The Effects of High-Intensity, Short-Duration and Low-Intensity, Long-Duration Hamstrings Static Stretching on Contralateral Limb Performance
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Design
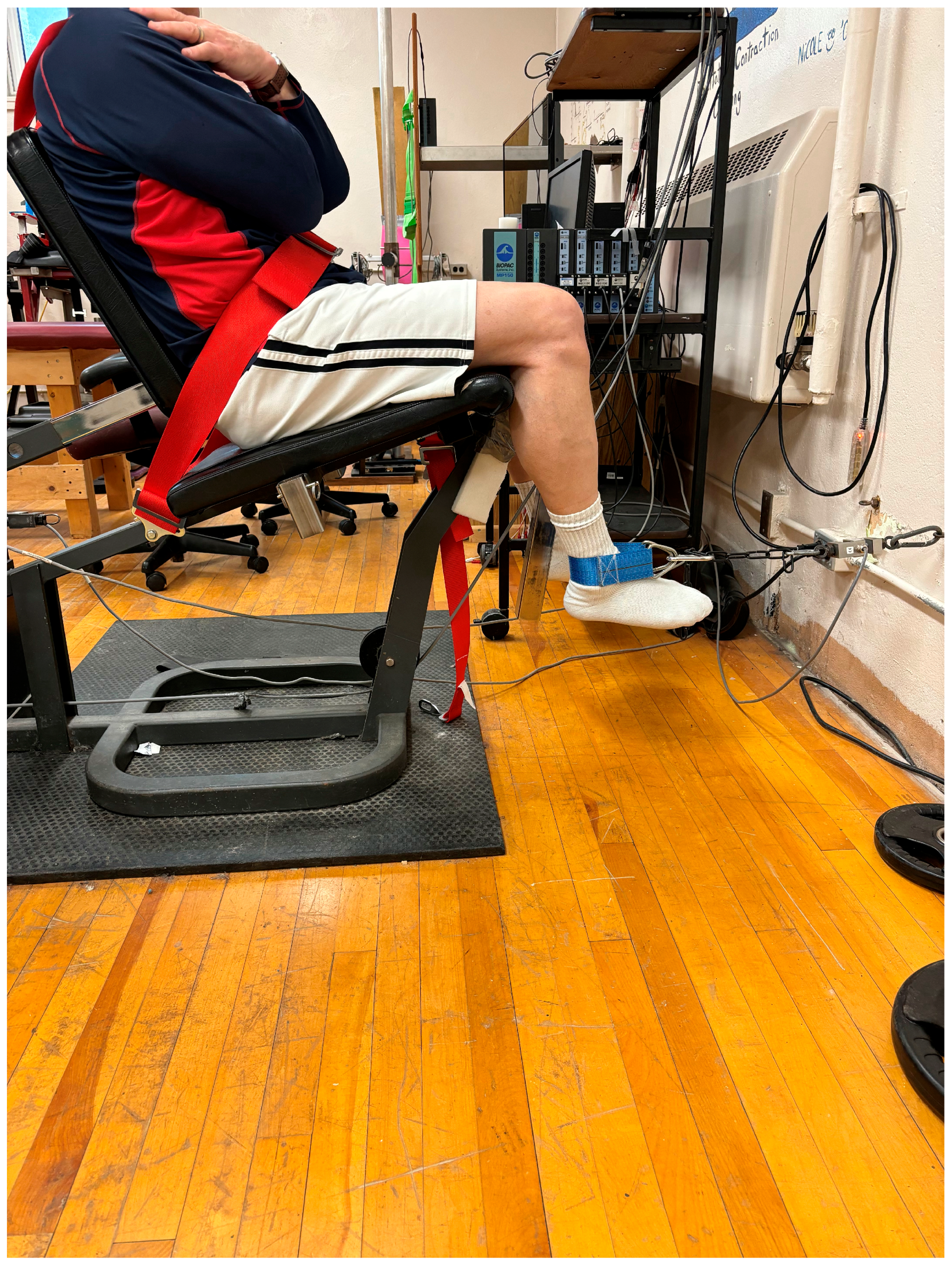
2.3. Pre- and Post-Test Measures
2.3.1. Hamstrings Range of Motion (ROM)
2.3.2. Knee Flexion Maximal Voluntary Isometric Contraction (MVIC) Force
2.3.3. Hamstrings Activation (Electromyography: EMG)
2.3.4. Unilateral Countermovement Jump (CMJ) and Drop Jump (DJ) Height
2.4. Control Session
2.5. Statistical Analysis
3. Results
3.1. Range of Motion
3.2. MVIC Force and Instantaneous Strength
3.3. Unilateral Countermovement (CMJ) and Drop Jump (DJ) Height
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alter, M.J. Science of Flexibility; Human Kinetics Publishers: Champaign, IL, USA, 1996; pp. 12–82. [Google Scholar]
- Magnusson, S.P.; Renstrom, P. The European College of Sports Sciences Position statement: The role of stretching exercises in sports. Eur. J. Sport Sci. 2006, 6, 87–91. [Google Scholar] [CrossRef]
- Nakamura, M.; Sato, S.; Murakami, Y.; Kiyono, R.; Yahata, K.; Sanuki, F.; Yoshida, R.; Fukaya, T.; Takeuchi, K. The Comparison of Different Stretching Intensities on the Range of Motion and Muscle Stiffness of the Quadriceps Muscles. Front. Physiol. 2021, 11, 628870. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Chaouachi, A. A review of the acute effects of static and dynamic stretching on performance. Eur. J. Appl. Physiol. 2011, 111, 2633–2651. [Google Scholar] [CrossRef]
- Behm, D.G.; Blazevich, A.J.; Kay, A.D.; McHugh, M. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: A systematic review. Appl. Physiol. Nutr. Metabol. 2016, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Kay, A.D.; Trajano, G.S.; Blazevich, A.J. Mechanisms underlying performance impairments following prolonged static stretching without a comprehensive warm-up. Eur. J. Appl. Physiol. 2021, 121, 67–94. [Google Scholar] [CrossRef]
- Kay, A.D.; Blazevich, A.J. Effect of Acute Static Stretch on Maximal Muscle Performance. Med. Sci. Sport Exerc. 2012, 44, 154–164. [Google Scholar] [CrossRef]
- Kay, A.D.; Blazevich, A.J. Reductions in active plantar flexors moment are significantly correlated with static stretch duration. Eur. J. Sport Sci. 2008, 8, 41–46. [Google Scholar] [CrossRef]
- Reid, C.J.; Greene, R.; Young, D.J.; Hodgson, D.D.; Blazevich, J.A.; Behm, D.G. The effects of different durations of static stretching within a comprehensive warm-up on voluntary and evoked contractile properties. Eur. J. Appl. Physiol. 2018, 118, 1427–1445. [Google Scholar] [CrossRef]
- Apostolopoulos, N.C.; Lahart, I.M.; Plyley, M.J.; Taunton, J.; Nevill, A.M.; Koutedakis, Y.; Wyon, M.; Metsios, G.S. The effects of different passive static stretching intensities on recovery from unaccustomed eccentric exercise—A randomized controlled trial. Appl. Physiol. Nutr. Metabol. 2018, 43, 806–815. [Google Scholar] [CrossRef]
- Bryant, J.C.; Peters, D.M.; Cook, M.D. The Effects of Static Stretching Intensity on Range of Motion and Strength: A Systematic Review. J. Funct. Morphol. Kinesiol. 2023, 8, 37. [Google Scholar] [CrossRef]
- Kataura, S.; Suzuki, S.; Matsuo, S.; Hatano, G.; Iwata, M.; Yokoi, K.; Tsuchida, W.; Banno, Y.; Asai, Y. Acute effects of the different intensity of static stretching on flexibility and isometric muscle force. J. Strength Cond. Res. 2017, 31, 3403–3410. [Google Scholar] [CrossRef]
- Arntz, F.; Markov, A.; Behm, D.G.; Behrens, M.; Negra, Y.; Nakamura, M.; Moran, J.; Chaabene, H. Chronic Effects of Static Stretching Exercises on Muscle Strength and Power in Healthy Individuals Across the Lifespan: A Systematic Review with Multi-level Meta-analysis. Sports Med. 2023, 53, 723–745. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Alizadeh, S.; Daneshjoo, A.; Anvar, H.S.; Graham, A.; Zahiri, A.; Goudini, R.; Edwards, C.; Scharf, C.; Behm, G.D. Chronic effects of stretching on range of motion with consideration of potential moderating variables: A systematic review with meta-analysis. J. Sport Health Sci. 2023, 13, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, E.Q.; Quirante, L.B.; Blanco, C.R.; Sendin, F.A. Immediate effects of the suboccipital muscle inhibition technique in subjects with short hamstring syndrome. J. Manip. Physiol. Ther. 2009, 32, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Hadjizadeh Anvar, H.S.; Granacher, U.; Konrad, A.; Alizadeh, S.; Culleton, R.; Edwards, C.; Goudini, R.; Behm, D.G. Corticospinal excitability and reflex modulation in a contralateral non-stretched muscle following unilateral stretching. Eur. J. Appl. Physiol. 2023, 123, 1837–1850. [Google Scholar] [CrossRef]
- Caldwell, S.; Bilodeau, R.; Cox, M.; Peddle, D.; Cavanaugh, T.; Young, J.; Behm, D.G. Unilateral Hamstrings Static Stretching Can Impair the Affected and Contralateral Knee Extension Force but Improve Unilateral Drop Jump Height. Eur. J. Appl. Physiol. 2019, 119, 1943–1949. [Google Scholar] [CrossRef]
- Behm, D.G.; Lau, J.R.; O’Leary, J.J.; Rayner, C.P.M.; Burton, A.E.; Lavers, L. Acute Effects of Unilateral Self-Administered Static Stretching on Contralateral Limb Performance. J. Perform. Health Res. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Chaouachi, A.; Padulo, J.; Kasmi, S.; Othmen, B.A.; Chatra, M.; Behm, G.D. Unilateral static and dynamic hamstrings stretching increases contralateral hip flexion range of motion. Clin. Physiol. Funct. Imaging 2017, 37, 23–29. [Google Scholar] [CrossRef]
- Ficarra, S.; Scardina, A.; Nakamura, M.; Patti, A.; Sahin, F.N.; Palma, A.; Bellafiore, M.; Bianco, A.; Thomas, E. Acute effects of static stretching and proprioceptive neuromuscular facilitation on non-local range of movement. Res. Sports Med. 2024, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, H.P.; Reis, G.R.; Gomes, A.W.; da Silva, J.J.; Soares, G.E.; de Freitas, S.F.; Behm, D.G. Static stretching of the pectoralis major decreases triceps brachii activation during maximal isometric bench press. Gaz. Medica Ital. Arch. Sci. Med. 2017, 176, 659–664. [Google Scholar] [CrossRef]
- Behm, D.G.; Alizadeh, S.; Anvar, S.; Drury, B.; Granacher, U.; Moran, J. Non-local acute passive stretching effects on range of motion in healthy adults: A systematic review with meta-analysis. Sports Med. 2021, 51, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Cavanaugh, T.; Quigley, P.J.; Reid, J.C. Acute bouts of upper and lower body static and dynamic stretching increase non-local joint range of motion. Eur. J. Appl. Physiol. 2016, 116, 241–249. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Simonsen, B.E.; Aagaard, P.; Sørensen, H.; Kjær, M. A mechanism for altered flexibility in human skeletal muscle. J. Physiol. 1996, 497, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.P.; Simonsen, E.B.; Aagaard, P.; Boesen, J.; Johannsen, F.; Kjaer, M. Determinants of musculoskeletal flexibility: Viscoelastic properties, cross-sectional area, EMG and stretch tolerance. Scand. J. Med. Sci. Sport 1997, 7, 195–202. [Google Scholar] [CrossRef]
- Stove, M.P.; Hirata, R.P.; Palsson, T.S. Muscle stretching—The potential role of endogenous pain inhibitory modulation on stretch tolerance. Scand. J. Pain 2019, 19, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Niederer, D.; Vogt, L.; Banzer, W. Remote effects of lower limb stretching: Preliminary evidence for myofascial connectivity? J. Sports Sci. 2016, 34, 2145–2148. [Google Scholar] [CrossRef]
- Ayala, F.; De Ste Croix, M.; Sainz De Baranda, P.; Santonja, F. Acute effects of static and dynamic stretching on hamstring eccentric isokinetic strength and unilateral hamstring to quadriceps strength ratios. J. Sports Sci. 2013, 31, 831–839. [Google Scholar] [CrossRef]
- Behm, D.G.; Kay, A.D.; Trajano, G.S.; Alizadeh, S.; Blazevich, A. Effects of Acute and Chronic Stretching on Pain Control. J. Clin. Exerc. Physiol. 2021, 10, 150–159. [Google Scholar] [CrossRef]
- Da Silva, J.; Behm, D.G.; Gomes, W.A.; Siva, F.H.; Soares, E.G.; Serpa, E.P.; Vieila, G.B.; Lopes, C.R.; Marchetti, P.H. Unilateral Plantar Flexors Static-Stretching Effects on Ipsilateral and Contralateral Jump Measures. J. Sports Sci. Med. 2015, 14, 315–321. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Lawrence Erlbaum Associates, Inc.: New York, NY, USA, 2013; pp. 34–96. [Google Scholar]
- Grabow, L.; Young, J.D.; Byrne, J.M.; Granacher, U.; Behm, D.G. Unilateral Rolling of the Foot Did Not Affect Non-local Range of Motion or Balance. J. Sports Sci. Med. 2017, 16, 209–218. [Google Scholar]
- Manieu, J.; Reiner, M.; Fischer, J.; Schopflin, A.; Tilp, M.; Behm, D.G.; Konrad, A. Seven weeks of pectoralis muscle stretching does not induce non-local effects in dorsiflexion ankle range of motion. Eur. J. Sport Sci. 2024, 24, 259–265. [Google Scholar]
- Konrad, A.; Reiner, M.; Manieu, J.; Fischer, L.; Schopflin, A.; Tilp, M.; Behm, D.G. The non-local effects of seven-week foot sole static stretching and foam rolling training on shoulder extension range of motion. Front. Sports Act. Living 2024, 5, 1335872. [Google Scholar] [CrossRef]
- Chaabene, H.; Behm, D.G.; Negra, Y.; Granacher, U. Acute effects of static stretching on muscle strength and power: An attempt to clarify previous caveats. Front. Physiol. Exerc. Physiol. 2019, 10, 1468. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G. The Science and Physiology of Flexibility and Stretching; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Konrad, A.; Tilp, M. The Time Course of Muscle-Tendon Unit Function and Structure Following Three Minutes of Static Stretching. J. Sports Sci. Med. 2020, 19, 52–58. [Google Scholar]
- Konrad, A.; Tilp, M. The Acute Time Course of Muscle and Tendon Tissue Changes Following One Minute of Static Stretching. Curr. Issues Sport Sci. 2020, 5, 3–6. [Google Scholar] [CrossRef]
- Weppler, H.C.; Magnusson, P.S. Increasing Muscle Extensibility: A Matter of Increasing Length or Modifying Sensation? Phys. Ther. Rehab. J. 2010, 3, 438–449. [Google Scholar] [CrossRef]
- Freitas, R.S.; Mendes, B.; Le Sant, G.; Andrade, J.R.; Nordez, A.; Milanovic, Z. Can chronic stretching change the muscle-tendon mechanical properties? A review. Scand. J. Med. Sci. Sports 2018, 28, 794–806. [Google Scholar] [CrossRef]
- Le Bars, D.; Villanueva, L.; Bouhassira, D.; Willer, J.C. Diffuse noxious inhibitory controls (DNIC) in animals and in man. Patol. Fiziol. Eksp. Ter. 1992, 4, 55–65. [Google Scholar] [CrossRef]
- Pud, D.; Granovsky, Y.; Yarnitsky, D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 2009, 144, 16–19. [Google Scholar] [CrossRef]
- Takeuchi, K.; Sato, S.; Kiyono, R.; Yahata, K.; Murakami, Y.; Sanuki, F.; Yoshida, R.; Nakamura, M. High-Intensity Static Stretching in Quadriceps Is Affected More by Its Intensity Than Its Duration. Front. Physiol. 2021, 12, 709655. [Google Scholar] [CrossRef]
- Takeuchi, K.; Akizuki, K.; Nakamura, M. The acute effects of high-intensity jack-knife stretching on the flexibility of the hamstrings. Sci. Rep. 2021, 11, 12115. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Alizadeh, S.; Daneshjoo, A.; Hadjizadeh Anvar, S.; Graham, A.; Zahiri, A.; Goudini, R.; Edwards, C.; Culleton, R.; Scharf, C.; et al. Acute effects of various stretching techniques on range of motion: A systematic review with meta-analysis. Sports Med. 2023, 9, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Blazevich, A.J.; Gill, N.D.; Kvorning, T.; Kay, A.D.; Goh, A.; Hilton, B.; Drinkwater, E.J.; Behm, D.G. Static and dynamic stretching within a comprehensive dynamic warm-up do not differentially affect flexibility or athletic performance: Randomized, controlled cross-over trial. Med. Sci. Sports Exerc. 2018, 50, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Trajano, G.S.; Blazevich, A.J. Static Stretching Reduces Motoneuron Excitability: The Potential Role of Neuromodulation. Exerc. Sport Sci. Rev. 2021, 49, 126–132. [Google Scholar] [CrossRef]
- Trajano, G.S.; Nosaka, K.; Blazevich, A.J. Neurophysiological Mechanisms Underpinning Stretch-Induced Force Loss. Sports Med. 2017, 47, 1531–1541. [Google Scholar] [CrossRef]
- Trajano, G.S.; Seitz, L.B.; Nosaka, K.; Blazevich, A.J. Can passive stretch inhibit motoneuron facilitation in the human plantar flexors? J. Appl. Physiol. 2014, 117, 1486–1492. [Google Scholar] [CrossRef]
- Trajano, G.S.; Taylor, J.L.; Orssatto, L.B.R.; McNulty, C.R.; Blazevich, A.J. Passive muscle stretching reduces estimates of persistent inward current strength in soleus motor units. J. Exper. Biol. 2020, 223 Pt 21, jeb229922. [Google Scholar] [CrossRef]
- Takeuchi, K.; Nakamura, M.; Fukaya, T.; Konrad, A.; Mizuno, T. Acute and Long-Term Effects of Static Stretching on Muscle-Tendon Unit Stiffness: A Systematic Review and Meta-Analysis. J. Sports Sci. Med. 2023, 22, 465–475. [Google Scholar] [CrossRef]
- Halperin, I.; Chapman, D.W.; Behm, D.G. Non-local muscle fatigue: Effects and possible mechanisms. Eur. J. Appl. Physiol. 2015, 115, 2031–2048. [Google Scholar] [CrossRef]
- Holgado, D.; Troya, E.; Perales, J.C.; Vadillo, M.A.; Sanabria, D. Does mental fatigue impair physical performance? A replication study. Eur. J. Sport Sci. 2020, 21, 762–770. [Google Scholar] [CrossRef]
- Marcora, S.M.; Staiano, W.; Manning, V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 2009, 106, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The Effects of Mental Fatigue on Physical Performance: A Systematic Review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef] [PubMed]
- Hakim, H.; Khemiri, A.; Chortane, O.G.; Boukari, S.; Chortane, S.G.; Bianco, A.; Marsigliante, S.; Patti, A.; Muscella, A. Mental Fatigue Effects on the Produced Perception of Effort and Its Impact on Subsequent Physical Performances. Int. J. Environ. Res. Public Health 2022, 19, 973. [Google Scholar] [CrossRef]
- Steele, J. What is (perception of) effort? Objective and subjective effort during task performance. PsyArχiv. 2020. [Google Scholar] [CrossRef]
- Aura, O.; Komi, P.V. The mechanical efficiency of locomotion in men and women with special emphasis on stretch-shortening cycle exercises. J. Appl. Physiol. 1986, 55, 37–43. [Google Scholar] [CrossRef]
- Bosco, C.; Tarkka, I.; Komi, P.V. Effect of elastic energy and myoelectrical potentiation of triceps surae during stretch-shortening cycle exercise. Int. J. Sports Med. 1982, 3, 137–140. [Google Scholar] [CrossRef]
- McCarthy, J.P.; Wood, D.S.; Bolding, M.S.; Roy, J.L.; Hunter, G.R. Potentiation of concentric force and acceleration only occurs early during the stretch-shortening cycle. J. Strength Cond. Res. 2012, 26, 2345–2355. [Google Scholar] [CrossRef]
- Chaouachi, A.; Castagna, C.; Chtara, M.; Brughelli, M.; Turki, O.; Galy, O.; Chamari, K.; Behm, D.G. Effect of warm-ups involving static or dynamic stretching on agility, sprinting, jumping performance in trained individuals. J. Strength Cond. Res. 2010, 24, 2001–2011. [Google Scholar] [CrossRef]
- Godges, J.J.; MacRae, H.; Longdon, C.; Tinberg, C. The effects of two stretching procedures on the economy of walking and jogging. J. Orthop. Sport Phys. Ther. 1989, 7, 350–357. [Google Scholar] [CrossRef]
- Wilson, G.J.; Elliott, B.C.; Wood, G.A. Stretching shorten cycle performance enhancement through flexibility training. Med. Sci. Sports Exerc. 1992, 24, 116–123. [Google Scholar]
- Konrad, A.; Budini, F.; Tilp, M. Acute Effects of Constant Torque and Constant Angle Stretching on the Muscle Tendon Tissue Properties. Eur. J. Appl. Physiol. 2017, 117, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ikezoe, T.; Ichihashi, N. Time Course of Changes in Passive Properties of the Gastrocnemius Muscle Tendon Unit During 5 Min of Static Stretching. Man. Ther. 2013, 18, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Henry, F.M. “Best” versus “Average” Individual Scores. Res. Q. 1967, 38, 317–320. [Google Scholar] [CrossRef]
- Whitley, J.D.; Smith, L.E. Larger correlations obtained by using average rather than “best” strength scores. Res. Q. 1963, 34, 248–249. [Google Scholar] [CrossRef]
| Stretched Leg | ||||||
|---|---|---|---|---|---|---|
| HISD Pre | HISD Post | LILD Pre | LILD Post | Control Pre | Control Post | |
| ROM (°) | 106.0± 9.0# p = 0.008 | 110.6 ± 12.6# p = 0.008 | 109.3 ± 16.2 + p = 0.06 | 112.2 ± 16.5 + p = 0.06 | 107.2 ± 11.9 | 107.8 ± 11.8 |
| MVIC (N) | 350.6 ± 99.1 | 360.1 ± 101.6 | 349.0 ± 101.6 | 336.3 ± 114.9 | 354.6 ± 100.7 | 347.4 ± 108.7 |
| MVIC EMG (mV) | 0.083 ± 0.038 | 0.086 ± 0.039 | 0.079 ± 0.038 | 0.081 ± 0.039 | 0.086 ± 0.041 | 0.081 ± 0.037 |
| IS (N) | 126.1 ± 77.4 | 106.8 ± 75.4 | 116.5 ± 77.6 | 111.8 ± 72.5 | 119.2 ± 74.8 | 118.1 ± 83.5 |
| IS EMG (mV) | 0.059 ± 0.038 | 0.055 ± 0.033 | 0.059 ± 0.028 | 0.057 ± 0.038 | 0.064 ± 0.030 | 0.063 ± 0.037 |
| CMJ (cm) | 11.2 ± 5.2 | 11.1 ± 4.9 | 10.6 ± 3.7 | 10.6 ± 4.3 | 10.7 ± 4.4 | 10.6 ± 5.1 |
| DJ (cm) | 11.0 ± 4.4 | 10.7 ± 4.9 | 10.3 ± 4.4 | 10.5 ± 3.7 * p < 0.05 | 11.9 ± 4.2 | 11.1 ± 4.2 |
| Contralateral Non-Stretched Leg | ||||||
| HISD Pre | HISD Post | LILD Pre | LILD Post | Control Pre | Control Post | |
| ROM (°) | 104.2 ± 11.1 | 105.3 ± 14.2 | 108.5 ± 12.7 | 109.1 ± 15.3 | 105.8 ± 12.7 | 107.2 ± 13.3 |
| MVIC (N) | 344.0 ± 107.0 | 344.8 ± 96.5 | 336.7 ± 101.1 | 320.2 ± 103.2 | 343.2 ± 99.6 | 330.8 ± 94.7 |
| MVIC EMG (mV) | 0.076 ± 0.030 | 0.074 ± 0.028 | 0.069 ± 0.027 | 0.066 ± 0.028 | 0.073 ± 0.023 | 0.072 ± 0.029 |
| IS | 104.0 ± 83.6 | 112.9 ± 75.3 | 103.9 ± 74.9 | 113.1 ± 82.8 | 113.1 ± 82.8 | 108.6 ± 85.1 |
| IS EMG (mV) | 0.061 ± 0.059 | 0.051 ± 0.029 | 0.045 ± 0.025 | 0.045 ± 0.028 | 0.052 ± 0.032 | 0.049 ± 0.030 |
| CMJ (cm) | 9.9 ± 4.6 | 10.2 ± 5.1 | 11.1 ± 4.3 | 9.7 ± 3.9 | 10.0 ± 4.6 | 9.0 ± 4.1 |
| DJ (cm) | 9.4 ± 3.8 | 9.3 ± 3.9 | 10.8 ± 4.8 | 9.1 ± 3.8 * p < 0.05 | 9.4 ± 4.1 | 10.2 ± 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philpott, E.J.; Bahrami, M.; Sardroodian, M.; Behm, D.G. The Effects of High-Intensity, Short-Duration and Low-Intensity, Long-Duration Hamstrings Static Stretching on Contralateral Limb Performance. Sports 2024, 12, 257. https://doi.org/10.3390/sports12090257
Philpott EJ, Bahrami M, Sardroodian M, Behm DG. The Effects of High-Intensity, Short-Duration and Low-Intensity, Long-Duration Hamstrings Static Stretching on Contralateral Limb Performance. Sports. 2024; 12(9):257. https://doi.org/10.3390/sports12090257
Chicago/Turabian StylePhilpott, Emily J., Mohammadmahdi Bahrami, Mahta Sardroodian, and David G. Behm. 2024. "The Effects of High-Intensity, Short-Duration and Low-Intensity, Long-Duration Hamstrings Static Stretching on Contralateral Limb Performance" Sports 12, no. 9: 257. https://doi.org/10.3390/sports12090257






