MALDI-TOF MS Profiling and Its Contribution to Mosquito-Borne Diseases: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
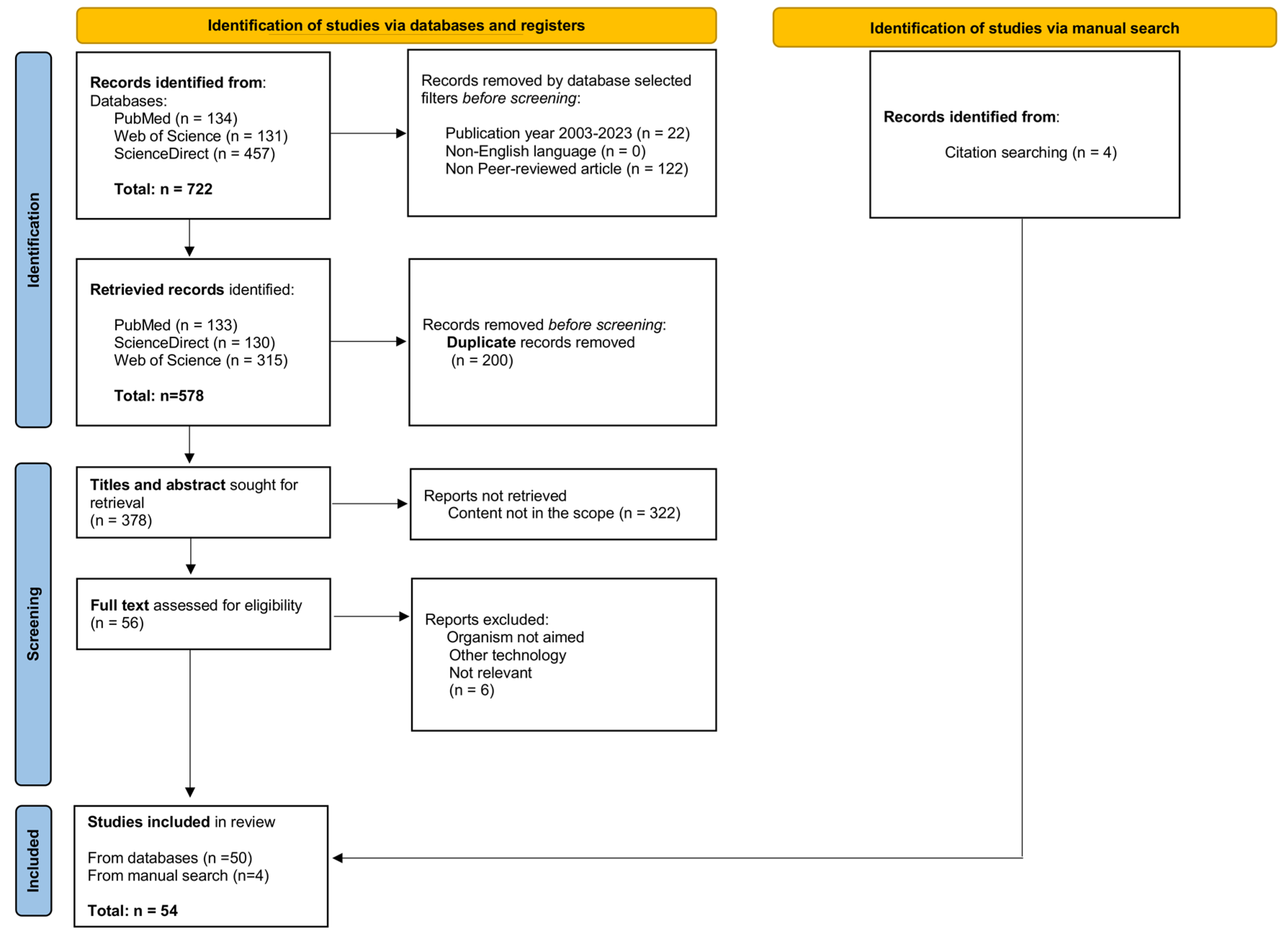
2. Methodology
3. Results and Discussion
3.1. Assessment of MALDI-TOF MS Profiling for Species Identification
3.2. Assessment of MALDI-TOF MS Profiling for Mosquito Surveillance
3.3. Assessment of MALDI-TOF MS Profiling for Mosquito Identification According to the Developmental Stages
3.4. Assessment of MALDI-TOF MS Profiling to Determine Mosquito Life Expectancy
3.5. Assessment of MALDI-TOF MS Profiling to Determine Mosquito Trophic Preferences
3.6. Impact of the Geographic Origin of Mosquitoes on the MALDI-TOF MS Profiling
3.7. Assessment of MALDI-TOF MS Profiling to Detect Pathogens in Mosquitoes
3.8. Assessment of MALDI-TOF MS Profiling to Study the Microbiota of Mosquitoes
3.9. Assessment of MALDI-TOF MS Profiling to Monitor Insecticide Resistance
3.10. Novel Applications of MALDI-TOF MS: Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yee, D.A.; Dean Bermond, C.; Reyes-Torres, L.J.; Fijman, N.S.; Scavo, N.A.; Nelsen, J.; Yee, S.H. Robust Network Stability of Mosquitoes and Human Pathogens of Medical Importance. Parasites Vectors 2022, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2023; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- The Lancet Global Health. Vector Control: Time for a Planetary Health Approach. Lancet Glob. Health 2017, 5, e556. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Rakotonirina, A.; Pol, M.; Raharimalala, F.N.; Ballan, V.; Kainiu, M.; Boyer, S.; Kilama, S.; Marcombe, S.; Russet, S.; Barsac, E.; et al. MALDI-TOF MS: An Effective Tool for a Global Surveillance of Dengue Vector Species. PLoS ONE 2022, 17, e0276488. [Google Scholar] [CrossRef]
- Vaux, A.G.C.; Dallimore, T.; Cull, B.; Schaffner, F.; Strode, C.; Pflüger, V.; Murchie, A.K.; Rea, I.; Newham, Z.; Mcginley, L.; et al. The Challenge of Invasive Mosquito Vectors in the U.K. during 2016-2018: A Summary of the Surveillance and Control of Aedes albopictus. Med. Vet. Entomol. 2019, 33, 443–452. [Google Scholar] [CrossRef]
- Osório, H.C.; Zé-Zé, L.; Neto, M.; Silva, S.; Marques, F.; Silva, A.S.; Alves, M.J. Detection of the Invasive Mosquito Species Aedes (Stegomyia) Albopictus (Diptera: Culicidae) in Portugal. Int. J. Environ. Res. Public Health 2018, 15, 820. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Versteirt, V.; Cull, B.; Kampen, H.; Fontenille, D.; Hendrickx, G.; Zeller, H.; Van Bortel, W.; Schaffner, F. An Entomological Review of Invasive Mosquitoes in Europe. Bull. Entomol. Res. 2015, 105, 637–663. [Google Scholar] [CrossRef]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with Chikungunya Virus in Italy: An Outbreak in a Temperate Region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Venturi, G.; Di Luca, M.; Fortuna, C.; Remoli, M.E.; Riccardo, F.; Severini, F.; Toma, L.; Del Manso, M.; Benedetti, E.; Caporali, M.G.; et al. Detection of a Chikungunya Outbreak in Central Italy, August to September 2017. Eurosurveillance 2017, 22, 17–00646. [Google Scholar] [CrossRef]
- Delisle, E.; Rousseau, C.; Broche, B.; Leparc-Goffart, I.; L’Ambert, G.; Cochet, A.; Prat, C.; Foulongne, V.; Ferre, J.B.; Catelinois, O.; et al. Chikungunya Outbreak in Montpellier, France, September to October 2014. Eurosurveillance 2015, 20, 21108. [Google Scholar] [CrossRef] [PubMed]
- Grandadam, M.; Caro, V.; Plumet, S.; Thiberge, J.M.; Souarès, Y.; Failloux, A.-B.; Tolou, H.J.; Budelot, M.; Cosserat, D.; Leparc-Goffart, I.; et al. Chikungunya Virus, Southeastern France. Emerg. Infect. Dis. 2011, 17, 910–913. [Google Scholar] [CrossRef]
- La Ruche, G.; Souarès, Y.; Armengaud, A.; Peloux-Petiot, F.; Delaunay, P.; Desprès, P.; Lenglet, A.; Jourdain, F.; Leparc-Goffart, I.; Charlet, F.; et al. First Two Autochthonous Dengue Virus Infections in Metropolitan France, September 2010. Eurosurveillance 2010, 15, 19676. [Google Scholar] [CrossRef]
- Marchand, E.; Prat, C.; Jeannin, C.; Lafont, E.; Bergmann, T.; Flusin, O.; Rizzi, J.; Roux, N.; Busso, V.; Deniau, J.; et al. Autochthonous Case of Dengue in France, October 2013. Eurosurveillance 2013, 18, 20661. [Google Scholar] [CrossRef] [PubMed]
- Giron, S.; Franke, F.; Decoppet, A.; Cadiou, B.; Travaglini, T.; Thirion, L.; Durand, G.; Jeannin, C.; L’Ambert, G.; Grard, G.; et al. Vector-Borne Transmission of Zika Virus in Europe, Southern France, August 2019. Eurosurveillance 2019, 24, 1900655. [Google Scholar] [CrossRef]
- Vasquez, M.I.; Notarides, G.; Meletiou, S.; Patsoula, E.; Kavran, M.; Michaelakis, A.; Bellini, R.; Toumazi, T.; Bouyer, J.; Petrić, D. Two Invasions at Once: Update on the Introduction of the Invasive Species Aedes Aegypti and Aedes albopictus in Cyprus—A Call for Action in Europe. Parasite 2023, 30, 41. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.Á.; Barceló, C.; Arnoldi, D.; Augsten, X.; Bakran-Lebl, K.; Balatsos, G.; Bengoa, M.; Bindler, P.; Boršová, K.; Bourquia, M.; et al. AIMSurv: First Pan-European Harmonized Surveillance of Aedes Invasive Mosquito Species of Relevance for Human Vector-Borne Diseases. GigaByte 2022, 2022, gigabyte57. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.; Jackson, C.E. Insecticide Resistance in Mosquitoes. Nature 1961, 190, 364–365. [Google Scholar] [CrossRef]
- Hemingway, J.; Field, L.; Vontas, J. An Overview of Insecticide Resistance. Science 2002, 298, 96–97. [Google Scholar] [CrossRef]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent Trends in Global Insecticide Use for Disease Vector Control and Potential Implications for Resistance Management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef]
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Müller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes Management for the Control of Aedes-Borne Diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006845. [Google Scholar] [CrossRef]
- Corbel, V.; Fonseca, D.M.; Weetman, D.; Pinto, J.; Achee, N.L.; Chandre, F.; Coulibaly, M.B.; Dusfour, I.; Grieco, J.; Juntarajumnong, W.; et al. International Workshop on Insecticide Resistance in Vectors of Arboviruses, December 2016, Rio de Janeiro, Brazil. Parasites Vectors 2017, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Wilson, M.E.; Touch, S.; McCloskey, B.; Mwaba, P.; Bates, M.; Dar, O.; Mattes, F.; Kidd, M.; Ippolito, G.; et al. Rapid Spread of Zika Virus in The Americas-Implications for Public Health Preparedness for Mass Gatherings at the 2016 Brazil Olympic Games. Int. J. Infect. Dis. 2016, 44, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Flacio, E.; Engeler, L.; Tonolla, M.; Lüthy, P.; Patocchi, N. Strategies of a Thirteen Year Surveillance Programme on Aedes albopictus (Stegomyia albopicta) in Southern Switzerland. Parasites Vectors 2015, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Yssouf, A.; Almeras, L.; Raoult, D.; Parola, P. Emerging Tools for Identification of Arthropod Vectors. Future Microbiol. 2016, 11, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Cuisance, D.; Antoine Rioux, J. Current Status of Medical and Veterinary Entomology in France: Endangered Discipline or Promising Science? Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 377–392. [Google Scholar] [CrossRef]
- Laroche, M.; Bérenger, J.-M.; Delaunay, P.; Charrel, R.; Pradines, B.; Berger, F.; Ranque, S.; Bitam, I.; Davoust, B.; Raoult, D.; et al. Medical Entomology: A Reemerging Field of Research to Better Understand Vector-Borne Infectious Diseases. Clin. Infect. Dis. 2017, 65, S30–S38. [Google Scholar] [CrossRef]
- Sevestre, J.; Diarra, A.Z.; Laroche, M.; Almeras, L.; Parola, P. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry: An Emerging Tool for Studying the Vectors of Human Infectious Diseases. Future Microbiol. 2021, 16, 323–340. [Google Scholar] [CrossRef]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Rapp, B.A.; Wheeler, D.L. GenBank. Nucleic Acids Res. 2000, 28, 15–18. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Fang, Q.; Keirans, J.E.; Mixson, T. The Use of the Nuclear Protein-Encoding Gene, RNA Polymerase II, for Tick Molecular Systematics. Exp. Appl. Acarol. 2002, 28, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wu, S.; Zhang, Y.; Chen, Y.; Feng, C.; Yuan, X.; Jia, G.; Deng, J.; Wang, C.; Wang, Q.; et al. Assessment of Four DNA Fragments (COI, 16S rDNA, ITS2, 12S rDNA) for Species Identification of the Ixodida (Acari: Ixodida). Parasites Vectors 2014, 7, 93. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.M. Species Differentiation of Insects and Other Multicellular Organisms Using Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry Protein Profiling. Syst. Entomol. 2005, 30, 186–190. [Google Scholar] [CrossRef]
- Perera, M.R.; Vargas, R.D.F.; Jones, M.G.K. Identification of Aphid Species Using Protein Profiling and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Entomol. Exp. Appl. 2005, 117, 243–247. [Google Scholar] [CrossRef]
- Dani, F.R.; Francese, S.; Mastrobuoni, G.; Felicioli, A.; Caputo, B.; Simard, F.; Pieraccini, G.; Moneti, G.; Coluzzi, M.; della Torre, A.; et al. Exploring Proteins in Anopheles gambiae Male and Female Antennae through MALDI Mass Spectrometry Profiling. PLoS ONE 2008, 3, e2822. [Google Scholar] [CrossRef]
- Müller, P.; Pflüger, V.; Wittwer, M.; Ziegler, D.; Chandre, F.; Simard, F.; Lengeler, C. Identification of Cryptic Anopheles Mosquito Species by Molecular Protein Profiling. PLoS ONE 2013, 8, e57486. [Google Scholar] [CrossRef]
- Yssouf, A.; Socolovschi, C.; Flaudrops, C.; Ndiath, M.O.; Sougoufara, S.; Dehecq, J.-S.; Lacour, G.; Berenger, J.-M.; Sokhna, C.S.; Raoult, D.; et al. Matrix-Assisted Laser Desorption Ionization—Time of Flight Mass Spectrometry: An Emerging Tool for the Rapid Identification of Mosquito Vectors. PLoS ONE 2013, 8, e72380. [Google Scholar] [CrossRef] [PubMed]
- Diarra, A.Z.; Laroche, M.; Berger, F.; Parola, P. Use of MALDI-TOF MS for the Identification of Chad Mosquitoes and the Origin of Their Blood Meal. Am. J. Trop. Med. Hyg. 2019, 100, 47–53. [Google Scholar] [CrossRef]
- Nabet, C.; Kone, A.K.; Dia, A.K.; Sylla, M.; Gautier, M.; Yattara, M.; Thera, M.A.; Faye, O.; Braack, L.; Manguin, S.; et al. New Assessment of Anopheles Vector Species Identification Using MALDI-TOF MS. Malar. J. 2021, 20, 33. [Google Scholar] [CrossRef]
- Bamou, R.; Costa, M.M.; Diarra, A.Z.; Martins, A.J.; Parola, P.; Almeras, L. Enhanced Procedures for Mosquito Identification by MALDI-TOF MS. Parasites Vectors 2022, 15, 240. [Google Scholar] [CrossRef]
- Huynh, L.N.; Diarra, A.Z.; Nguyen, H.S.; Tran, L.B.; Do, V.N.; Ly, T.D.A.; Ho, V.H.; Nguyen, X.Q.; Parola, P. MALDI-TOF Mass Spectrometry Identification of Mosquitoes Collected in Vietnam. Parasites Vectors 2022, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Niare, S.; Berenger, J.-M.; Dieme, C.; Doumbo, O.; Raoult, D.; Parola, P.; Almeras, L. Identification of Blood Meal Sources in the Main African Malaria Mosquito Vector by MALDI-TOF MS. Malar. J. 2016, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Niare, S.; Tandina, F.; Davoust, B.; Doumbo, O.; Raoult, D.; Parola, P.; Almeras, L. Accurate Identification of Anopheles gambiae Giles Trophic Preferences by MALDI-TOF MS. Infect. Genet. Evol. 2018, 63, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Niare, S.; Almeras, L.; Tandina, F.; Yssouf, A.; Bacar, A.; Toilibou, A.; Doumbo, O.; Raoult, D.; Parola, P. MALDI-TOF MS Identification of Anopheles gambiae Giles Blood Meal Crushed on Whatman Filter Papers. PLoS ONE 2017, 12, e0183238. [Google Scholar] [CrossRef] [PubMed]
- Tandina, F.; Niare, S.; Almeras, L.; Davoust, B.; Doumbo, O.K.; Raoult, D.; Parola, P.; Laroche, M. Identification of Mixed and Successive Blood Meals of Mosquitoes Using MALDI-TOF MS Protein Profiling. Parasitology 2020, 147, 329–339. [Google Scholar] [CrossRef]
- Fall, F.K.; Laroche, M.; Bossin, H.; Musso, D.; Parola, P. Performance of MALDI-TOF Mass Spectrometry to Determine the Sex of Mosquitoes and Identify Specific Colonies from French Polynesia. Am. J. Trop. Med. Hyg. 2021, 104, 1907–1916. [Google Scholar] [CrossRef]
- Tahir, D.; Almeras, L.; Varloud, M.; Raoult, D.; Davoust, B.; Parola, P. Assessment of MALDI-TOF Mass Spectrometry for Filariae Detection in Aedes Aegypti Mosquitoes. PLoS Negl. Trop. Dis. 2017, 11, e0006093. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Almeras, L.; Pecchi, E.; Bechah, Y.; Raoult, D.; Viola, A.; Parola, P. MALDI-TOF MS as an Innovative Tool for Detection of Plasmodium Parasites in Anopheles Mosquitoes. Malar. J. 2017, 16, 5. [Google Scholar] [CrossRef]
- Raharimalala, F.N.; Andrianinarivomanana, T.M.; Rakotondrasoa, A.; Collard, J.M.; Boyer, S. Usefulness and Accuracy of MALDI-TOF Mass Spectrometry as a Supplementary Tool to Identify Mosquito Vector Species and to Invest in Development of International Database. Med. Vet. Entomol. 2017, 31, 289–298. [Google Scholar] [CrossRef]
- Rakotonirina, A.; Pol, M.; Kainiu, M.; Barsac, E.; Tutagata, J.; Kilama, S.; O’Connor, O.; Tarantola, A.; Colot, J.; Dupont-Rouzeyrol, M.; et al. MALDI-TOF MS: Optimization for Future Uses in Entomological Surveillance and Identification of Mosquitoes from New Caledonia. Parasites Vectors 2020, 13, 359. [Google Scholar] [CrossRef]
- Nebbak, A.; Willcox, A.C.; Bitam, I.; Raoult, D.; Parola, P.; Almeras, L. Standardization of Sample Homogenization for Mosquito Identification Using an Innovative Proteomic Tool Based on Protein Profiling. Proteomics 2016, 16, 3148–3160. [Google Scholar] [CrossRef]
- Vega-Rúa, A.; Pagès, N.; Fontaine, A.; Nuccio, C.; Hery, L.; Goindin, D.; Gustave, J.; Almeras, L. Improvement of Mosquito Identification by MALDI-TOF MS Biotyping Using Protein Signatures from Two Body Parts. Parasites Vectors 2018, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Nebbak, A.; Willcox, A.C.; Koumare, S.; Berenger, J.-M.; Raoult, D.; Parola, P.; Fontaine, A.; Briolant, S.; Almeras, L. Longitudinal Monitoring of Environmental Factors at Culicidae Larval Habitats in Urban Areas and Their Association with Various Mosquito Species Using an Innovative Strategy. Pest Manag. Sci. 2019, 75, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Kaufmann, C.; Pflüger, V.; Mathis, A. Rapid Protein Profiling Facilitates Surveillance of Invasive Mosquito Species. Parasites Vectors 2014, 7, 142. [Google Scholar] [CrossRef]
- Briolant, S.; Costa, M.M.; Nguyen, C.; Dusfour, I.; Pommier de Santi, V.; Girod, R.; Almeras, L. Identification of French Guiana Anopheline Mosquitoes by MALDI-TOF MS Profiling Using Protein Signatures from Two Body Parts. PLoS ONE 2020, 15, e0234098. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Guidez, A.; Briolant, S.; Talaga, S.; Issaly, J.; Naroua, H.; Carinci, R.; Gaborit, P.; Lavergne, A.; Dusfour, I.; et al. Identification of Neotropical Culex Mosquitoes by MALDI-TOF MS Profiling. Trop. Med. Infect. Dis. 2023, 8, 168. [Google Scholar] [CrossRef]
- Suter, T.; Flacio, E.; Fariña, B.F.; Engeler, L.; Tonolla, M.; Müller, P. First Report of the Invasive Mosquito Species Aedes Koreicus in the Swiss-Italian Border Region. Parasites Vectors 2015, 8, 402. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mewara, A.; Sharma, M.; Kaura, T.; Zaman, K.; Yadav, R.; Sehgal, R. Rapid Identification of Medically Important Mosquitoes by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Parasites Vectors 2018, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Yssouf, A.; Parola, P.; Lindström, A.; Lilja, T.; L’Ambert, G.; Bondesson, U.; Berenger, J.-M.; Raoult, D.; Almeras, L. Identification of European Mosquito Species by MALDI-TOF MS. Parasitol. Res. 2014, 113, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Tandina, F.; Niaré, S.; Laroche, M.; Koné, A.K.; Diarra, A.Z.; Ongoiba, A.; Berenger, J.M.; Doumbo, O.K.; Raoult, D.; Parola, P. Using MALDI-TOF MS to Identify Mosquitoes Collected in Mali and Their Blood Meals. Parasitology 2018, 145, 1170–1182. [Google Scholar] [CrossRef]
- Lawrence, A.L.; Batovska, J.; Webb, C.E.; Lynch, S.E.; Blacket, M.J.; Šlapeta, J.; Parola, P.; Laroche, M. Accurate Identification of Australian Mosquitoes Using Protein Profiling. Parasitology 2019, 146, 462–471. [Google Scholar] [CrossRef]
- Nebbak, A.; Monteil-Bouchard, S.; Berenger, J.-M.; Almeras, L.; Parola, P.; Desnues, C. Virome Diversity among Mosquito Populations in a Sub-Urban Region of Marseille, France. Viruses 2021, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Abdellahoum, Z.; Nebbak, A.; Lafri, I.; Kaced, A.; Bouhenna, M.M.; Bachari, K.; Boumegoura, A.; Agred, R.; Boudchicha, R.H.; Smadi, M.A.; et al. Identification of Algerian Field-Caught Mosquito Vectors by MALDI-TOF MS. Vet. Parasitol. Reg. Stud. Rep. 2022, 31, 100735. [Google Scholar] [CrossRef]
- Loaiza, J.R.; Almanza, A.; Rojas, J.C.; Mejía, L.; Cervantes, N.D.; Sanchez-Galan, J.E.; Merchán, F.; Grillet, A.; Miller, M.J.; De León, L.F.; et al. Application of Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry to Identify Species of Neotropical Anopheles Vectors of Malaria. Malar. J. 2019, 18, 95. [Google Scholar] [CrossRef]
- Dieme, C.; Yssouf, A.; Vega-Rúa, A.; Berenger, J.-M.; Failloux, A.-B.; Raoult, D.; Parola, P.; Almeras, L. Accurate Identification of Culicidae at Aquatic Developmental Stages by MALDI-TOF MS Profiling. Parasites Vectors 2014, 7, 544. [Google Scholar] [CrossRef]
- Nebbak, A.; Koumare, S.; Willcox, A.C.; Berenger, J.-M.; Raoult, D.; Almeras, L.; Parola, P. Field Application of MALDI-TOF MS on Mosquito Larvae Identification. Parasitology 2018, 145, 677–687. [Google Scholar] [CrossRef]
- Nebbak, A.; Almeras, L. Identification of Aedes Mosquitoes by MALDI-TOF MS Biotyping Using Protein Signatures from Larval and Pupal Exuviae. Parasites Vectors 2020, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Juanes, F.; Calvo Sánchez, N.; Belhassen García, M.; Vieira Lista, C.; Román, R.M.; Álamo Sanz, R.; Muro Álvarez, A.; Muñoz Bellido, J.L. Applications of MALDI-TOF Mass Spectrometry to the Identification of Parasites and Arthropod Vectors of Human Diseases. Microorganisms 2022, 10, 2300. [Google Scholar] [CrossRef]
- Karger, A. Current Developments to Use Linear MALDI-TOF Spectra for the Identification and Typing of Bacteria and the Characterization of Other Cells/Organisms Related to Infectious Diseases. Proteom. Clin. Appl. 2016, 10, 982–993. [Google Scholar] [CrossRef]
- Suarez, E.; Nguyen, H.P.; Ortiz, I.P.; Lee, K.J.; Kim, S.B.; Krzywinski, J.; Schug, K.A. Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry of Cuticular Lipid Profiles Can Differentiate Sex, Age, and Mating Status of Anopheles gambiae Mosquitoes. Anal. Chim. Acta 2011, 706, 157–163. [Google Scholar] [CrossRef]
- Caputo, B.; Dani, F.R.; Horne, G.L.; N’Fale, S.; Diabate, A.; Turillazzi, S.; Coluzzi, M.; Costantini, C.; Priestman, A.A.; Petrarca, V.; et al. Comparative Analysis of Epicuticular Lipid Profiles of Sympatric and Allopatric Field Populations of Anopheles gambiae s.s. Molecular Forms and An. arabiensis from Burkina Faso (West Africa). Insect Biochem. Mol. Biol. 2007, 37, 389–398. [Google Scholar] [CrossRef]
- Caputo, B.; Dani, F.R.; Horne, G.L.; Petrarca, V.; Turillazzi, S.; Coluzzi, M.; Priestman, A.A.; della Torre, A. Identification and Composition of Cuticular Hydrocarbons of the Major Afrotropical Malaria Vector Anopheles gambiae s.s. (Diptera: Culicidae): Analysis of Sexual Dimorphism and Age-Related Changes. J. Mass. Spectrom. 2005, 40, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.E.; Sinkins, S.P. Transcriptional Profiling of Anopheles gambiae Mosquitoes for Adult Age Estimation. Insect Mol. Biol. 2010, 19, 745–751. [Google Scholar] [CrossRef]
- Wang, M.-H.; Marinotti, O.; James, A.A.; Walker, E.; Githure, J.; Yan, G. Genome-Wide Patterns of Gene Expression during Aging in the African Malaria Vector Anopheles gambiae. PLoS ONE 2010, 5, e13359. [Google Scholar] [CrossRef]
- Nabet, C.; Chaline, A.; Franetich, J.-F.; Brossas, J.-Y.; Shahmirian, N.; Silvie, O.; Tannier, X.; Piarroux, R. Prediction of Malaria Transmission Drivers in Anopheles Mosquitoes Using Artificial Intelligence Coupled to MALDI-TOF Mass Spectrometry. Sci. Rep. 2020, 10, 11379. [Google Scholar] [CrossRef]
- Mohammad, N.; Naudion, P.; Dia, A.K.; Boëlle, P.-Y.; Konaté, A.; Konaté, L.; Niang, E.H.A.; Piarroux, R.; Tannier, X.; Nabet, C. Predicting the Age of Field Anopheles Mosquitoes Using Mass Spectrometry and Deep Learning. Sci. Adv. 2024, 10, eadj6990. [Google Scholar] [CrossRef] [PubMed]
- Tempelis, C.H. Host-Feeding Patterns of Mosquitoes, with a Review of Advances in Analysis of Blood Meals by Serology. J. Med. Entomol. 1975, 11, 635–653. [Google Scholar] [CrossRef]
- Washino, R.K.; Tempelis, C.H. Mosquito Host Bloodmeal Identification: Methodology and Data Analysis. Annu. Rev. Entomol. 1983, 28, 179–201. [Google Scholar] [CrossRef]
- Börstler, J.; Jöst, H.; Garms, R.; Krüger, A.; Tannich, E.; Becker, N.; Schmidt-Chanasit, J.; Lühken, R. Host-Feeding Patterns of Mosquito Species in Germany. Parasites Vectors 2016, 9, 318. [Google Scholar] [CrossRef]
- Tandina, F.; Laroche, M.; Davoust, B.; Doumbo, O.K.; Parola, P. Blood Meal Identification in the Cryptic Species Anopheles gambiae and Anopheles coluzzii Using MALDI-TOF MS. Parasite 2018, 25, 40. [Google Scholar] [CrossRef] [PubMed]
- Ricci, I.; Damiani, C.; Capone, A.; DeFreece, C.; Rossi, P.; Favia, G. Mosquito/Microbiota Interactions: From Complex Relationships to Biotechnological Perspectives. Curr. Opin. Microbiol. 2012, 15, 278–284. [Google Scholar] [CrossRef]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and Function of Bacterial Microbiota in the Mosquito Holobiont. Parasites Vectors 2013, 6, 146. [Google Scholar] [CrossRef]
- Carvajal-Lago, L.; Ruiz-López, M.J.; Figuerola, J.; Martínez-de la Puente, J. Implications of Diet on Mosquito Life History Traits and Pathogen Transmission. Environ. Res. 2021, 195, 110893. [Google Scholar] [CrossRef]
- Strand, M.R. Composition and Functional Roles of the Gut Microbiota in Mosquitoes. Curr. Opin. Insect Sci. 2018, 28, 59–65. [Google Scholar] [CrossRef]
- Vinayagam, S.; Rajendran, D.; Sekar, K.; Renu, K.; Sattu, K. The Microbiota, the Malarial Parasite, and the Mosquito [MMM]—A Three-Sided Relationship. Mol. Biochem. Parasitol. 2023, 253, 111543. [Google Scholar] [CrossRef] [PubMed]
- Dada, N.; Sheth, M.; Liebman, K.; Pinto, J.; Lenhart, A. Whole Metagenome Sequencing Reveals Links between Mosquito Microbiota and Insecticide Resistance in Malaria Vectors. Sci. Rep. 2018, 8, 2084. [Google Scholar] [CrossRef] [PubMed]
- Caragata, E.P.; Tikhe, C.V.; Dimopoulos, G. Curious Entanglements: Interactions between Mosquitoes, Their Microbiota, and Arboviruses. Curr. Opin. Virol. 2019, 37, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Tandina, F.; Almeras, L.; Koné, A.K.; Doumbo, O.K.; Raoult, D.; Parola, P. Use of MALDI-TOF MS and Culturomics to Identify Mosquitoes and Their Midgut Microbiota. Parasites Vectors 2016, 9, 495. [Google Scholar] [CrossRef]
- Gazzoni Araújo Gonçalves, G.; Feitosa, A.P.S.; Portela-Júnior, N.C.; de Oliveira, C.M.F.; de Lima Filho, J.L.; Brayner, F.A.; Alves, L.C. Use of MALDI-TOF MS to Identify the Culturable Midgut Microbiota of Laboratory and Wild Mosquitoes. Acta Trop. 2019, 200, 105174. [Google Scholar] [CrossRef]
- E Silva, B.; Matsena Zingoni, Z.; Koekemoer, L.L.; Dahan-Moss, Y.L. Microbiota Identified from Preserved Anopheles. Malar. J. 2021, 20, 230. [Google Scholar] [CrossRef]
- Sicard, M.; Bonneau, M.; Weill, M. Wolbachia Prevalence, Diversity, and Ability to Induce Cytoplasmic Incompatibility in Mosquitoes. Curr. Opin. Insect Sci. 2019, 34, 12–20. [Google Scholar] [CrossRef]
- Ogunlade, S.T.; Meehan, M.T.; Adekunle, A.I.; Rojas, D.P.; Adegboye, O.A.; McBryde, E.S. A Review: Aedes-Borne Arboviral Infections, Controls and Wolbachia-Based Strategies. Vaccines 2021, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Rakotonirina, A.; Caruzzo, C.; Ballan, V.; Kainiu, M.; Marin, M.; Colot, J.; Richard, V.; Dupont-Rouzeyrol, M.; Selmaoui-Folcher, N.; Pocquet, N. Wolbachia Detection in Aedes Aegypti Using MALDI-TOF MS Coupled to Artificial Intelligence. Sci. Rep. 2021, 11, 21355. [Google Scholar] [CrossRef] [PubMed]
- Dusfour, I.; Vontas, J.; David, J.-P.; Weetman, D.; Fonseca, D.M.; Corbel, V.; Raghavendra, K.; Coulibaly, M.B.; Martins, A.J.; Kasai, S.; et al. Management of Insecticide Resistance in the Major Aedes Vectors of Arboviruses: Advances and Challenges. PLoS Negl. Trop. Dis. 2019, 13, e0007615. [Google Scholar] [CrossRef]
- Devillers, J.; David, J.-P.; Barrès, B.; Alout, H.; Lapied, B.; Chouin, S.; Dusfour, I.; Billault, C.; Mekki, F.; Attig, I.; et al. Integrated Plan of Insecticide Resistance Surveillance in Mosquito Vectors in France. Insects 2023, 14, 457. [Google Scholar] [CrossRef]
- Almeras, L.; Costa, M.M.; Amalvict, R.; Guilliet, J.; Dusfour, I.; David, J.-P.; Corbel, V. Potential of MALDI-TOF MS Biotyping to Detect Deltamethrin Resistance in the Dengue Vector Aedes aegypti. PLoS ONE 2024, 19, e0303027. [Google Scholar] [CrossRef]
- Hlavackova, K.; Dvorak, V.; Chaskopoulou, A.; Volf, P.; Halada, P. A Novel MALDI-TOF MS-Based Method for Blood Meal Identification in Insect Vectors: A Proof of Concept Study on Phlebotomine Sand Flies. PLoS Negl. Trop. Dis. 2019, 13, e0007669. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, K.R.; Gibb, S.; Kalkhof, S.; Arroyo-Abad, U.; Schulz, C.; Hoffmann, B.; Stubbins, F.; Carpenter, S.; Beer, M.; von Bergen, M.; et al. Species Determination of Culicoides Biting Midges via Peptide Profiling Using Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. Parasites Vectors 2014, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.S.; Boerwinkle, E.; Doris, P.A. High-Throughput Multiplex SNP Genotyping with MALDI-TOF Mass Spectrometry: Practice, Problems and Promise. Hum. Mutat. 2001, 17, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Gijavanekar, C.; Drabek, R.; Soni, M.; Jackson, G.W.; Strych, U.; Fox, G.E.; Fofanov, Y.; Willson, R.C. Detection and Typing of Viruses Using Broadly Sensitive Cocktail-PCR and Mass Spectrometric Cataloging: Demonstration with Dengue Virus. J. Mol. Diagn. 2012, 14, 402–407. [Google Scholar] [CrossRef]
- Mao, Y.; Tan, F.; Yan, S.-G.; Wu, G.-X.; Qiao, C.-L.; Zhang, W.-X.; Cui, F. High-Throughput Genotyping of Single-Nucleotide Polymorphisms in Ace-1 Gene of Mosquitoes Using MALDI-TOF Mass Spectrometry. Insect Sci. 2013, 20, 167–174. [Google Scholar] [CrossRef]
- Mu, Q.; Zhao, X.; Li, F.; Li, W.; Zhou, X.; Lun, X.; Wang, Y.; Hua, D.; Liu, Q.; Xiao, D.; et al. A Novel Strategy for Screening Mutations in the Voltage-Gated Sodium Channel Gene of Aedes albopictus Based on Multiplex PCR-Mass Spectrometry Minisequencing Technology. Infect. Dis. Poverty 2023, 12, 74. [Google Scholar] [CrossRef]
- Stillger, M.N.; Li, M.J.; Hönscheid, P.; von Neubeck, C.; Föll, M.C. Advancing Rare Cancer Research by MALDI Mass Spectrometry Imaging: Applications, Challenges, and Future Perspectives in Sarcoma. Proteomics 2024, 24, e2300001. [Google Scholar] [CrossRef]
- Sikulu, M.T.; Monkman, J.; Dave, K.A.; Hastie, M.L.; Dale, P.E.; Kitching, R.L.; Killeen, G.F.; Kay, B.H.; Gorman, J.J.; Hugo, L.E. Proteomic Changes Occurring in the Malaria Mosquitoes Anopheles gambiae and Anopheles Stephensi during Aging. J. Proteom. 2015, 126, 234–244. [Google Scholar] [CrossRef]
- Khalil, S.M.; Römpp, A.; Pretzel, J.; Becker, K.; Spengler, B. Phospholipid Topography of Whole-Body Sections of the Anopheles Stephensi Mosquito, Characterized by High-Resolution Atmospheric-Pressure Scanning Microprobe Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Anal. Chem. 2015, 87, 11309–11316. [Google Scholar] [CrossRef]

| Genus (n = 9) | Species (n = 67) | Pre-Immature and Immature Stages | Imago (Compartment) | Reference * |
|---|---|---|---|---|
| Anopheles | An. aquasalis | Adult (legs and thorax) | [57] | |
| An. braziliensis | Adult (legs and thorax) | [57] | ||
| An. darlingi | Adult (legs and thorax) | [57] | ||
| An. nuneztovari (s.l.) | Adult (legs and thorax) | [57] | ||
| An. triannulatus (s.l.) | Adult (legs and thorax) | [57] | ||
| An. oswaldoi (s.l.) | Adult (legs and thorax) | [57] | ||
| An. intermedius | Adult (legs and thorax) | [57] | ||
| An. peryassui | Adult (legs and thorax) | [57] | ||
| An. gambiae | Larvae, pupae | Adult (head, legs, and thorax) | [39,41,42,53,70] | |
| An. coluzzii | Larvae, pupae | Adult (legs and thorax) | [42,53,70] | |
| An. funestus | Adult (head, legs, and thorax) | [39] | ||
| An. ziemanni | Adult (legs) | [39] | ||
| An. arabiensis | Adult (head, legs, and thorax) | [39,41] | ||
| An. wellcomei | Adult (legs) | [39] | ||
| An. rufipes | Adult (legs) | [39] | ||
| An. pharoensis | Adult (legs) | [39] | ||
| An. maculipennis | Larvae | [71] | ||
| An. claviger | Adult (legs) | [64] | ||
| An. hyrcanus | Adult (legs) | [64] | ||
| An. maculipennis | Adult (legs) | [64] | ||
| An. bancroftii | Adult (legs) | [52] | ||
| Culex | Cx. dunni | Adult (legs and thorax) | [58] | |
| Cx. nigripalpus | Adult (legs and thorax) | [58] | ||
| Cx. quinquefasciatus | Adult (legs and thorax) | [39,52,54] | ||
| Cx. usquatus | Adult (legs and thorax) | [58] | ||
| Cx. adamesi | Adult (legs and thorax) | [58] | ||
| Cx. dunni | Adult (legs and thorax) | [58] | ||
| Cx. eastor | Adult (legs and thorax) | [58] | ||
| Cx. idottus | Adult (legs and thorax) | [58] | ||
| Cx. pedroi | Adult (legs and thorax) | [58] | ||
| Cx. phlogistus | Adult (legs and thorax) | [58] | ||
| Cx. portesi | Adult (legs and thorax) | [58] | ||
| Cx. rabanicolus | Adult (legs and thorax) | [58] | ||
| Cx. spissipes | Adult (legs and thorax) | [58] | ||
| Cx. nigripalpus | Adult (legs and thorax) | [54] | ||
| Cx. pipiens | Larvae, pupae | Adult (legs) | [39,64,70,71] | |
| Cx. modestus | Adult (legs) | [64] | ||
| Cx. hortensis | Larvae | [71] | ||
| Cx. atratus (s.l.) | Adult (legs and thorax) | [54] | ||
| Cx. iyengari | Adult (legs) | [52] | ||
| Cx. sitiens | Adult (legs) | [52] | ||
| Cx. annulirostris | Adult (legs) | [52] | ||
| Cx. molestus | Larvae and pupae | [70] | ||
| Aedes | Ae. aegypti | Eggs, larvae, pupae, and exuviae of larvae and pupae | Adult (legs and thorax) | [39,42,52,54,56,70,72] |
| Ae. albopictus | Eggs, larvae, pupae, and exuviae of larvae and pupae | Adult (legs and thorax) | [39,42,53,54,56,70,71,72] | |
| Ae. atropalpus | Eggs | [56] | ||
| Ae. cretinus | Eggs | [56] | ||
| Ae. geniculatus | Eggs | [56] | ||
| Ae. japonicus | Eggs | [56] | ||
| Ae. koreicus | Eggs | [56] | ||
| Ae. phoeniciae | Eggs | [56] | ||
| Ae. triseriatus | Eggs | [56] | ||
| Ae. taeniorhynchus | Adult (legs and thorax) | [54] | ||
| Ae. cinereus | Adult (legs) | [64] | ||
| Ae. vexans | Adult (legs) | [52,64] | ||
| Ae. caspius | Larvae | Adult (legs) | [62,68] | |
| Ae. rusticus | Adult (legs) | [64] | ||
| Ae. excrucians | Adult (legs) | [64] | ||
| Ae. scutellaris | Adult (legs) | [52] | ||
| Ae. notoscriptus | Adult (legs) | [52] | ||
| Ae. vigilax | Adult (legs) | [52] | ||
| Mansonia | M. uniformis | Adult (legs) | [39] | |
| Culiseta | Cs. longiareolata | Larvae | [71] | |
| Deinocerites | D. magnus | Adult (legs and thorax) | [54] | |
| Psorophora | P. cingulata | Adult (legs and thorax) | [54] | |
| Coquillettidia | Cq. richiardii | Adult (legs) | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.M.; Corbel, V.; Ben Hamouda, R.; Almeras, L. MALDI-TOF MS Profiling and Its Contribution to Mosquito-Borne Diseases: A Systematic Review. Insects 2024, 15, 651. https://doi.org/10.3390/insects15090651
Costa MM, Corbel V, Ben Hamouda R, Almeras L. MALDI-TOF MS Profiling and Its Contribution to Mosquito-Borne Diseases: A Systematic Review. Insects. 2024; 15(9):651. https://doi.org/10.3390/insects15090651
Chicago/Turabian StyleCosta, Monique Melo, Vincent Corbel, Refka Ben Hamouda, and Lionel Almeras. 2024. "MALDI-TOF MS Profiling and Its Contribution to Mosquito-Borne Diseases: A Systematic Review" Insects 15, no. 9: 651. https://doi.org/10.3390/insects15090651







