff, D.S.; Zhu, K.Y.; Arthur, F.H.; Campbell, J.F. Mobility of Adult Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) after Exposure to Long-Lasting Insecticide-Incorporated Netting. J. Econ. Entomol. 2018, 111, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Ngwenyama, P.; Mvumi, B.M.; Stathers, T.E.; Nyanga, L.K.; Siziba, S. How Different Hermetic Bag Brands and Maize Varieties Affect Grain Damage and Loss during Smallholder Farmer Storage. Crop Prot. 2022, 153, 105861. [Google Scholar] [CrossRef]
- Scheff, D.S.; Arthur, F.H. Fecundity of Tribolium castaneum and Tribolium confusum Adults after Exposure to Deltamethrin Packaging. J. Pest Sci. 2018, 91, 717–725. [Google Scholar] [CrossRef]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and Natural Insecticides: Gas, Liquid, Gel and Solid Formulations for Stored-Product and Food-Industry Pest Control. Insects 2021, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Williams, S.B.; Murdock, L.L.; Baributsa, D. Hermetic Storage of Wheat and Maize Flour Protects against Red Flour Beetle (Tribolium castaneum Herbst). PLoS ONE 2017, 12, e0185386. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Benito-Jardón, M.; López-Galiano, M.J.; Real, M.D.; Rausell, C. Tribolium castaneum Immune Defense Genes Are Differentially Expressed in Response to Bacillus thuringiensis Toxins Sharing Common Receptor Molecules and Exhibiting Disparate Toxicity. Dev. Comp. Immunol. 2015, 50, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xiong, W.; Wei, L.; Liu, J.; Liu, X.; Xie, J.; Song, X.; Bi, J.; Li, B. Transcriptome Profiling Analysis Reveals the Role of Latrophilin in Controlling Development, Reproduction and Insecticide Susceptibility in Tribolium castaneum. Genetica 2018, 146, 287–302. [Google Scholar] [CrossRef]
- Kramarz, P.E.; Mordarska, A.; Mroczka, M. Response of Tribolium Castaneum to Elevated Copper Concentrations Is Influenced by History of Metal Exposure, Sex-Specific Defences, and Infection by the Parasite Steinernema feltiae. Ecotoxicology 2014, 23, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, S.; Opit, G.P.; Arthur, F.H.; Bingham, G.V.; Gautam, S.G. Contact Toxicity of Deltamethrin against Tribolium castaneum (Coleoptera: Tenebrionidae), Sitophilus oryzae (Coleoptera: Curculionidae), and Rhyzopertha dominica (Coleoptera: Bostrichidae) Adults. J. Econ. Entomol. 2016, 109, 1936–1942. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Hooshmandi, M. Insecticidal and Repellent Activity of Three Satureja Species against Adult Red Flour Beetles, Tribolium castaneum (Coleoptera: Tenebrionidae). Acta Ecol. Sin. 2017, 37, 201–206. [Google Scholar] [CrossRef]
- Andreev, D.; Rocheleau, T.; Phillips, T.W.; Beeman, R.W.; ffrench-Constant, R.H. A PCR Diagnostic for Cyclodiene Insecticide Resistance in the Red Flour Beetle Tribolium Castaneum. Pestic. Sci. 1994, 41, 345–349. [Google Scholar] [CrossRef]
- Rösner, J.; Wellmeyer, B.; Merzendorfer, H. Tribolium castaneum: A Model for Investigating the Mode of Action of Insecticides and Mechanisms of Resistance. Curr. Pharm. Des. 2020, 26, 3554–3568. [Google Scholar] [CrossRef]
- Zettler, L.J.; Cuperus, G.W. Pesticide Resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) in Wheat. J. Econ. Entomol. 1990, 83, 1677–1681. [Google Scholar] [CrossRef]
- Zhu, F.; Parthasarathy, R.; Bai, H.; Woithe, K.; Kaussmann, M.; Nauen, R.; Harrison, D.A.; Palli, S.R. A Brain-Specific Cytochrome P450 Responsible for the Majority of Deltamethrin Resistance in the QTC279 Strain of Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2010, 107, 8557–8562. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Scully, E.D.; Campbell, J.F. Towards Developing Areawide Semiochemical-Mediated, Behaviorally-Based Integrated Pest Management Programs for Stored Product Insects. Pest Manag. Sci. 2021, 77, 2667–2682. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Koh, L.; Ma, Y.; Huang, Y.; Sim, K.Y. The Oil of Garlic, Allium sativum L. (Amaryllidaceae), as a Potential Grain Protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol. Technol. 1996, 9, 41–48. [Google Scholar] [CrossRef]
- Ho, S.H.; Cheng, L.P.L.; Sim, K.Y.; Tan, H.T.W. Potential of Cloves (Syzygium aromaticum (L.) Merr. and Perry as a Grain Protectant against Tribolium castaneum (Herbst) and Sitophilus Zeamais Motsch. Postharvest Biol. Technol. 1994, 4, 179–183. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ho, S.H. Bioactivity of the Essential Oil Extracted from Evodia rutaecarpa Hook f. et Thomas against the Grain Storage Insects, Sitophilus zeamais Motsch. and Tribolium castaneum (Herbst). J. Stored Prod. Res. 1999, 35, 317–328. [Google Scholar] [CrossRef]
- Islam, W. Eco-Friendly Approaches for the Management of Red Flour Beetle: Tribolium castaneum (Herbst). Sci. Lett. 2017, 5, 105–114. [Google Scholar]
- Upadhyay, N.; Dwivedy, A.K.; Kumar, M.; Prakash, B.; Dubey, N.K. Essential Oils as Eco-Friendly Alternatives to Synthetic Pesticides for the Control of Tribolium Castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Essent. Oil Bear. Plants 2018, 21, 282–297. [Google Scholar] [CrossRef]
- Khaliq, A.; Ullah, M.I.; Afzal, M.; Ali, S.; Sajjad, A.; Ahmad, A.; Khalid, S. Management of Tribolium Castaneum Using Synergism between Conventional Fumigant and Plant Essential Oils. Int. J. Trop. Insect Sci. 2020, 40, 781–788. [Google Scholar] [CrossRef]
- Gross, A.D.; Norris, E.J.; Kimber, M.J.; Bartholomay, L.C.; Coats, J.R. Essential Oils Enhance the Toxicity of Permethrin against Aedes aegypti and Anopheles gambiae. Med. Vet. Entomol. 2017, 31, 55–62. [Google Scholar] [CrossRef]
- O’Neal, S.T.; Johnson, E.J.; Rault, L.C.; Anderson, T.D. Vapor Delivery of Plant Essential Oils Alters Pyrethroid Efficacy and Detoxification Enzyme Activity in Mosquitoes. Pestic. Biochem. Physiol. 2019, 157, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Rault, L.C.; O’Neal, S.T.; Johnson, E.J.; Anderson, T.D. Vaporous Essential Oils and Isolates Restore Pyrethroid-Treated Netting Efficacy to Aedes aegypti (Diptera: Culicidae). bioRxiv 2022, 2022.12.13.520257. [Google Scholar]
- Gerken, A.R.; Scully, E.D.; Campbell, J.F. Red Flour Beetle (Coleoptera: Tenebrionidae) Response to Volatile Cues Varies with Strain and Behavioral Assay. Environ. Entomol. 2018, 47, 1252–1265. [Google Scholar] [CrossRef]
- Duehl, A.J.; Arbogast, R.T.; Teal, P.E.A. Density-Related Volatile Emissions and Responses in the Red Flour Beetle, Tribolium castaneum. J. Chem. Ecol. 2011, 37, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, F.; Valbon, W.R.; Wang, Q.; Liu, F.; Xu, P.; Bandason, E.; Chen, M.; Wu, S.; Smith, L.B.; Scott, J.G.; et al. Sodium Channel Activation Underlies Transfluthrin Repellency in Aedes aegypti. PLoS Negl. Trop. Dis. 2021, 15, e0009546. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Grosdidier, R.F.; Arthur, F.H.; Myers, S.W.; Domingue, M.J. Attraction, Arrestment, and Preference by Immature Trogoderma variabile and Trogoderma granarium to Food and Pheromonal Stimuli. J. Pest Sci. 2020, 93, 135–147. [Google Scholar] [CrossRef]
- Bruce, A.; Wilson, A.N.; Ranabhat, S.; Montgomery, J.; Nicholson, S.; Harris, K.; Morrison, W.R., III. A Biomass Pyrolysis Oil as a Novel Insect Growth Regulator Mimic for a Variety of Stored Product Beetles. J. Econ. Entomol. 2022, 115, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, T.; Ponce, M.; Quellhorst, H.; Bruce, A.; Albin, C.E.; Kim, T.N.; Zhu, K.Y.; Morrison, W.R. Microbial Volatile Organic Compounds from Tempered and Incubated Grain Mediate Attraction by a Primary but Not Secondary Stored Product Insect Pest in Wheat. J. Chem. Ecol. 2022, 48, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Noldus, L.P.J.J.; Spink, A.J.; Tegelenbosch, R.A.J. EthoVision: A Versatile Video Tracking System for Automation of Behavioral Experiments. Behav. Res. Methods Instrum. Comput. 2001, 33, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Kang, C.-S.; Lee, J.-K.; Kim, Y.-R.; Han, H.-Y.; Yun, H.K. Evaluation of Repellency Effect of Two Natural Aroma Mosquito Repellent Compounds, Citronella and Citronellal*. Entomol. Res. 2005, 35, 117–120. [Google Scholar] [CrossRef]
- Ponce, M.A.; Ranabhat, S.; Bruce, A.; Van Winkle, T.; Campbell, J.F.; Morrison, W.R., III. Density-mediated foraging behavioral responses of Rhyzopertha dominica (Coleoptera: Bostrichidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). Sci. Rep. 2024, 14, 12259. [Google Scholar] [CrossRef] [PubMed]
- Licciardello, F.; Muratore, G.; Suma, P.; Russo, A.; Nerín, C. Effectiveness of a Novel Insect-Repellent Food Packaging Incorporating Essential Oils against the Red Flour Beetle (Tribolium castaneum). Innov. Food Sci. Emerg. Technol. 2013, 19, 173–180. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Merino, S.; Lobato, E.; Rivero-de Aguilar, J.; del Cerro, S.; Ruiz-de-Castañeda, R. Testing the Use of a Citronella-Based Repellent as an Effective Method to Reduce the Prevalence and Abundance of Biting Flies in Avian Nests. Parasitol. Res. 2009, 104, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Page, P.C.; Labuschagne, K.; Nurton, J.P.; Venter, G.J.; Guthrie, A.J. Duration of Repellency of N,N-Diethyl-3-Methylbenzamide, Citronella Oil and Cypermethrin against Culicoides Species When Applied to Polyester Mesh. Vet. Parasitol. 2009, 163, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.J.; Labuschagne, K.; Boikanyo, S.N.B.; Morey, L. Assessment of the Repellent Effect of Citronella and Lemon Eucalyptus Oil against South African Culicoides Species: Original Research. J. S. Afr. Vet. Assoc. 2014, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Campbell, J.F. Capture of Tribolium castaneum and Tribolium confusum (Coleoptera: Tenebrionidae) in Floor Traps: The Effect of Previous Captures. J. Econ. Entomol. 2016, 109, 461–466. [Google Scholar] [CrossRef]
- Harman, R.R.; Morrison, W.R., III; Bruce, A.; Ranabhat, S.; Quellhorst, H.E.; Wilkins, R.V.; Campbell, J.F.; Gerken, A.R. The Behavioral Response to the Putative Necromones from Dead Tribolium castaneum (Coleoptera: Tenebrionidae) in Traps by Conspecifics as a Function of Density and Time since Capture. Environ. Entomol. 2023, 52, 1020–1032. [Google Scholar] [CrossRef]
- Cosimi, S.; Rossi, E.; Cioni, P.L.; Canale, A. Bioactivity and Qualitative Analysis of Some Essential Oils from Mediterranean Plants against Stored-Product Pests: Evaluation of Repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). J. Stored Prod. Res. 2009, 45, 125–132. [Google Scholar] [CrossRef]
- Vendl, T.; Stejskal, V.; Kadlec, J.; Aulicky, R. New Approach for Evaluating the Repellent Activity of Essential Oils against Storage Pests Using a Miniaturized Model of Stored-Commodity Packaging and a Wooden Transport Pallet. Ind. Crops Prod. 2021, 172, 114024. [Google Scholar] [CrossRef]
- Elnabawy, E.-S.M.; Hassan, S.; Taha, E.-K.A. Repellent and Toxicant Effects of Eight Essential Oils against the Red Flour Beetle, Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Biology 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zou, K.; Zhang, W.; Guo, S.; Liu, H.; Sun, J.; Li, J.; Huang, D.; Wu, Y.; Du, S.; et al. Efficacy of Compounds Isolated from the Essential Oil of Artemisia lavandulaefolia in Control of the Cigarette Beetle, Lasioderma serricorne. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 343. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Signal, F.A.; Campion, S.H.; Motion, R.L. Citronella as an Insect Repellent in Food Packaging. J. Agric. Food Chem. 2005, 53, 4633–4636. [Google Scholar] [CrossRef] [PubMed]


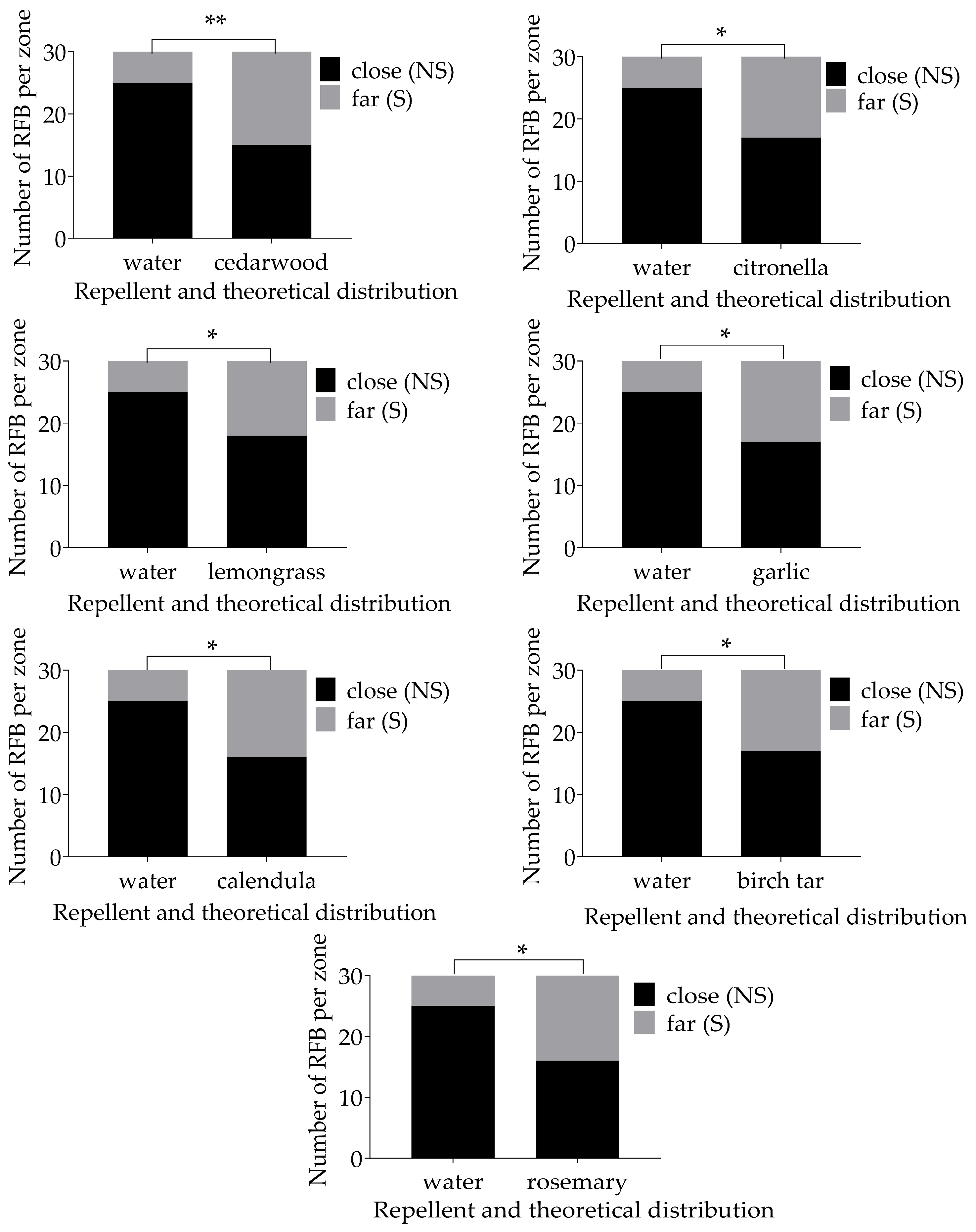

| Oils | χ2 | p-Value | Signif. Level | |
|---|---|---|---|---|
| Comparison to a water control—individuals | citronella | 5.079 | 0.024 | * |
| cedarwood | 7.5 | 0.003 | ** | |
| lemongrass | 4.022 | 0.045 | * | |
| garlic | 5.079 | 0.024 | * | |
| calendula | 6.239 | 0.013 | * | |
| birch tar | 5.079 | 0.024 | * | |
| rosemary | 6.239 | 0.013 | * | |
| bergamot | 0.8838 | 0.3472 | ns | |
| lime | 0.4167 | 0.5186 | ns | |
| wintergreen | 0.1113 | 0.7386 | ns | |
| verbena | 3.068 | 0.0798 | ns | |
| eucalyptus | 1.491 | 0.2221 | ns | |
| peppermint | 0.4167 | 0.5186 | ns | |
| black pepper | 2.222 | 0.136 | ns | |
| DEET 25% | 1.491 | 0.2221 | ns | |
| Comparison to a water control—group | citronella | 17.01 | <0.0001 | **** |
| lime | 24.9 | <0.0001 | **** | |
| wintergreen | 6.81 | 0.009 | ** | |
| eucalyptus | 1.263 | 0.2611 | ns | |
| bergamot | 1.263 | 0.2611 | ns | |
| black pepper | 1.313 | 0.2518 | ns | |
| peppermint | 0.04547 | 0.8311 | ns | |
| verbena | 0.4301 | 0.5119 | ns |
| Comparison | χ2 S vs. NS | p-Value S vs. NS | χ2 A vs. B | p-Value A vs. B |
|---|---|---|---|---|
| citronella | 1.176 | 0.2781 | 0.3175 | 0.5731 |
| lime | 0.2182 | 0.6404 | 0.6932 | 0.4051 |
| verbena | 1.92 | 0.1659 | 0.6932 | 0.4051 |
| DEET 25% | 0.1617 | 0.6876 | 0.08889 | 0.7656 |
| cedarwood | 0.5769 | 0.2238 | 0.6932 | 0.2025 |
| garlic | 1.92 | 0.1659 | 1.2 | 0.2733 |
| calendula | 0.1617 | 0.6876 | 0.8838 | 0.3472 |
| rosemary | 1.176 | 0.2781 | 0 | >0.9999 |
| transfluthrin 1 mg/mL | 0.0982 | 0.754 | 0.07326 | 0.7866 |
| methylbenzoquinone 40 mg/mL | 0.1113 | 0.7386 | 0.3 | 0.5839 |
| Storgard * | 0 | >0.9999 | 2.836 | 0.0922 |
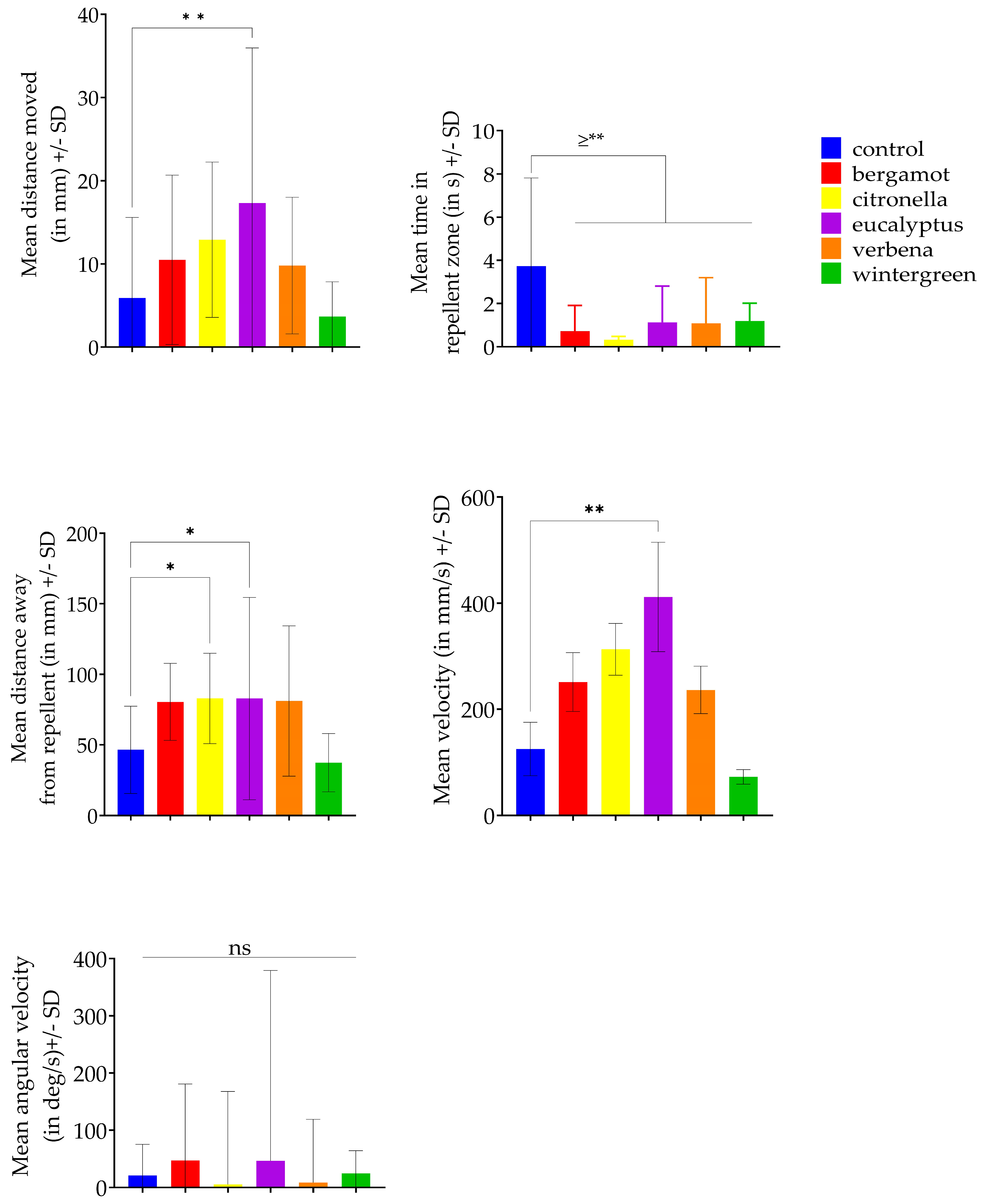
| Variable | Oils Significantly Different from the Control | Df | Mean Difference with the Control | Adjusted p-Value | Significance Level |
|---|---|---|---|---|---|
| Mean distance moved | Eucalyptus | 112 | −11.41 | 0.0061 | ** |
| Mean velocity | Eucalyptus | 101 | −286.4 | 0.0036 | ** |
| Mean time spent in the repellent zone | Bergamot | 106 | 3.008 | 0.0001 | *** |
| Citronella | 106 | 3.405 | <0.0001 | **** | |
| Eucalyptus | 106 | 2.598 | 0.0012 | ** | |
| Verbena | 106 | 2.645 | 0.0014 | ** | |
| Wintergreen | 106 | 2.54 | 0.0011 | ** | |
| Mean distance from the repellent zone | Citronella | 112 | −36.35 | 0.0398 | * |
| Eucalyptus | 112 | −36.37 | 0.0363 | * |
| Citronella | Lime | Eucalyptus | Verbena | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 8 h | 24 h | 1 h | 8 h | 24 h | 1 h | 8 h | 24 h | 1 h | 8 h | 24 h | |
| χ2 | 0.497 | 2.375 | 1.382 | 0.010 | 8.807 | 4.844 | 0.010 | 0.010 | 0.145 | 0.488 | 3.391 | 5.420 |
| p-value | 0.481 | 0.123 | 0.240 | 0.922 | 0.003 | 0.028 | 0.919 | 0.921 | 0.703 | 0.485 | 0.066 | 0.020 |
| Level | ns | ns | ns | ns | ** | * | ns | ns | ns | ns | ns | * |
| Citronella | Lime | Eucalyptus | Verbena | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 8 h | 24 h | 1 h | 8 h | 24 h | 1 h | 8 h | 24 h | 1 h | 8 h | 24 h | |
| Zones | ||||||||||||
| F | 1.382 | 13.18 | 7.057 | 5.566 | 1.351 | 2.354 | 2.03 | 8.817 | 3.812 | 4.607 | 0.436 | 0.9729 |
| p-value | 0.257 | <0.0001 | 0.0002 | 0.0012 | 0.271 | 0.070 | 0.109 | <0.0001 | 0.010 | 0.004 | 0.782 | 0.4331 |
| Level | ns | ** | ** | ** | ns | ns | ns | ** | * | ** | ns | ns |
| Treatment | ||||||||||||
| F | 0 | 0 | 0 | 0 | 2.535 | 0 | 0 | 0 | 0 | 0 | 2.04 × 10−29 | 0 |
| p-value | >0.9999 | >0.9999 | >0.9999 | >0.9999 | 0.120 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
| Level | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Interaction | ||||||||||||
| F | 0.372 | 1.771 | 0.337 | 0.170 | 0.683 | 1.561 | 0.305 | 0.498 | 1.707 | 0.838 | 1.629 | 2.440 |
| p-value | 0.827 | 0.154 | 0.851 | 0.952 | 0.608 | 0.203 | 0.873 | 0.738 | 0.168 | 0.509 | 0.186 | 0.0625 |
| Level | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rault, L.C.; Morrison, W.R., III; Gerken, A.R.; Bingham, G.V. Challenges in Assessing Repellency via the Behavioral Response by the Global Pest Tribolium castaneum to Protect Stored Grains. Insects 2024, 15, 626. https://doi.org/10.3390/insects15080626
Rault LC, Morrison WR III, Gerken AR, Bingham GV. Challenges in Assessing Repellency via the Behavioral Response by the Global Pest Tribolium castaneum to Protect Stored Grains. Insects. 2024; 15(8):626. https://doi.org/10.3390/insects15080626
Chicago/Turabian StyleRault, Leslie C., William R. Morrison, III, Alison R. Gerken, and Georgina V. Bingham. 2024. "Challenges in Assessing Repellency via the Behavioral Response by the Global Pest Tribolium castaneum to Protect Stored Grains" Insects 15, no. 8: 626. https://doi.org/10.3390/insects15080626







