1. Introduction
Traditional metal additive manufacturing techniques, such as selective laser sintering, electron beam melting, and laser engineered net shaping, notably depend on powder-centric variables such as the particle size distribution, morphology, and flowability of powders used in said processes. As reported previously, the powders’ sphericity and morphology—which captures two powder-centric variables for traditional powder-based metal additive manufacturing methods—will affect the powder’s flow through the powder feeder in such systems [
1]. These variables matter in laser-engineered net shaping because a specific size range of powder, 36–150 μm in diameter, must be continuously injected onto the powder bed in a controlled fashion as the laser passes over it [
2]. In contrast, the size distribution and morphology matter to a lesser degree (although they remain significant and non-negligible, as discussed by Valente et al. in [
3] and pointed out by Hussain et al. in [
4]) for the cold spray additive manufacturing (CSAM) process. Nevertheless, in CSAM processing, small particles may not bond to the respective target substrate under a given set of processing conditions for one or both of the following reasons: (i) fine particles can suffer from an inability to sufficiently plastically deform (due to increased yield strengths being associated with smaller atomized particles, as they experience higher solidification rates) and therefore bond with the substrate; and (ii) from the bow shock effect [
5,
6].
One should note that successful attempts at utilizing sub-10 μm diameter feedstock particles have been reported in the literature [
7]. When particles are too large, they will not reach the critical impact velocity due to their relatively increased mass on a per-particle basis [
8]. However, for those particles belonging to the appropriate range of feedstock powder particulate sizes, one of the essential powders for CSAM is the microstructure (including microstructural crystallinity and intermetallic constituents and phases). The microstructure matters so much for CSAM because CSAM is an entirely solid-state process; therefore, the initial properties and phases within the microstructure of an alloyed metallic powder greatly influence the final properties of the CSAM consolidated material. Most of the literature suggests that the phases present in the powder remain in the CSAM material.
To date, gas-atomized aluminum powder undergoes thermal pre-processing to degas the powder prior to consumption during manufacturing or modify the as-atomized internal microstructures. While powder degassing remains a common industrial practice prior to introducing the feedstock into powder-based manufacturing systems of relevance [
9,
10], thermal pre-processing for microstructural modification of gas-atomized powder has historically been of limited interest to the materials processing sector because conventional powder metallurgy methods remain, at least in large part, dependent on complete or partial melting; thus, generally nullifying the need for understanding the as-atomized microstructure, since particulate melting remains intrinsic to the respective methods themselves [
11]. That said, thermal processing of heat-treatable alloyed aluminum systems, including Al 6061, remains sensitive to the solutionizing temperature, heating rate, quench rate, aging temperature (if precipitation hardening is sought after), and hold time. At the same time, wrought- or bulk-inspired heat-treating parameters are non-transferrable to rapidly solidified, highly non-equilibrium, and gas-atomized alloyed aluminum particulates [
12]. Such non-direct transference follows from the fact that the size of the particles and the resultant solidification structure enable much more rapid atomic diffusion along sub-granular and granular boundaries, as detailed in [
13]. As a result, one must formulate and identify appropriate thermal pre-processing parameters and their significant influences on the internal microstructures of gas-atomized Al 6061 to properly understand and approach the implementation of thermal pre-processing of Al 6061 feedstock for optimized and tunable CSAM processing and consolidated material performance. In turn, such matters were considered herein.
The aim of heat treating particulate CSAM feedstock significantly deviates from those traditionally affiliated with classical thermal or thermomechanical processing. More specifically, thermal pre-processing of CSAM feedstock stems from the desire to tune the resultant consolidated components’ performance and enhance the particulate deformability and, therefore, suitability for CSAM [
14]. Increasing the deformability of the feedstock microparticles lowers the critical impact velocity required for particle–substrate bonding. At the same time, one may consider that the previous work has highlighted that powder particles of variable diameters for a single batch of bulk powder may very well maintain microstructural intermetallic constituents that are notably variable as a function of microparticle diameter [
15]. Hence, heat-treating or thermally pre-processing feedstock for CSAM can also enable greater quality assurance and normalization of the internal phase compositions, concentrations, and the like, across the range of particle sizes able to be processed via CSAM, especially when compared with the aforenoted variability of the as-atomized condition.
When applying heat treatments, two main microstructural effects are those related to grain size and the phases present. Firstly, holding an alloy at elevated temperatures for a considerable time will cause grain coarsening. Grain growth is an important consideration when heat treating any metal because of the Hall–Petch relationship, where the strength of the alloy is inversely proportional to the square root of the grain size. The second consideration when heat treating aluminum alloys centers upon the phase transformations at elevated temperatures. Two strengthening mechanisms in aluminum alloys are solid solution strengthening and precipitation hardening. During a solutionization heat treatment step, alloying elements will partially or wholly dissolve into the aluminum matrix, causing a supersaturated solid solution (SSS) to form. This causes local distortion in the aluminum lattice and will thus increase the material’s strength by obstructing dislocation motion. An appropriate solutionization temperature is 5 °C below the solidus temperature. The alloy is then quenched to room temperature to preserve the high-temperature composition of the SSS [
16]. Unfortunately, it is difficult to simply apply heat treatment schedules from literature to the powder for cold spray, as it has been shown that powders will not respond to heat treatments in the same fashion as rods, sheets, plates, for example [
12,
17]. Therefore, powders have no universally accepted and specific heat treatment schedule to date.
It has not yet been determined which thermally processed condition and processing parameters are the most suitable for gas-atomized Al 6061 feedstock-based CSAM. Accordingly, the present work presents experimentally observed thermal pre-processing effects that will enable continued research and development throughout the CSAM community in identifying a thermal processing window for alloyed Al 6061 powders. Furthermore, the present work focused on homogenizing or solutionization of the gas-atomized Al 6061 powder microstructures, as there has been an initial success in cold spraying the powder in this condition. An as-atomized powder can be considered a cast structure where the rapid cooling or solidification rate causes intermetallic secondary phases to nucleate along inter-dendritic regions or grain boundaries. However, a homogenous solid solution is a more desirable microstructure for many applications. Sheppard and Raghunathan conducted several different homogenization treatments on Al 5056 samples to study the effects on the microstructure [
18]. The homogenization treatment aims to put the segregated solute atoms into the solution. It is hypothesized that homogenization times for the powder will be less than what is seen in the literature because the grain sizes are much smaller than the cast materials studied. Therefore, there is less distance for the atoms to diffuse.
As discussed in the aforementioned introductory remarks, the microstructural and mechanical properties of non-equilibrium, rapidly solidified, and gas-atomized aluminum alloys are of interest to the CSAM materials consolidation research and engineering communities [
19]. As previously mentioned, such interest arises from the fact that CSAM processing is microstructurally retentive (although refined), and the mechanical properties of particulate feedstock directly affect the critical parameter known as the critical impact velocity required to achieve mechanical interlocking as well as intimate metallurgical bonding between the powder particles and a target substrate material together with the particle–particle bonding too [
20]. Given the well-established fact that the mechanical properties of a metallic material are directly dependent upon the metal’s microstructure and processing history [
21], one can pre-process polycrystalline, rapidly solidified, CSAM feedstock powders prior to use in the CSAM process to finely-tune and control the resultant consolidated materials performance, behavior, and properties [
22].
Rapidly solidified, gas-atomized alloyed aluminum (and other matrix materials) powders can be preprocessed before consumption during the CSAM materials consolidation process. Preprocessing methods include milling [
23], vacuum degassing [
9], thermally assisted degassing [
24], thermal heat treating [
25], plasma spheroidized [
26], granulation [
27], spray drying [
28], plating [
29], and more [
30]. Degassing, milling, and thermal heat-treating capture most preprocessing methods that have been researched and developed in the CSAM literature. However, degassing, milling, and thermal heat treatments are not universally applicable across gas-atomized aluminum alloys. For example, degassing preprocessing parameters must be optimized according to a trial-and-error approach, even when two powders are of the same nominal composition [
31]. At the same time, thermal preprocessing requires knowledge of the powders’ composition and classification to target desired secondary phase formation and deleterious phase dissolution or achieve a homogenized and solutionized state [
32].
Growing interest exists surrounding the industrial implementation of thermal pre-processing of CSAM particulate feedstock materials [
33]. Therefore, this work considers how experimental characterization, coupled with a previously developed through-process framework for aluminum alloy CSAM [
34], can be invoked to control CSAM particulate feedstock mechanical properties and microstructures for improved CSAM consolidations performance in service and optimal processing parameter selection. Beyond influencing the critical velocity required for bonding, among other noteworthy CSAM parameters, thermal pre-processing of CSAM alloyed aluminum feedstock systems can be utilized to address CSAM component ductility and toughness without further post-processing. Thus, one way to improve ductility is to thermally pre-process powder before CSAM deposition. This is carried out for two reasons: hydroxides are removed from the surface of the powder, which is a typical degassing step for powder processing, and secondly, the microstructure is modified from the as-atomized condition to a more “sprayable” condition.
2. Materials and Methods
The gas-atomized Al 6061 powder utilized during this research was procured from F. J. Bromann & Co. (Harvey, LA, USA). By direct current plasma emission spectroscopy-based analysis, performed per ASTM E 1097-12 and outsourced to Luvak Laboratories, Inc. (Boylston, MA, USA), the chemical composition of the gas-atomized Al 6061 powder was unveiled. More specifically, the measured chemical composition of the gas-atomized Al 6061 CSAM feedstock was as follows: 0.11 wt.% Cr, 0.26 wt.% Cu, 0.28 wt.% Fe, 0.922 wt.% Mg, 0.078 wt.% Mn, 0.024 wt.% Ti, 0.02 wt.% Zn, 0.591 wt.% Si, and 97.7 wt.% Al. Said chemical composition falls within the standard Al 6061 compositional tolerances and ranges specified for each alloying element [
16].
Rapidly solidified, gas-atomized aluminum alloy powder particle properties have previously been shown to depend on particle diameters [
35]. Accordingly, the powder was sieved before characterization was performed. Concerning the powder sieving, fine particles of less than 20 μm in diameter were removed by introducing a stream of nitrogen gas through a modified fluidized bed wherein the fine particles were ejected from the bulk batch of powder and the fluidized bed’s gas stream. The removal of said fine particulates ensured that the sieve meshes employed would not clog and also ensured that amorphous powder particles were removed from the analysis in light of prior reports that made mention of amorphous particulates forming from the very rapid cooling rates associated with sub-10 μm sized microparticles manufactured via gas atomization [
36]. Using a mechanical sieve, which was equipped with interwoven stainless-steel wire-based meshes, resulted in sieve size categories ranging from 25–32 μm, 32–38 μm, 38–45 μm, 45–53 μm, and 53–63 μm in particulate diameter. Particle size distributions of the sieved gas-atomized Al 6061 powder—that is, particle size distributions for each of the size categories—were determined by way of laser diffraction-based analysis at the United Technologies Research Center (now part of Raytheon Technologies Corporation and located in East Hartford, CT, USA), as shown in
Figure 1.
For studying the heat-treated powders, small samples of powder (approximately 30 mg) were thermally processed in a TA Instruments, Inc., (part of Waters Corp. and located in New Castle, DE, USA) Q20 differential scanning calorimeter (DSC). As needed, heat treatment times, temperatures, and heating rates are stated hereafter for each respective sample in the Results and Discussion sections. Still, in DSC analysis, the DSC data were collected with a heating rate of 5 °C min−1 and scanned from 20–530 °C. The DSC testing atmosphere comprised nitrogen with a flow rate of 50 mL min−1. A Tzero Al pan and lid were used, with an empty Tzero Al pan and lid for the reference sample. While the generally favorable and hermetically sealable Tzero Al lid option was not used herein, as has been performed in similar studies, to ensure that degassed oxygen-rich and hydroxide-rich volatilized outgassing species could potentially escape the pan housing the powder, the hypothetical risk of nitriding the particle surfaces was shown to have been avoided during heat treating via electron microscopy and localized EDS-based elemental analysis of the DSC-processed powders.
Nevertheless, the powder was removed from the DSC immediately after the allotted time and rapid quenching in the DSC sample reservoir. Feedstock powders were cold mounted in epoxy, ground with 800-grit silicon carbide paper, and then mechanically polished using a Struers (part of Roper Technologies and located in Ballerup, Denmark) Tegramin-20 auto-polisher to a final 0.05 μm silica polish. For microstructural examination, the gas-atomized Al 6061 powders were etched with a 0.5% hydrofluoric acid solution for 60 s.
The microstructural examination was performed with a NIKON Epiphot 200 light optical microscope (Nikon Corporation, Minato City, Tokyo, Japan), and a JEOL, Ltd. (Akishima, Tokyo, Japan), JSM-7000F field emission scanning electron microscopy (FESEM) with an Oxford (Oxford Instruments, Abingdon, Oxfordshire, UK) X-MAXN silicon drift detector for energy dispersive spectrometry (EDS). For further examination, a TEM lamella was cross-sectioned and prepared by a Thermo Fisher Scientific (Waltham, MA, USA) Helios NanoLab G3 UC DualBeam scanning electron microscope and focused ion beam (SEM/FIB) at the Institute of Materials Science (IMS) at the University of Connecticut (Mansfield, CT, USA). The lamellas were imaged in a Thermo Fisher Scientific FEI Talos F200X scanning electron microscope (STEM), also housed at UConn’s IMS. Copper was not included in the EDS analysis because the sample was mounted on a copper grid. This would cause more copper to appear on the EDS map than in the material. The limitations imposed by using such a grid were considered as detailed hereafter in the Discussion section of the present manuscript. Transmission electron backscatter diffraction (t-EBSD) was conducted in a JEOL JSM-7000F field emission SEM at a voltage of 30 V. Phase fraction and precipitate size measurements were performed in the image analysis software Olympus Stream Essentials (Olympus Corporation, Japan). Automatic thresholds were employed. Area fraction results from image analysis were converted to volume fractions using a method described by Corti et al. in [
37].
Conventionally static nanoindentation hardness measurements of the gas-atomized Al 6061 feedstock particulates, as a function of processing condition and even size, were performed using a Keysight Nano Indenter G200 (Keysight Technologies, Santa Rosa, CA, USA). However, one ought to note that the G200 nanoindentation system has since been acquired by the KLA Corporation and rebranded as The Nano Indenter
® G200 system under the umbrella of KLA Instruments (part of the KLA Corporation, Milpitas, CA, USA, and has incorporated Nanomechanics, Inc. (Oak Ridge, TN, USA), too). In any case, the Keysight model of the nanoindentation system, formally known as the Keysight Nano Indenter G200, was equipped with a Berkovich diamond pyramidal nanoindenter tip from Micro Star Technologies (Huntsville, TX, USA). Nanoindentation measurements were made at an indentation depth of 250 nm, which compliments the recommended depth limits for proper indentation testing of metallurgical powder particles mounted in compliant matrix materials by Sousa et al. [
36]. For each of the processed gas-atomized Al 6061 powder conditions, at least 30 nanoindentation measurements were recorded. The displacement resolution of the Keysight Nano Indenter G200 was less than 0.01 nm, and a passive antivibration isolation mount below the G200 nanoindenter ensured well-controlled testing conditions.
3. Results and Discussion
One of the most critical features of powder feedstock for CSAM processing is the microstructure [
38]. CSAM is an entirely solid-state process, so feedstock powder particles’ initial properties and microstructural constituents dramatically influence the CSAM consolidated material [
39]. Stated another way, the phases present in the powder remain in the CSAM material. This was evident by formulating
Figure 2a,b. One may readily note that the XRD patterns in
Figure 2c suggest that no significant phase transformations occurred during CSAM-based processing of the as-atomized Al 6061 studied during the present work. Accordingly, when SEM analysis was considered alongside the XRD analysis presented in
Figure 2c, the same two phases, which were indicated by the arrows in the micrograph of the powder presented in
Figure 2a, can also be found in the corresponding CSAM sample presented in
Figure 2b.
As already mentioned, Al 6061 has been highly regarded and considered within the CSAM community since the 2000s and remains as such [
40]. Still, consideration of the current state of the literature surrounding the use of rapidly solidified and gas-atomized Al 6061 particulate feedstock within CSAM processing unveils an ongoing discrepancy surrounding the microstructural solidification type or class such rapidly solidified alloyed aluminum microparticulate powders belong. Stated otherwise, researchers have claimed that gas-atomized metallic droplets’ rapidly solidified, non-equilibrium, and undercooled nature may vary between dendritic, mixed dendritic/cellular (also known as compound), and cellular, as evidenced by the work of Behulova et al. in [
41]. Considering such microstructural variability and sensitivity to processing conditions, etc., during atomization suggests that care must be taken when attempting to directly compare one reported microstructure with another for the same nominal alloy system. Nevertheless, one ought to still consider the claims reported within the academic literature of relevance.
Per the need for continued consideration of the literature reported to date surrounding gas-atomized Al 6061 powder particle microstructures, Bedard et al. was considered first herein. More to the point, Bedard et al. claimed to have observed “cell-like solidification microstructures” in their Al 6061 feedstock [
42]. Beyond the work of Bedard et al., Ernst et al. purported that “cellular-dendritic”, i.e., compound-like, solidification microstructures were observable in another gas-atomized Al 6061 powder [
43]. However, according to Vijayan et al., gas-atomized Al 6061 powder particles reportedly maintained a pronounced cellular solidification microstructure that was identified as being more cellular than the cellular/dendritic or cell-like microstructures reported by Bedard et al. and Ernst et al. [
44]. At the same time, Sabard et al. reportedly noted a dendritic microstructure during their research concerned with gas-atomized Al 6061, even though they state that a compound-like or “cellular/dendritic microstructure” follows from the high cooling rates achieved during the solidification of the particles in the introduction to [
45]. Work by Evans et al. also asserted that a “cellular rapid solidification structure” could be found within gas-atomized Al 6061 [
46]. Nevertheless, contemporary work by Wei et al. focused on identifying equiaxed grains within their as-atomized Al 6061 feedstock rather than compound or cellular grains, which offers an alternative example of the rapidly solidified structures reported upon to date within the scholarly literature of relevance [
47].
In keeping with the consideration of alternative interpretations of the resultant microstructural classification of gas-atomized Al 6061 and observed microstructural solidification types reported upon as of late, the recent work of Sousa et al. may be considered next. Interestingly, Sousa et al. demonstrated how secondary/primary dendritic solidification modeling can accurately predict the resultant grain size of gas-atomized Al 6061 particles as a function of the atomization gas utilized, molten droplet/particle diameter, cooling rate [
12]. The notable agreement between the computed and measured effective grain sizes attests to dendritic or compound-based solidification of rapidly solidified gas-atomized Al 6061. One may also note that Sousa et al. not only substantiated the dendritic solidification class of gas-atomized Al 6061 in [
12]; rather, Sousa et al. also substantiated the dendritic nature of the non-equilibrium microstructure associated with gas-atomized Al 6061 in [
48] and [
36] as well. Additional works of relevance also serve to either substantiate or detract from the suitability of a dendritic solidification frame of reference, as put forth by Sousa et al. (and Belsito in [
49]), for gas-atomized Al 6061. For example, Rokni et al. identified the polycrystalline microstructure in Al 6061 particulates gas-atomized as equiaxed with low-angled sub-grains comprising high-angled grains [
50]. However, as a counter example, work by Tsaknopoulos et al. and Walde et al. identified the possibility of cellular precipitation or granular nucleation in [
51] and [
52], respectively.
With the discussion above in mind, it stands to reason that it is still unclear from the literature’s interpretations if commonly gas-atomized aluminum powder microstructures are primarily comprised of dendrites, a cellular structure, or a compound microstructural constituent. For example, dendritic solidification microstructures were also observed in a non-6061 alloyed aluminum powder [
53]. Thus, for the present work, a preliminary EBSD study was conducted on the as-atomized Al 6061 powder studied herein to give more information about the grain structure in the powders. Recall that the powder particles were polished, etched, and viewed as cross-sections of the respective particulates via SEM and optical light microscopy. The Materials and Methods section noted that the mounted and polished gas-atomized Al 6061 cross-sections were etched with a 0.5% hydrofluoric acid solution. Grain size parameters were measured from SEM and optical microscopy micrographs using the ASTM E112-13 intercept method [
54]. It was revealed that smaller particles contain smaller grains while larger particles contain larger grains. Such an observation may be relatively intuitive because larger particles have been shown to solidify with lower cooling rates, whereas smaller particles solidified with greater cooling rates during the gas-atomization production process [
55].
For the present work, a preliminary EBSD study was conducted on the as-atomized Al 6061 powder studied herein to yield more information about the internal polycrystalline grain structure associated with the powders. As shown in the inverse pole sub-image of
Figure 3, larger, high-angled grains contain smaller sub-grains, or low-angled grains, with the same orientation. Other individual grains with different orientations are not contained within larger grains. Herein, the term “grains” essentially encapsulates sub-granular low-angled grains since (i) high-angled grains were found to house multiple low-angled and similarly oriented grains, (ii) have previously been shown to act as the adequate grain size that dictates mechanical properties, and (iii) matches modeled secondary dendrite arm spacing (SDAS) as a function of cooling rate and therefore gas-atomized powder particle size [
12,
34,
36]. Since sample preparation was considered essential for EBSD analysis, the sample associated with
Figure 3 was thinned through focused ion beam milling.
Beyond the isolated realm of microstructural polycrystalline solidification class identification and grain size/orientation analysis, STEM has also emerged as a beneficial and experimentally rooted characterization tool for the study of gas-atomized, rapidly solidified microparticles in general, as was highlighted by Srinivasan in [
56], and Al 6061 powders in particular herein. More to the point, STEM is suitable or adequate to unveil the possible identities and approximate compositions of secondary intermetallic precipitates along the sub-grain and high-angled grain boundaries within the gas-atomized feedstock. Moreover, McNally previously demonstrated the well-suited nature of STEM to compare gas-atomized aluminum alloyed intermetallic phases with the equilibrium and non-equilibrium predictions via computational thermodynamics and computational kinetics of the secondary phases for the experimentally determined chemical composition of the powder. Stated otherwise, the respective chemical composition of the bulk powder had been utilized as an input for computational thermodynamic and kinetic predictive analysis of prospective phases and their potential volume fractions [
57]. In any case,
Figure 4a captures fine precipitates along the grain boundaries, around 100 nm to 200 nm in size. The white arrow within
Figure 4 points to what was labeled as the “white phase”. Similarly, the dotted white arrow in
Figure 4 points to what was labeled as the “gray phase”, and the black arrow identifies what was labeled as the “dark phase”.
Figure 4b presents a higher magnification micrograph of the phases along the grain boundaries affiliated with a region captured in
Figure 4a.
The average composition, in atomic percent, was assessed for each of the phases associated with
Figure 4 and was provided in
Table 1. The dark phase was identified as a form of β-Mg
2Si; however, a measured diffraction pattern did not identify the β-Mg
2Si intermetallic as the stable Mg
2Si phase. Said non-stable β-Mg
2Si was identified as a metastable β-Mg
2Si precursor precipitate phase formed during gas-atomization of the Al 6061 CSAM feedstock powder. The identification of a metastable form of β-Mg
2Si, rather than an equilibrium or stable β-Mg
2Si phase, was also consistent with another original research article by Sousa et al., wherein the authors noted the fact that the cooling rates achieved during gas-atomization of Al 6061 will not intersect the stable β-Mg
2Si cooling curves computed and presented in a plot of a computationally derived graphical depiction of the respective continuous cooling transformation (CCT) curves in [
34].
In any case, the identity of the white and gray phases was difficult to pinpoint in exact terms because of their respective similarity in chemical compositions and the stoichiometric similarity of the phases predicted by computational thermodynamic analysis, as detailed in [
49,
57]. Moreover, the lack of copper-EDS data associated with
Figure 4 and
Table 1 limited the ability to confidently assess copper’s presence within two of the non-(β-Mg
2Si) intermetallic phases. Therefore, further TEM analysis was applied to another gas-atomized Al 6061 CSAM feedstock powder particle, approximately 47 μm in diameter, as shown in
Figure 5. Again, equiaxed dendritic grains were found within the respective powder particle, with discrete smaller phases along the grain boundaries.
The micrograph presented in
Figure 5 highlighted how an equiaxed or compound dendrite-like microstructure within gas-atomized Al 6061 feedstock could be observed. Said solidification structure observed within the particulate captured in
Figure 5 was consistent with the work of Molnárová et al. for another gas-atomized aluminum alloy [
58]. As alluded to, the micrograph presented in
Figure 5 also captured the location of secondary intermetallic phases along the grain boundaries in the gas-atomized Al 6061 powder. However, the need for a more sensitively quantitative analysis of regional and site-specific compositions remained. To address such a need, EDS mapping of various regions of interest within the gas-atomized Al 6061 FIB lamella shown in
Figure 5 was performed. The EDS maps of said regions of relevance are shown in
Figure 6,
Figure 7 and
Figure 8. Once again, one ought to note that copper was not included in the EDS maps because of the influence that would follow from the copper grid upon which the lamellae was seated.
Figure 6 showed interesting precipitate structures along grain boundaries. An Mg-Si precipitate appeared to be a “backbone” type structure, which was identified as a Chinese script eutectic non-stable form of β-Mg
2Si, with primarily Al-Fe intermetallic particles within the spaces between the “spines” of the non-stable form of a β-Mg-Si precipitate.
An inclined grain boundary was observed and recorded in
Figure 8 and contains the same interesting “backbone” structured Mg-Si precipitates noted in
Figure 6, i.e., the Chinese script eutectic non-stable form of β-Mg
2Si. The Mg-Si precipitate structure at said grain boundaries was dendritic, with the Al-Fe particles located in between the secondary dendrite arms. This was not found in the first STEM sample shown in
Figure 4; however, it was evident across the sample introduced initially in
Figure 5. Notably, the recorded microstructural intermetallic arrangements and morphologies were like the structure of the precipitates presented by Chakrabarti et al. in [
16]. In addition, though there were still similarly dendritic Mg-Si precipitates captured within
Figure 7, there were also observable and discrete Al-Fe particles that did not reside between the secondary dendrite arms but were still contained along the polycrystalline grain boundaries.
Image analysis was performed upon the EDS mapping areas, i.e., locations captured in
Figure 6,
Figure 7 and
Figure 8, and their respective micrographs taken at several locations within the TEM sample presented in
Figure 5. A histogram of the intermetallic size results of the Mg-Si and Al-Fe particles is presented in
Figure 9. It was shown that most Mg-Si particles were around 40 nm to 80 nm in size. The Mg-Si phase was also found to yield an approximately 2.7% +/− 0.7% area fraction from the images analyzed. The Al-Fe intermetallic phases seemed to range widely in size, from 20 nm to over 140 nm, and were also found to yield an approximately 2.25% +/− 0.01% area fraction. Following the observable solidification structures documented for gas-atomized aluminum alloys, the interesting Chinese script-like eutectic structure of the non-stable form of β-Mg
2Si precipitates ought to be considered dendritic; consequently, a secondary dendrite arm width and spacing were able to be reported within the regions of relevance as well. The secondary dendrite arm widths were measured and found to be approximately 53 nm +/− 16 nm, while the dendritic SDAS within said regions was measured as 78 nm +/− 17 nm. Such sub-100 nm sized SDAS and SDA values suggest that nanostructured regions exist within the gas-atomized Al 6061 feedstock particulates.
The solutionized sample was scanned in the DSC, described in the Methods and Materials section previously documented, and held at 540 °C for 1 h. The DSC curve is plotted in
Figure 10. The typical peaks for the precipitation and dissolution of the β phase were found, like the literature data in
Figure 10. A powder particle approximately 40 μm in diameter was examined in STEM and shown in
Figure 11. Fairly equiaxed grains are found in the sample, with precipitates speckled at the grain boundaries. Upon closer inspection of the right side of
Figure 11, there are bright, small precipitates containing heavier elements and larger dark precipitates at triple points of the grain boundaries. EDS was used to probe the identities of these phases once again. The DSC curve presented in
Figure 10 was consistent with the work of Sousa et al. in [
12].
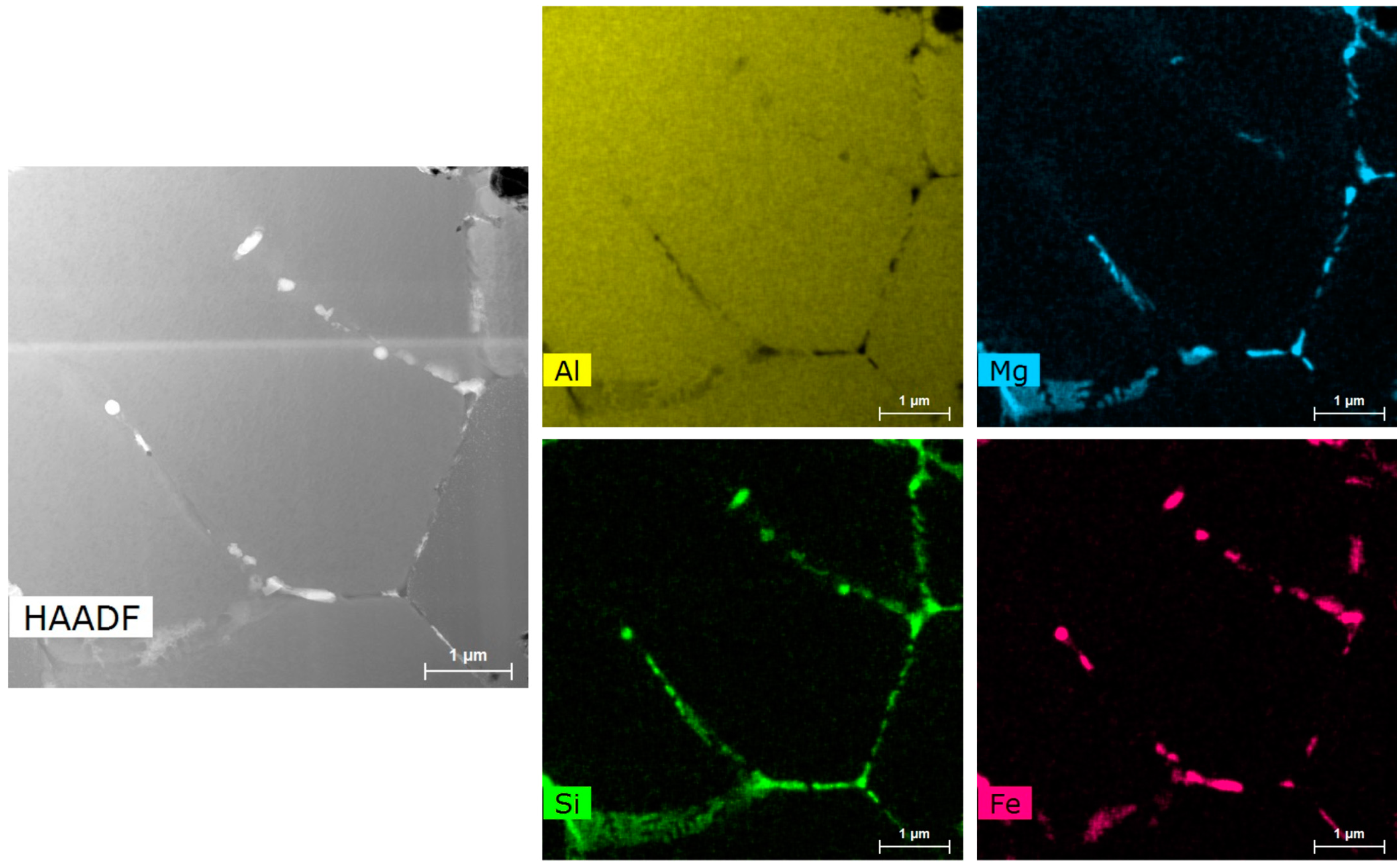
Through EDS mapping in
Figure 12 and
Figure 13, it was found that the small bright white precipitates at the grain boundary contain almost all of the elements in the alloy; aluminum, silicon, iron, chromium, and manganese. This means these phases are difficult to identify and could be any combination of the following phases from the predictive tools: Al
13Fe
4, Al
8Fe
2Si, Al
15Si
2Fe
4, Al
9Fe
2Si
2, and Al
18Fe
2Mg
7Si
20. The darker, larger precipitates mostly contained magnesium and silicon at triple points and were hypothesized to be Mg
2Si. The precipitate radius size distribution of Al-Fe-Si-Cr-Mn and Mg
2Si particles is shown in
Figure 14. It is found that the Al-Fe-Si-Cr-Mn precipitates are small, with the majority around 40 nm to 60 nm and with a few over 200 nm in size. The average area fraction of these particles is approximately 4.1 +/− 1.3%. The Mg
2Si phase has seemed to be lower in number but coarser in size. Most of the precipitates are over 200 nm, with an average of 324 +/− 154 nm in size.
There was a significant change in the morphology of the phases during the solutionization step. Firstly, the Al-Fe-Si-Cr-Mn particles seemed to have refined in size. There was an extensive size range in the as-received sample, from 20 nm to over 140 nm in size. In the solutionized sample, most of these particles were around 40 nm to 60 nm, with only a few over 200 nm in size. Secondly, the solutionization step dissolved the interesting dendritic structure of the Mg2Si phase. The non-dendritic precipitates found in the as-atomized sample were around 40 nm to 80 nm in size, with a 2.7 +/− 0.7% area fraction. After solutionization, the Mg2Si phase does not contain any precipitates in the dendritic structure, but it lessened in number and became coarser in size. Most of these precipitates were between 200 nm and 500 nm in radius. The sample had approximately 15 Mg2Si precipitates, mostly at triple points.
Nanoindentation was applied to the gas-atomized Al 6061 powder, both with and without thermal processing. The average nanoindentation hardness (, wherein is the contact area function dependent upon the reporting indentation depth) of the as-atomized Al 6061 powder, with a size range of 38–45 μm was 1.12 GPa, and an average nanoindentation hardness value of 1.26 ± 0.15 GPa for randomly selected particulates of variable size categories, i.e., within the un-sieved powder. The variation in nanoindentation hardness values for difference solutionization hold times at an elevated temperature of thermal processing temperature of 540 °C was recorded as follows: 1.29 GPa at zero minutes, 1.23 GPa at 20 min, 1.19 GPa at 60 min, 1.14 GPa at 120 min, and 0.88 GPa at 240 min. It was shown that an initial increase in nanoindentation hardness existed at 0 h when the sample was brought to 540 °C and immediately quenched. As the time held at the solutionization temperature increased, the nanoindentation hardness tended to decrease, with the lowest value after 4 h of elevated temperature exposure. This could have been due to the coarsening and loss of coherency with the main strengthening phase, Mg2Si. These results show that heat treating the powder will reduce its hardness, thus reducing the flow stress of the material and making it more “sprayable”.
Power law curve fitting was applied to a multi-curve fit dataset obtained using each loading cycle load–depth data captured via nanoindentation testing as a function of specimen solutionization condition. Accordingly, the load–depth data were found to take the following form:
, wherein the magnitude of the coefficients (
) were found to be 0.00003, 0.0005, 0.00006, 0.0002, and 0.000002 for the 0, 20, 60, 120, and 240 min solutionized powder particles, respectively. In said order, the power exponents (
) were determined to be 1.9912, 1.4508, 1.8486, 1.6753, and 2.4268, respectively. Finally, the statistical regression scoring metric known as the coefficient of determination (
) was also computed per hold time, in the same order, yielding 0.9959, 0.9987, 0.9970, 0.9979, and 0.9937, respectively. Finally,
Figure 15 presents the power-law curve fit load–depth data associated with the loading portion of the test for each solutionization hold time to provide additional contextualization of the nanoindentation-based mechanical behavior across each condition studied.
Since the point in time when cold spray processing was first reported within the early scholastic literature of relevance, let alone cold spray’s recent and formalized extension into the realm of metal additive manufacturing via CSAM, numerable mechanistic models and relations have emerged relating impact phenomena with material properties and the like. For example, CSAM researchers not only modified [
59] and applied [
60] the Johnson–Cook plasticity model for computationally exploring impact-induced mechanical, microstructural, and thermal evolution, as highlighted in [
61] through [
62]. Instead, the materials research community focused upon CSAM processing development also invoked, recalled, and refined numerable relations between the following, for instance: the critical impact velocity and the ultimate tensile strength of a microparticle [
63]; the critical impact velocity and the yield/flow stress of a microparticle [
64]; the strain rate experienced during particle impact and the microhardness of the material [
33]; the dependence of deposition efficiency on particle size and thermophysical properties of the powder; the critical impact velocity as a function of spall strength, the bulk modulus and speed of sound, etc. [
65]; the critical impact velocity as a function of oxygen content in gas-atomized pure Cu, for example [
36]; and the mean impact pressure as a function of particle impact velocity; among others.
Of course, such an itemization only captures a few of the notable developments made to date following the continued evolution of our collective understanding of the feedstock material properties and microstructural relations underpinning and CSAM processing and thus resultant consolidated component performance. In so far as such mechanistic developments underlying CSAM are related to the experimental results presented and discussed herein, such formulations and theoretical, computational, or empirical relations can be coupled with said findings better to understand their implications for CSAM processing optimization and tunability.