Transcriptomic and Gene Expression Analysis of Chemosensory Genes from White Grubs of Hylamorpha elegans (Coleoptera: Scarabaeidae), a Subterranean Pest in South America
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. White Grub Collection and RNA Extraction
2.2. Sequencing and De Novo Transcriptome Assembly
2.3. Transcriptome Assessment, Abundance Estimation, and Differential Expression
2.4. Transcriptome Annotation and Candidate Gene Identification
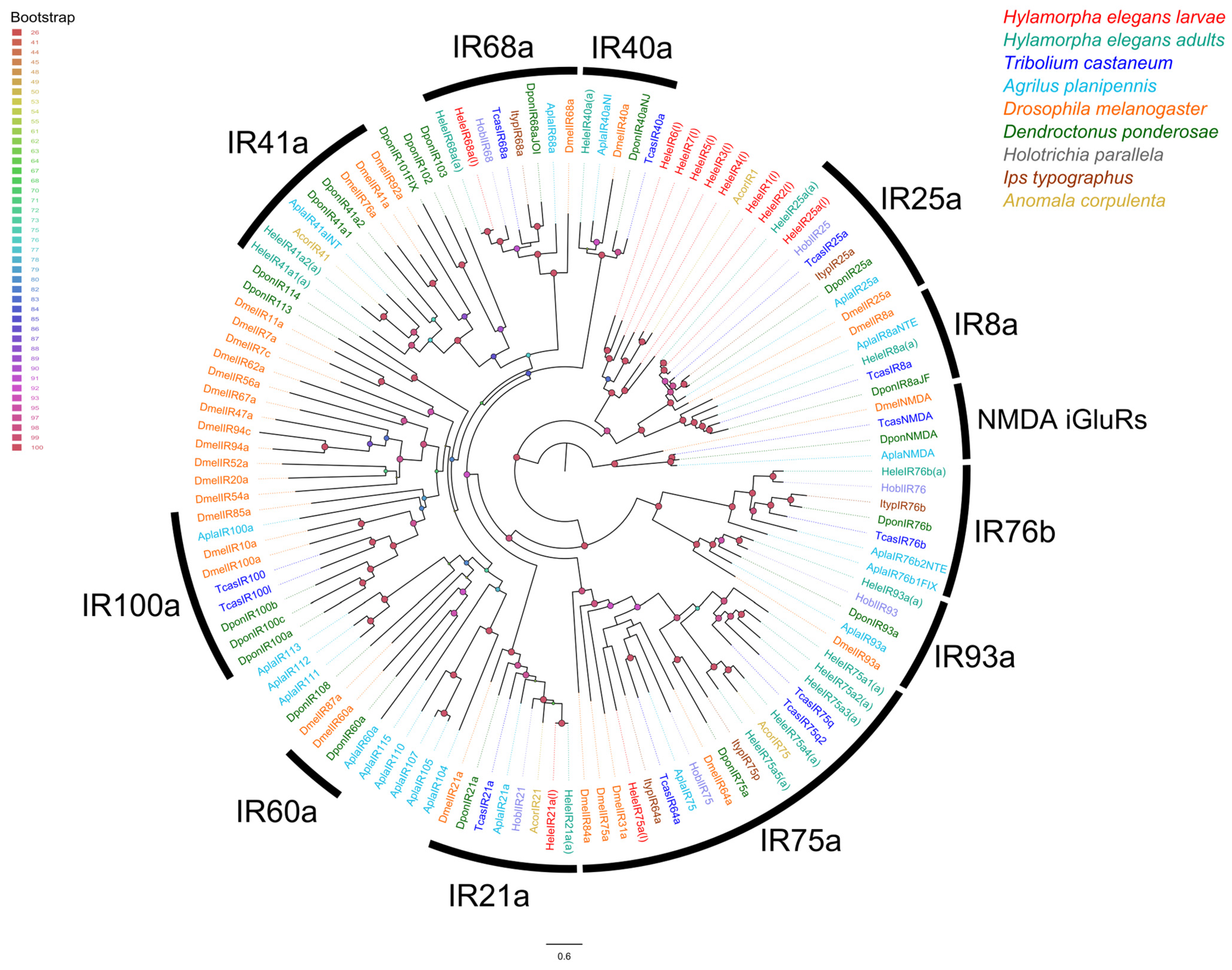
2.5. Phylogenetic Analysis
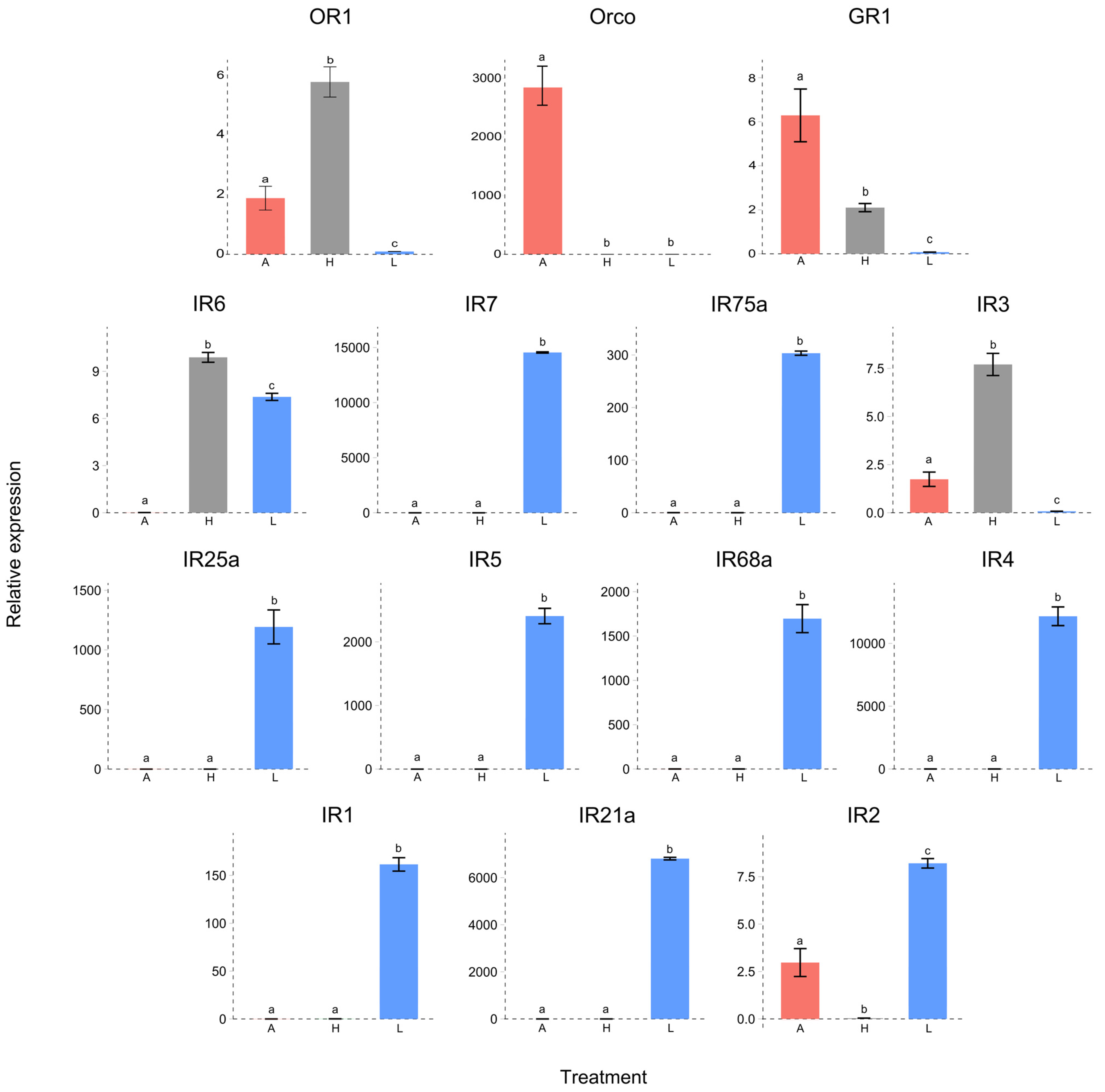
2.6. Tissue-Based Relative Expression Determined by qRT–PCR
3. Results
3.1. Sequencing and De Novo Assembly
3.2. Transcript Annotation and Abundance Estimation of Chemoreceptors
3.3. Chemoreceptor–Phylogenetic Relationships
3.4. Differential Expression of Chemoreceptors
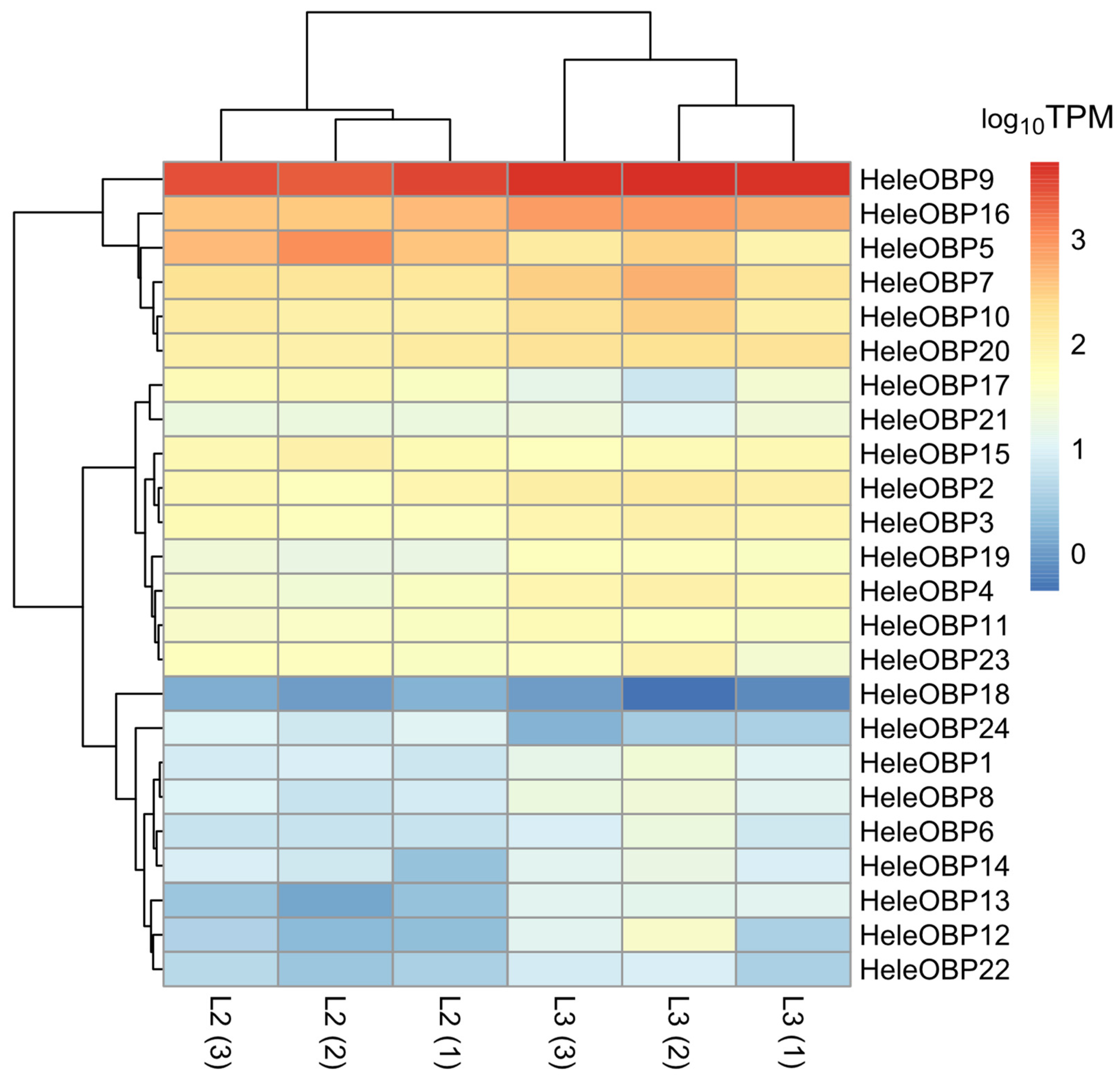
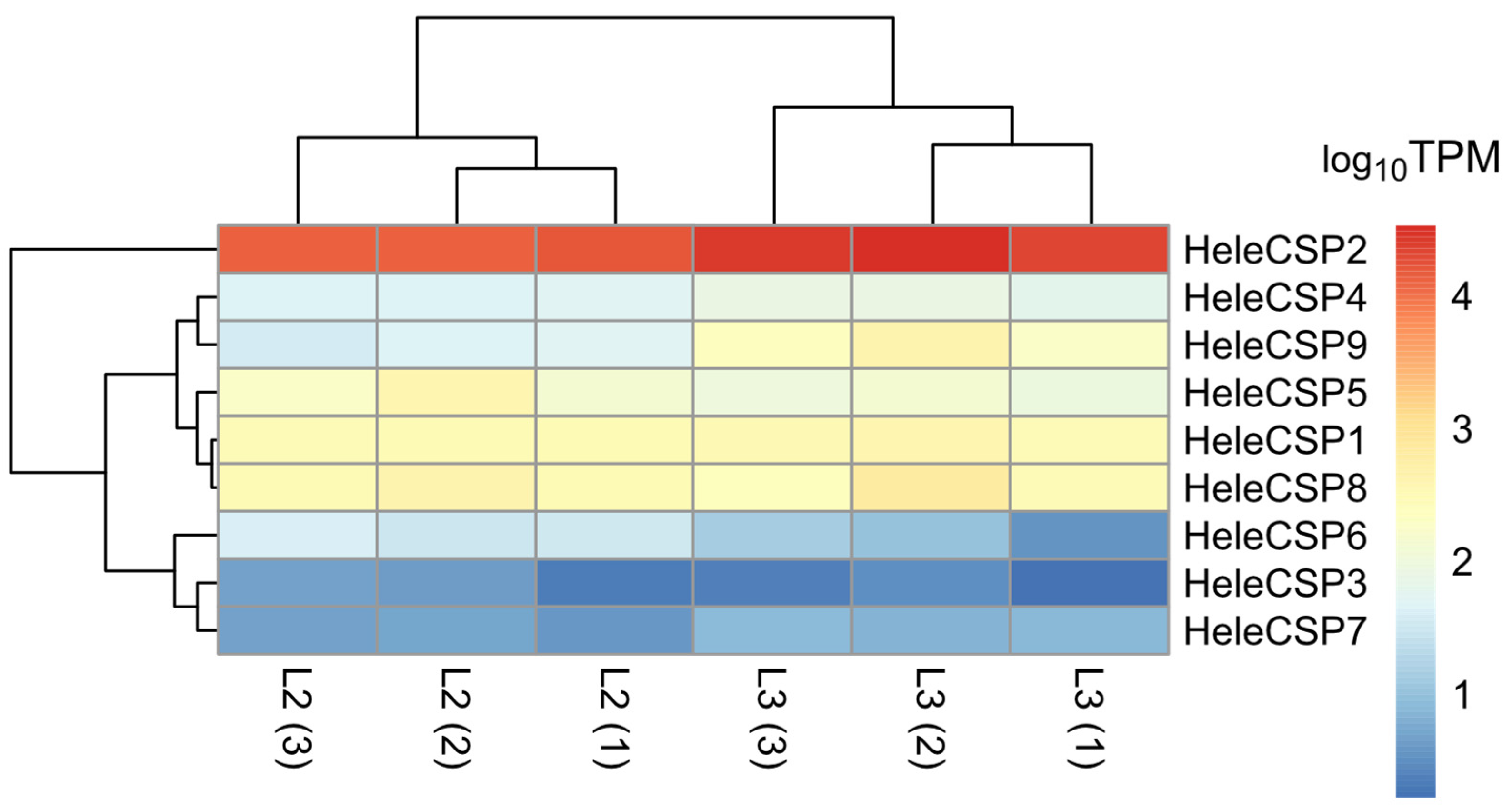
3.5. Transcript Annotation and Abundance Estimation of Soluble Proteins
3.6. Phylogenetic Relationships of Soluble Proteins
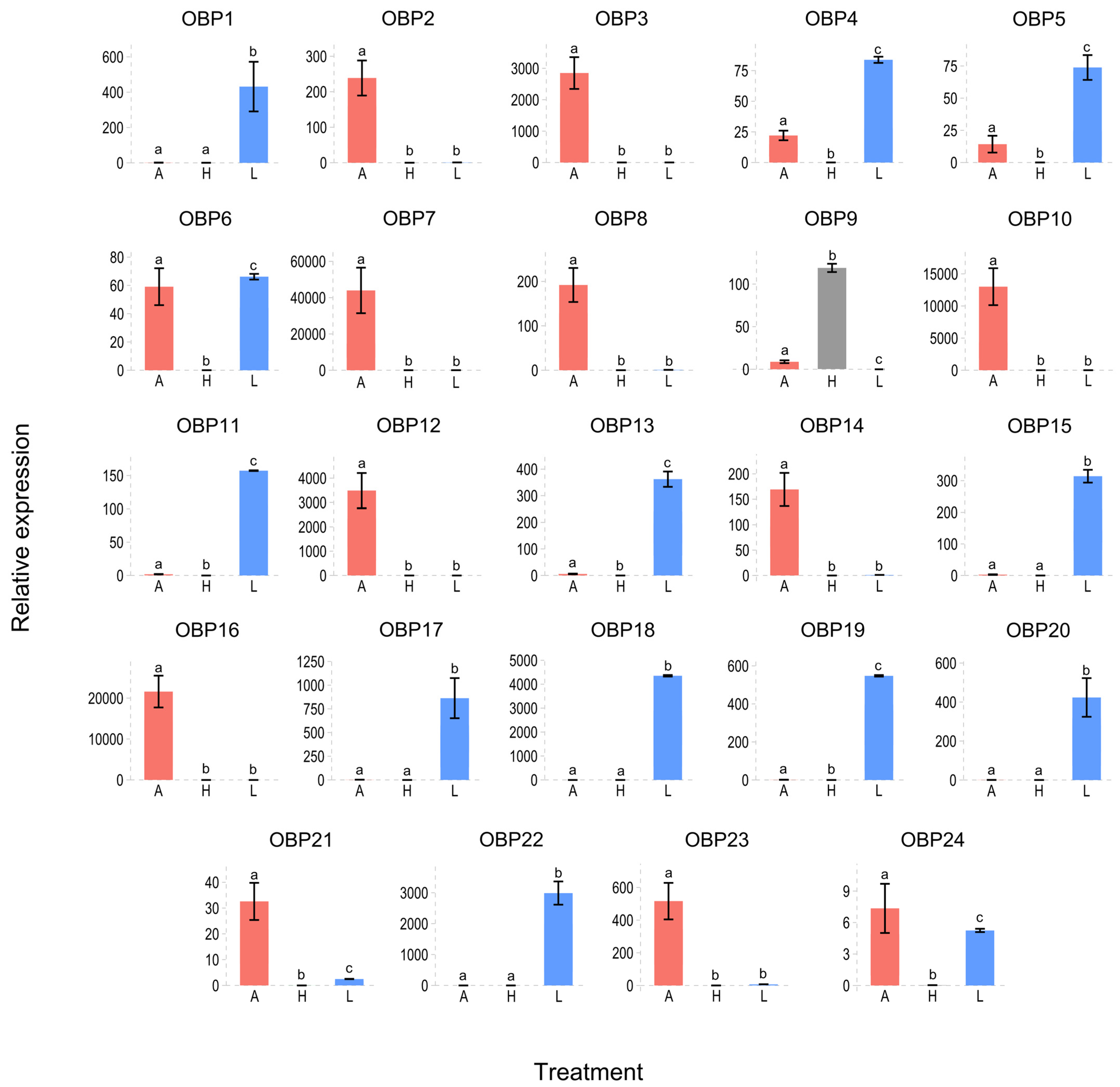
3.7. Differential Expression of Soluble Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anton, S.; Rössler, W. Plasticity and Modulation of Olfactory Circuits in Insects. Cell Tissue Res. 2021, 383, 149–164. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in Insect Olfaction: To Smell or Not to Smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef]
- Venthur, H.; Zhou, J.J. Odorant Receptors and Odorant-Binding Proteins as Insect Pest Control Targets: A Comparative Analysis. Front. Physiol. 2018, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S.; Nikonova, L.; Peng, G. Disulfide Structure of the Pheromone Binding Protein from the Silkworm Moth, Bombyx mori. FEBS J. 1999, 464, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Scaloni, A.; Monti, M.; Angeli, S.; Pelosi, P. Structural Analysis and Disulfide-Bridge Pairing of Two Odorant-Binding Proteins from Bombyx mori. Biochem. Biophys. Res. Commun. 1999, 266, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Breer, H.; Raming, K.; Krieger, J. Signal Recognition and Transduction in Olfactory Neurons. Biochim. Biophys. Acta Mol. Cell Res. 1994, 1224, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Calvello, M.; Guerra, N.; Brandazza, A.; D’Ambrosio, C.; Scaloni, A.; Dani, F.R.; Turillazzi, S.; Pelosi, P. Soluble Proteins of Chemical Communication in the Social Wasp Polistes dominulus. Cell. Mol. Life Sci. 2003, 60, 1933–1943. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, S.; Li, K.; Liu, J.; Zheng, Y.; Shan, S.; Yang, Y.; Li, R.; Zhang, Y.; Guo, Y. Identification of Odorant Binding Proteins and Chemosensory Proteins in Microplitis mediator as Well as Functional Characterization of Chemosensory Protein 3. PLoS ONE 2017, 12, e0180775. [Google Scholar] [CrossRef]
- Wicher, D.; Marion-Poll, F. Editorial: Function and Regulation of Chemoreceptors. Front. Cell. Neurosci. 2018, 12, 496. [Google Scholar] [CrossRef]
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect Olfactory Receptors Are Heteromeric Ligand-Gated Ion Channels. Nature 2008, 452, 1002–1006. [Google Scholar] [CrossRef]
- Wicher, D.; Schäfer, R.; Bauernfeind, R.; Stensmyr, M.C.; Heller, R.; Heinemann, S.H.; Hansson, B.S. Drosophila Odorant Receptors Are Both Ligand-Gated and Cyclic-Nucleotide-Activated Cation Channels. Nature 2008, 452, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Knecht, Z.A.; Silbering, A.F.; Cruz, J.; Yang, L.; Croset, V.; Benton, R.; Garrity, P.A. Ionotropic Receptor-Dependent Moist and Dry Cells Control Hygrosensation in Drosophila. eLife 2017, 6, e26654. [Google Scholar] [CrossRef]
- Slone, J.; Daniels, J.; Amrein, H. Sugar Receptors in Drosophila. Curr. Biol. 2007, 17, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Wanner, K.W.; Robertson, H.M. The Gustatory Receptor Family in the Silkworm Moth Bombyx mori Is Characterized by a Large Expansion of a Single Lineage of Putative Bitter Receptors. Insect Mol. Biol. 2008, 17, 621–629. [Google Scholar] [CrossRef]
- Jiao, Y.; Moon, S.J.; Wang, X.; Ren, Q.; Montell, C. Gr64f Is Required in Combination with Other Gustatory Receptors for Sugar Detection in Drosophila. Curr. Biol. 2008, 18, 1797. [Google Scholar] [CrossRef]
- Kim, S.M.; Wang, J.W. Hygrosensation: Feeling Wet and Cold. Curr. Biol. 2016, 26, R408–R410. [Google Scholar] [CrossRef]
- Enjin, A.; Zaharieva, E.E.; Frank, D.D.; Mansourian, S.; Suh, G.S.B.; Gallio, M.; Stensmyr, M.C. Humidity Sensing in Drosophila. Curr. Biol. 2016, 26, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Saint-Germain, M.; Drapeau, P.; Buddle, C.M. Host-Use Patterns of Saproxylic Phloeophagous and Xylophagous Coleoptera Adults and Larvae along the Decay Gradient in Standing Dead Black Spruce and Aspen. Ecography 2007, 30, 737–748. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G. Effect of Larval Density on Food Utilization Efficiency of Tenebrio molitor (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2015, 108, 2259–2267. [Google Scholar] [CrossRef]
- Mankin, R.W.; Smith, M.T.; Tropp, J.M.; Atkinson, E.B.; Jong, D.Y. Detection of Anoplophora glabripennis (Coleoptera: Cerambycidae) Larvae in Different Host Trees and Tissues by Automated Analyses of Sound-Impulse Frequency and Temporal Patterns. J. Econ. Entomol. 2008, 101, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, G.; Buscariollo, D.; Pitts, R.J.; Wenger, H.; Zwiebel, L.J. The Molecular and Cellular Basis of Olfactory-Driven Behavior in Anopheles gambiae Larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 6433–6438. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, X.; Gui, H.; Lan, M.; Zhu, J.; Xie, Y.; Zhan, Y.; Wang, Z.; Li, Z.; Ye, M.; et al. Identification and Preliminary Characterization of Chemosensory-Related Proteins in the Gall Fly, Procecidochares utilis by Transcriptomic Analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 36, 100724. [Google Scholar] [CrossRef]
- Kreher, S.A.; Kwon, J.Y.; Carlson, J.R. The Molecular Basis of Odor Coding in the Drosophila Larva. Neuron 2005, 46, 445–456. [Google Scholar] [CrossRef]
- Fishilevich, E.; Domingos, A.I.; Asahina, K.; Naef, F.; Vosshall, L.B.; Louis, M. Chemotaxis Behavior Mediated by Single Larval Olfactory Neurons in Drosophila. Curr. Biol. 2005, 15, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Poivet, E.; Gallot, A.; Montagné, N.; Glaser, N.; Legeai, F.; Jacquin-Joly, E. A Comparison of the Olfactory Gene Repertoires of Adults and Larvae in the Noctuid Moth Spodoptera littoralis. PLoS ONE 2013, 8, e60263. [Google Scholar] [CrossRef]
- Chang, H.; Liu, Y.; Ai, D.; Jiang, X.; Dong, S.; Wang, G. A Pheromone Antagonist Regulates Optimal Mating Time in the Moth Helicoverpa armigera. Curr. Biol. 2017, 27, 1610–1615.e3. [Google Scholar] [CrossRef]
- Llopis-Giménez, A.; Carrasco-Oltra, T.; Jacquin-Joly, E.; Herrero, S.; Crava, C.M. Coupling Transcriptomics and Behaviour to Unveil the Olfactory System of Spodoptera exigua Larvae. J. Chem. Ecol. 2020, 46, 1017–1031. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Miao, C.; Wang, G.; Zhao, M.; Yuan, G.; Guo, X. Identication of the Differences in Olfactory System Between Male and Female Oriental Tobacco Budworm Helicoverpa assulta. Arch. Insect Biochem. Physiol. 2021, 107, e21829. [Google Scholar] [CrossRef]
- Wu, Z.R.; Fan, J.T.; Tong, N.; Guo, J.M.; Li, Y.; Lu, M.; Liu, X.L. Transcriptome Analysis and Identification of Chemosensory Genes in the Larvae of Plagiodera versicolora. BMC Genom. 2022, 23, 845. [Google Scholar] [CrossRef]
- Arce, C.; Theepan, V.; Schimmel, B.; Jaffuel, G.; Erb, M.; Machado, R. Plant-associated CO2 mediates long-distance host location and foraging behaviour of a root herbivore. eLife 2021, 10, e65575. [Google Scholar] [CrossRef]
- Eilers, E.J.; Talarico, G.P.; Hansson, B.S.; Hilker, M.; Reinecke, A. Sensing the Underground—Ultrastructure and function of sensory organs in Root-Feeding Melolontha Melolontha (Coleoptera: Scarabaeinae) larvae. PLoS ONE 2012, 7, e41357. [Google Scholar] [CrossRef] [PubMed]
- Prado, E. Artrópodos y Enemigos Naturales Asociados a Plantas Cultivadas en Chile; Boletín Técnico, Instituto de Investigaciones Agropecuarias: Santiago, Chile, 1991; Volume 16, Available online: https://hdl.handle.net/20.500.14001/37317 (accessed on 20 May 2023).
- Alfonso, A. Plagas Subterráneas; Serie Carillanca, Instituto de Investigaciones Agropecuarias: Temuco, Chile, 1995; Available online: https://hdl.handle.net/20.500.14001/27495 (accessed on 20 May 2023).
- Trombert, M. Eficacia en Laboratorio del Nemátodo Parásito Pristionchus sp. (Nematoda: Diplogasteridae) y de Cuatro Insecticidas Orgánicos Para el Control de larvas de Hylamorpha elegans (Burmeister) (Coleoptera: Scarabaeidae). Agronomist Degree, Universidad de La Frontera, Temuco, Chile, 1998. [Google Scholar]
- Ratcliffe, B.; Ocampo, F. A Review of the Genus Hylamorpha Arrow (Coleoptera: Scarabaeidae: Rutelinae: Anoplognathini: Brachysternina). Coleopt. Bull. 2002, 56, 367–378. [Google Scholar] [CrossRef]
- Cisternas, E.; Carrillo, R. Description of the Larvae of Hylamorpha Elegans (Burmeister, 1844) and Aulacopalpus Punctatus (Fairmaire and Germain, 1860) (Coleoptera: Scarabaeidae: Rutelinae: Anoplognathini). Coleopt. Bull. 2012, 66, 37–44. [Google Scholar] [CrossRef]
- Quiroz, A.; Palma, R.; Etcheverría, P.; Navarro, V.; Rebolledo, R. Males of Hylamorpha Elegans Burmeister (Coleoptera: Scarabaeidae) Are Attracted to Odors Beleased from Conspecific Females. Environ. Entomol. 2007, 36, 272–280. [Google Scholar] [CrossRef]
- González-González, A.; Palma-Millanao, R.; Yáñez, O.; Rojas, M.; Mutis, A.; Venthur, H.; Quiroz, A.; Ramírez, C.C. Virtual Screening of Plant Volatile Compounds Reveals a High Affinity of Hylamorpha Elegans (Coleoptera: Scarabaeidae) Odorant-Binding Proteins for Sesquiterpenes From Its Native Host. J. Insect Sci. 2016, 16, 30. [Google Scholar] [CrossRef]
- Venthur, H.; Zhou, J.J.; Mutis, A.; Ceballos, R.; Mella-Herrera, R.; Larama, G.; Avila, A.; Iturriaga-Vásquez, P.; Faundez-Parraguez, M.; Alvear, M.; et al. β-Ionone as Putative Semiochemical Suggested by Ligand Binding on an Odorant-Binding Protein of Hylamorpha Elegans and Electroantennographic Recordings. Entomol. Sci. 2016, 19, 188–200. [Google Scholar] [CrossRef]
- Cisternas, E. Biologia y Control de Insectos Plagas en Praderas; Serie Remehue, Instituto de Investigaciones Agropecuarias: Santiago, Chile, 1992; Available online: https://biblioteca.inia.cl/bitstream/handle/20.500.14001/37637/NR14287.pdf (accessed on 20 May 2023).
- Cisternas, E. Descripción de Los Estados Preimaginales de Escarabeidos Asociados a Praderas Antropogénicas de La Zona Sur de Chile; Universidad Austral de Chile: Valdivia, Chile, 1986. [Google Scholar]
- Artigas, J. Hylamorpha elegans(Burmeister). In Pololo San Juan verde (San juan Green Beetle); Universidad de Concepción, Ed.; Entomología Económica, Insectos de Interés Agrícola, Forestal, Médico y Veterinario; Editorial Universidad de Concepción: Concepción, Chile, 1994; pp. 344–346. [Google Scholar]
- Palma, R. Relaciones Entre Densidades Larvales de Hylamorpha Elegans (Burmeister) (Coleoptera: Scarabaeidae), y El Establecimiento y Desarrollo de La Especie Pratense Lolium Perenne; Universidad Austral de Chile: Valdivia, Chile, 2004. [Google Scholar]
- Villalobos-Hernandez, F.J. The Role of Soil Organic Matter in the Sustainable Management of the Grass Grub Costelytra Zealandica (White) in Canterbury Pastures. Doctor of Philosophy Degree, Lincoln University, Lincoln, New Zealand, 1994. [Google Scholar]
- Millas, P.; Carrillo, R. Live Weight and Relative Faecal Production of Hylamorpha Elegans (Burm.) (Coleoptera: Scarabaeidae) Larvae Fed on Soils with Different Particulate Organic Matter Content. Aust. J. Entomol. 2014, 53, 275–279. [Google Scholar] [CrossRef]
- Millas, P.; Carrillo, R. Larval Damage of Hylamorpha Elegans (Burm.) on Wheat Plants Sown in Soils with Different Particulate Organic Matter Content. Aust. J. Entomol. 2011, 50, 125–129. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for Clustering the next-Generation Sequencing Data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-H.; Zhou, J.-J.; Gao, S.; Wang, D.-H.; Li, X.-C.; Guo, Y.-Y.; Zhang, Y.-J. Identification and Comparative Expression Analysis of Odorant Binding Protein Genes in the Tobacco Cutworm Spodoptera litura. Sci. Rep. 2015, 5, 13800. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.; Madden, T.; Schäffer, A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D. Peripheral Olfactory Signaling in Insects. Curr. Opin. Insect Sci. 2014, 25, 3389–3402. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.; Wong, T.; von Haeseler, A.; Jermiin, L. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.; Chernomor, O.; von Haeseler, A.; Minh, B.; Vinh, L. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, E45. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Li, J.; Mei, X.; Liu, Y.; Huang, H. QPCRtools: An R Package for QPCR Data Processing and Visualization. Front. Genet. 2022, 13, 1002704. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.F.; Schneider, T.; Schwartz, A.M.; Andersson, M.; McKenna, D.D. The diversity and evolution of odorant receptors in beetles (Coleoptera). Insect Mol. Biol. 2019, 29, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; He, P.; Zhang, L.; Fang, S.Q.; Dong, S.L.; Zhang, Y.J.; Li, F. Large-Scale Identification of Odorant-Binding Proteins and Chemosensory Proteins from Expressed Sequence Tags in Insects. BMC Genom. 2009, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Vuts, J.; Imrei, Z.; Birkett, M.A.; Pickett, J.A.; Woodcock, C.M.; Tóth, M. Semiochemistry of the Scarabaeoidea. J. Chem. Ecol. 2014, 40, 190–210. [Google Scholar] [CrossRef]
- Krell, F. Fossil Record and Evolution of Scarabaeoidea (Coleoptera: Polyphaga). Coleopt. Bull. 2006, 60, 120–143. [Google Scholar] [CrossRef]
- Ahrens, D.; Schwarzer, J.; Vogler, A.P. The evolution of scarab beetles tracks the sequential rise of angiosperms and mammals. Proc. Royal Soc. B 2014, 281, 20141470. [Google Scholar] [CrossRef]
- McKenna, D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.; Donath, A.; Escalona, H.; Friedrich, F.; Letsch, H.; et al. The Evolution and Genomic Basis of Beetle Diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef]
- Jackson, T.; Klein, M. Scarabs as Pests: A Continuing Problem. Coleopt. Bull. 2006, 60, 102–119. [Google Scholar] [CrossRef]
- Tan, C.L.; Kasran, R.; Lee, W.W.; Leong, W.M. Transcriptome Analysis of Developmental Stages of Cocoa Pod Borer, Conopomorpha Cramerella: A Polyphagous Insect Pest of Economic Importance in Southeast Asia. bioRxiv 2021. [Google Scholar] [CrossRef]
- Tapia, T.; Perich, F.; Pardo, F.; Palma, G.M.; Quiróz, A. Identification of volatiles from differently aged red clover (Trifolium pratense) root extracts and behavioural responses of clover root borer (Hylastinus obscurus) (Marsham) (Coleoptera: Scolytidae) to them. Biochem. Syst. Ecol. 2007, 35, 61–67. [Google Scholar] [CrossRef]
- Palma, R.; Mutis, A.; Manosalva, L.; Ceballos, R.; Quiróz, A. Behavioral and electrophysiological responses of Hylastinus obscurus to volatiles released from the roots of Trifolium pratense L. J. Soil Sci. Plant Nutr. 2012, 12, 183–193. [Google Scholar] [CrossRef]
- Quiróz, A.; Méndez, L.; Mutis, A.; Hormazábal, E.; Ortega, F.; Birkett, M.A.; Parra, L. Antifeedant activity of red clover root isoflavonoids on Hylastinus obscurus. J. Soil Sci. Plant Nutr. 2017, 17, 231–239. [Google Scholar] [CrossRef]
- Kong, C.; Zhang, S.; Li, Y.; Xia, Z.; Yang, X.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun. 2018, 9, 3867. [Google Scholar] [CrossRef]
- González-González, A.; Rubio-Meléndez, M.E.; Ballesteros, G.I.; Ramírez, C.C.; Palma-Millanao, R. Sex- And Tissue-Specific Expression of Odorant-Binding Proteins and Chemosensory Proteins in Adults of the Scarab Beetle Hylamorpha elegans (Burmeister) (Coleoptera: Scarabaeidae). PeerJ 2019, 7, e7054. [Google Scholar] [CrossRef]
- Lizana, P.; Mutis, A.; Palma-Millanao, R.; González-González, A.; Ceballos, R.; Quiróz, A.; Bardehle, L.; Hidalgo, A.; Torres, F.; Romero-López, A.; et al. Comparative transcriptomic analysis of chemoreceptors in two sympatric scarab beetles, Hylamorpha elegans and Brachysternus prasinus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101174. [Google Scholar] [CrossRef]
- Bohbot, J.; Pitts, R.J.; Kwon, H.W.; Rützler, M.; Robertson, H.M.; Zwiebel, L.J. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol. Biol. 2007, 16, 525–537. [Google Scholar] [CrossRef]
- Cheng, D.; Lu, Y.; Ling, Z.; Liang, G.; Xiao, H. Si-CSP9 regula el tegumento y el proceso de muda de las larvas de la hormiga roja importada, Solenopsis invicta. Sci. Rep. 2015, 5, 9245. [Google Scholar] [CrossRef]
- Giglio, A.; Brandmayr, P.; Ferrero, E.; Giulianini, P.G.; Perrotta, E.; Talarico, F.; Brandmayr, T.Z. Ultrastructure of the antennal sensorial appendage of larvae of Ophonus ardosiacus (Lutshnik, 1922) (Coleoptera, carabidae) and possible correlations between size and shape and the larval feeding habits. Zool. Anz. 2008, 247, 209–221. [Google Scholar] [CrossRef]
- Benton, R.; Sachse, S.; Michnick, S.W.; Vosshall, L.B. Atypical membrane topology and heteromeric function of drosophila odorant receptors in vivo. PLoS ONE 2006, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.C.; Domingos, A.I.; Jones, W.D.; Chiappe, M.E.; Amrein, H.; Vosshall, L.B. Or83b Encodes a Broadly Expressed Odorant Receptor Essential for Drosophila Olfaction. Neuron 2004, 43, 703–714. [Google Scholar] [CrossRef]
- Jones, W.D.; Nguyen, T.; Kloss, B.; Lee, K.J.; Vosshall, L.B. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr. Biol. 2005, 15, R119–R121. [Google Scholar] [CrossRef]
- Missbach, C.; Dweck, H.K.M.; Vogel, H.; Vilcinskas, A.; Stensmyr, M.C.; Hansson, B.S.; Grosse-Wilde, E. Evolution of Insect Olfactory Receptors. eLife 2014, 3, e02115. [Google Scholar] [CrossRef] [PubMed]
- Soffan, A.; Subandiyah, S.; Makino, H.; Watanabe, T.; Horiike, T. Evolutionary Analysis of the Highly Conserved Insect Odorant Coreceptor (Orco) Revealed a Positive Selection Mode, Implying Functional Flexibility. J. Insect Sci. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, G.; Chen, H. Silencing of the olfactory co-receptor gene in Dendroctonus armandi leads to EAG response declining to major host volatiles. Sci. Rep. 2016, 6, 23136. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, C.; Peng, T.; Zhang, H. Odorant receptor co-receptor Orco is upregulated by methyl eugenol in male Bactrocera dorsalis (Diptera: Tephritidae). J. Insect Physiol. 2012, 58, 1122–1127. [Google Scholar] [CrossRef]
- Sun, H.; Bu, L.-A.; Su, S.-C.; Guo, D.; Gao, C.-F.; Wu, S.-F. Knockout of the odorant receptor co-receptor, orco, impairs feeding, mating and egg-laying behavior in the fall armyworm Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2023, 152, 103889. [Google Scholar] [CrossRef]
- Gonzalez, F.; Witzgall, P.; Walker, W.B. Antennal Transcriptomes of Three Tortricid Moths Reveal Putative Conserved Chemosensory Receptors for Social and Habitat Olfactory Cues. Sci. Rep. 2017, 7, 41829. [Google Scholar] [CrossRef]
- Xu, W.; Papanicolaou, A.; Zhang, H.-J.; Anderson, A. Expansion of a Bitter Taste Receptor Family in a Polyphagous Insect Herbivore. Sci. Rep. 2016, 6, 23666. [Google Scholar] [CrossRef]
- Thorne, N.; Chromey, C.; Bray, S.; Amrein, H. Taste Perception and Coding in Drosophila. Curr. Biol. 2004, 14, 1065–1079. [Google Scholar] [CrossRef]
- Jung, J.W.; Park, K.W.; Ahn, Y.J.; Kwon, H.W. Functional Characterization of Sugar Receptors in the Western Honeybee, Apis mellifera. J. Asia-Pac. Entomol. 2015, 18, 19–26. [Google Scholar] [CrossRef]
- Kumar, A.; Tauxe, G.M.; Perry, S.L.; Scott, C.A.; Dahanukar, A.; Ray, A. Contributions of the conserved insect carbon dioxide receptor subunits to odor detection. Cell Rep. 2020, 31, 107510. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, T.; Chen, Z.; Jiang, L.; Guo, Y.; Liu, J.; Li, S.; Taniai, K.; Asaoka, K.; Kadono-Okuda, K.; et al. Expression Map of a Complete Set of Gustatory Receptor Genes in Chemosensory Organs of Bombyx mori. Insect Biochem. Mol. Biol. 2017, 82, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.B.; Gonzalez, F.; Garczynski, S.F.; Witzgall, P. The chemosensory receptors of codling moth Cydia pomonella–expression in larvae and adults. Sci. Rep. 2016, 6, 23518. [Google Scholar] [CrossRef]
- Abuin, L.; Bargeton, B.; Ulbrich, M.H.; Isacoff, E.Y.; Kellenberger, S.; Benton, R. Functional Architecture of Olfactory Ionotropic Glutamate Receptors. Neuron 2011, 69, 44–60. [Google Scholar] [CrossRef]
- Li, K.; Wei, H.; Shu, C.; Zhang, S.; Cao, Y.; Luo, C.; Yin, J. Identification and Comparison of Candidate Odorant Receptor Genes in the Olfactory and Non-Olfactory Organs of Holotrichia oblita Faldermann by Transcriptome Analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Klein, M.; Svec, K.V.; Budelli, G.; Chang, E.C.; Ferrer, A.J.; Benton, R.; Samuel, A.D.T.; Garrity, P.A. The Ionotropic Receptors IR21a and IR25a Mediate Cool Sensing in Drosophila. eLife 2016, 5, e13254. [Google Scholar] [CrossRef]
- Maher, N.; Thiery, D.; Städler, E. Oviposition by Lobesia Botrana Is Stimulated by Sugars Detected by Contact Chemoreceptors. Physiol. Entomol. 2006, 31, 14–22. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; van Loon, J.J.A.; Wang, C.-Z. Tarsal Taste Neuron Activity and Proboscis Extension Reflex in Response to Sugars and Amino Acids in Helicoverpa armigera (Hübner). J. Exp. Biol. 2010, 213, 2889–2895. [Google Scholar] [CrossRef]
- Koh, T.W.; He, Z.; Gorur-Shandilya, S.; Menuz, K.; Larter, N.K.; Stewart, S.; Carlson, J.R. The Drosophila IR20a Clade of Ionotropic Receptors Are Candidate Taste and Pheromone Receptors. Neuron 2014, 83, 850–865. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Liu, C.; Liu, Y.; Mei, X.; Zhang, T. Identification and Expression of Candidate Chemosensory Receptors in the White-Spotted Flower Chafer, Protaetia brevitarsis. Sci. Rep. 2019, 9, 3339. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-H.; Sun, L.; Yang, R.-N.; Wu, K.-M.; Guo, Y.-Y.; Li, X.-C.; Zhou, J.-J.; Zhang, Y.-J. Molecular Characterization and Differential Expression of Olfactory Genes in the Antennae of the Black Cutworm Moth Agrotis ipsilon. PLoS ONE 2014, 9, e103420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ju, Q.; Jie, W.; Li, F.; Jiang, X.; Hu, J.; Qu, M. Chemosensory Gene Families in Adult Antennae of Anomala corpulenta Motschulsky (Coleoptera: Scarabaeidae: Rutelinae). PLoS ONE 2015, 10, e0121504. [Google Scholar] [CrossRef]
- Wu, Z.; Tong, N.; Li, Y.; Guo, J.; Lu, M.; Liu, X. Foreleg Transcriptomic Analysis of the Chemosensory Gene Families in Plagiodera versicolora (Coleoptera: Chrysomelidae). Insects 2022, 13, 763. [Google Scholar] [CrossRef]
- Sánchez-Gracia, A.; Vieira, F.G.; Rozas, J. Molecular Evolution of the Major Chemosensory Gene Families in Insects. Heredity 2009, 103, 208–216. [Google Scholar] [CrossRef]
- Li, F.; Zhao, X.; Li, M.; He, K.; Huang, C.; Zhou, Y.; Li, Z.; Walters, J.R. Insect Genomes: Progress and Challenges. Insect Mol. Biol. 2019, 28, 739–758. [Google Scholar] [CrossRef]
- Khuhro, S.A.; Yan, Q.; Liao, H.; Zhu, G.H.; Bin Sun, J.; Dong, S.L. Expression Profile and Functional Characterization Suggesting the Involvement of Three Chemosensory Proteins in Perception of Host Plant Volatiles in Chilo suppressalis (Lepidoptera: Pyralidae). J. Insect Sci. 2018, 18, 6. [Google Scholar] [CrossRef]
- Gong, L.; Luo, Q.; Rizwan-Ul-Haq, M.; Hu, M.Y. Cloning and Characterization of Three Chemosensory Proteins from Spodoptera exigua and Effects of Gene Silencing on Female Survival and Reproduction. Bull. Entomol. Res. 2012, 102, 600–609. [Google Scholar] [CrossRef]
- Sun, H.; Guan, L.; Feng, H.; Yin, J.; Cao, Y.; Xi, J.; Li, K. Functional Characterization of Chemosensory Proteins in the Scarab Beetle, Holotrichia oblita Faldermann (Coleoptera: Scarabaeida). PLoS ONE 2014, 9, e107059. [Google Scholar] [CrossRef]
- Yin, J.; Wang, C.; Fang, C.; Zhang, S.; Cao, Y.; Li, K.; Leal, W.S. Functional Characterization of Odorant-Binding Proteins from the Scarab Beetle Holotrichia oblita Based on Semiochemical-Induced Expression Alteration and Gene Silencing. Insect Biochem. Mol. Biol. 2019, 104, 11–19. [Google Scholar] [CrossRef]
- Jaworski, C.C.; Allan, C.W.; Matzkin, L.M. Chromosome-Level Hybrid de Novo Genome Assemblies as an Attainable Option for Nonmodel Insects. Mol. Ecol. Resour. 2020, 20, 1277–1293. [Google Scholar] [CrossRef]






| Metric | Data |
|---|---|
| Trinity transcripts | 48,112 |
| Trinity “genes” | 28,215 |
| GC (%) | 37.09 |
| Contig N50 (bp) | 2305 |
| Median contig length (bp) | 1061 |
| Average contig. (bp) | 1540 |
| Total assembled bases | 74,092,714 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizana, P.; Mutis, A.; Palma-Millanao, R.; Larama, G.; Antony, B.; Quiroz, A.; Venthur, H. Transcriptomic and Gene Expression Analysis of Chemosensory Genes from White Grubs of Hylamorpha elegans (Coleoptera: Scarabaeidae), a Subterranean Pest in South America. Insects 2024, 15, 660. https://doi.org/10.3390/insects15090660
Lizana P, Mutis A, Palma-Millanao R, Larama G, Antony B, Quiroz A, Venthur H. Transcriptomic and Gene Expression Analysis of Chemosensory Genes from White Grubs of Hylamorpha elegans (Coleoptera: Scarabaeidae), a Subterranean Pest in South America. Insects. 2024; 15(9):660. https://doi.org/10.3390/insects15090660
Chicago/Turabian StyleLizana, Paula, Ana Mutis, Rubén Palma-Millanao, Giovanni Larama, Binu Antony, Andrés Quiroz, and Herbert Venthur. 2024. "Transcriptomic and Gene Expression Analysis of Chemosensory Genes from White Grubs of Hylamorpha elegans (Coleoptera: Scarabaeidae), a Subterranean Pest in South America" Insects 15, no. 9: 660. https://doi.org/10.3390/insects15090660






