Effect of Nanosized Ni Reinforcements on the Structure of the Sn-3.0Ag-0.5Cu Alloy in Liquid and After-Reflow Solid States
Abstract
:1. Introduction
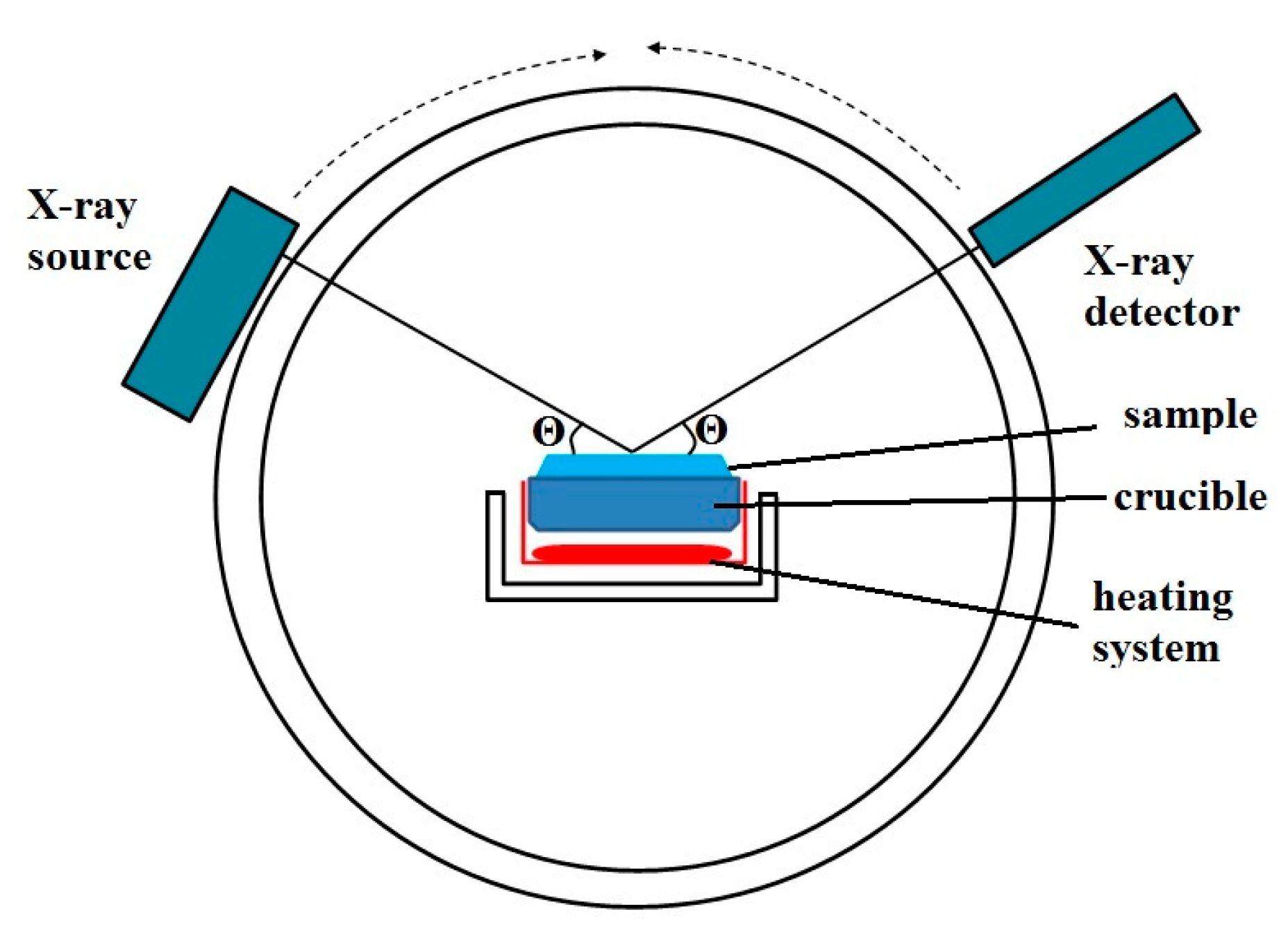
2. Materials and Methods
3. Results and Discussion
3.1. High-Temperature XRD Analysis
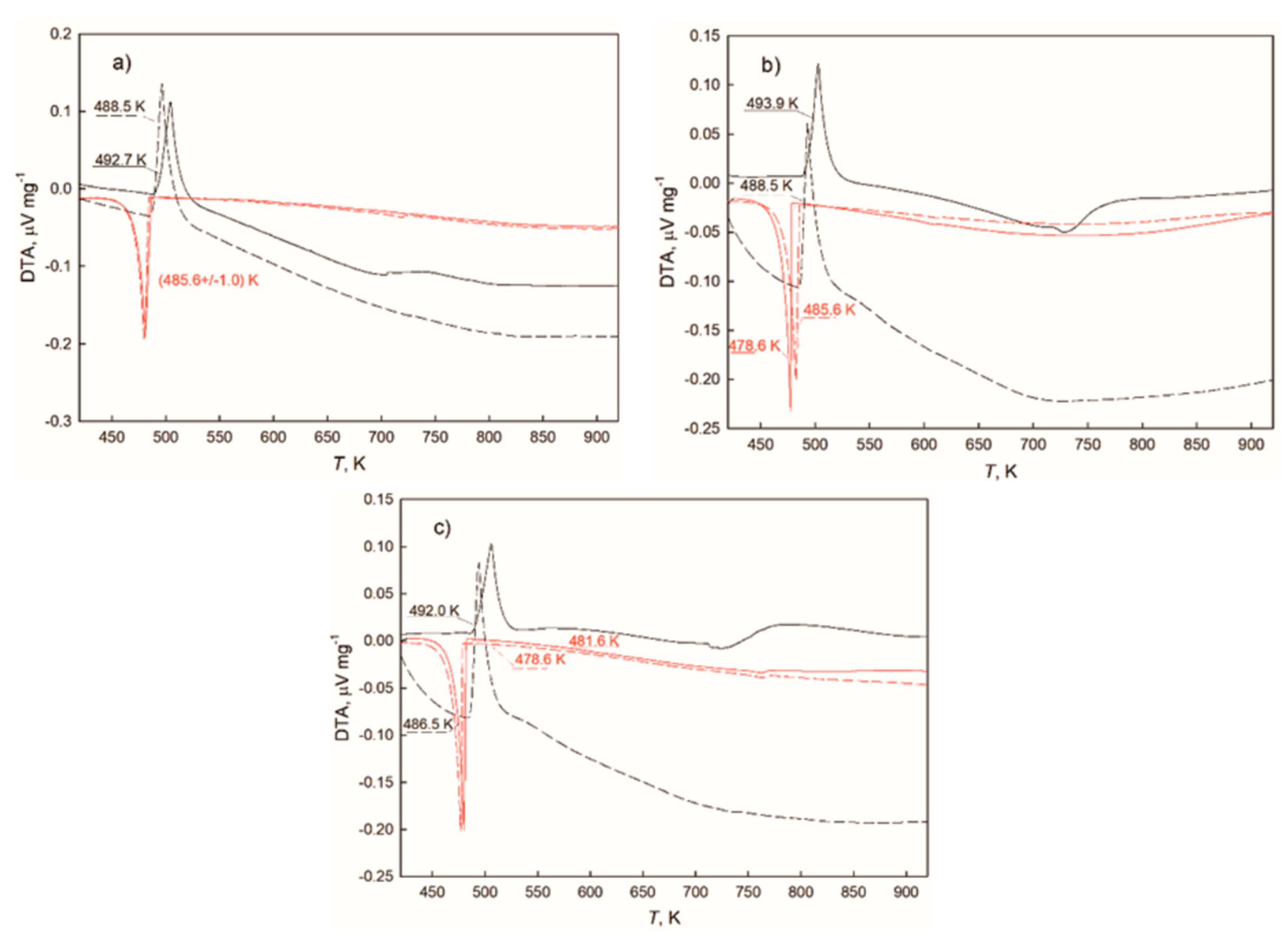
3.2. DTA Study
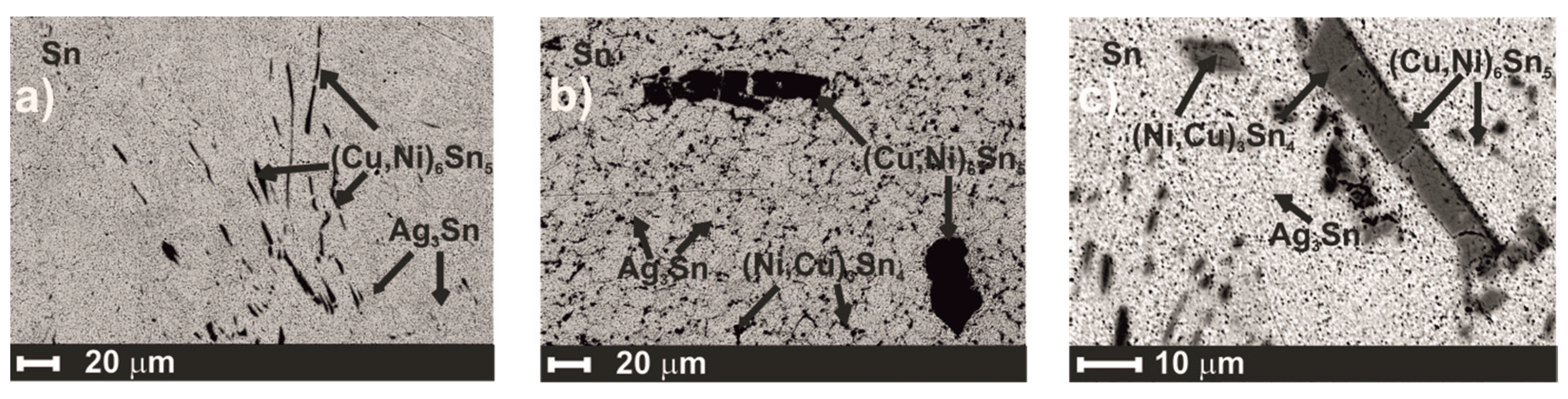
3.3. The Microstructural Study in the Solid State
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.-L.; Zhang, L.; Jiang, N.; Zhang, L.; Zhong, S.-J. Materials modification of the lead-free solders incorporated with micro/nano-sized particles: A review. Mater. Design. 2021, 197, 109224. [Google Scholar] [CrossRef]
- Tian, R.; Wang, C.; Huang, Y.; Guo, X. Effects of nanoparticle addition on the reliability of Sn-based Pb-free solder joints under various conditions: A review. NANO 2023, 18, 2330001. [Google Scholar] [CrossRef]
- Schmetterer, C.; Ipser, H.; Pearce, J. ELFNET COST Action 531—Handbook of Properties of SAC Solders and Joints; Vydavatelstvi KNIHAR: Brno, Czech Republic, 2008. [Google Scholar]
- Tamizi, M.; Movahedi, M.; Kokabi, A.H. Real-time monitoring of soldering process in SAC composite solder paste: Movement and interaction of dopant particles in solder paste. J. Mater. Sci. Mater. Electron. 2023, 34, 507. [Google Scholar] [CrossRef]
- Tikale, S.; Narayan Prabhu, K. Bond shear strength of Al2O3 nanoparticles reinforced 2220-capacitor/SAC305 solder interconnects reflowed on bare and Ni-coated copper substrate. J. Mater. Sci. Mater. Electron. 2021, 32, 2865–2886. [Google Scholar] [CrossRef]
- Rajendran, S.H.; Hwang, S.J.; Jung, J.P. Shear strength and aging characteristics of Sn-3.0Ag-0.5Cu/Cu solder joint reinforced with ZrO2 nanoparticles. Metals 2020, 10, 1295. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Xi, W.; Li, M.-I. Structure and properties of low-Ag SAC solders for electronic packaging. J. Mater. Sci. Mater. Electron. 2022, 33, 22668–22705. [Google Scholar] [CrossRef]
- Aamir, M.; Muhammad, R.; Tolouei-Rad, M.; Giasin, K.; Silberschmidt, V. A review: Microstructure and properties of tin-silver-copper lead-free solder series for the applications of electronics. Solder. Surf. Mount. Technol. 2020, 32, 115–126. [Google Scholar] [CrossRef]
- Skwarek, A.; Krammer, O.; Hurtony, T.; Ptak, P.; Górecki, K.; Wroński, S.; Straubinger, D.; Witek, K.; Illés, B. Application of ZnO nanoparticles in Sn99Ag0.3Cu0.7 based composite solder alloys. Nanomaterials 2021, 11, 1545. [Google Scholar] [CrossRef]
- Skwarek, A.; Ptak, P.; Górecki, K.; Hurtony, T.; Illés, B. Microstructure influence of SACX0307-TiO2 composite solder joints on thermal properties of power LED assemblies. Materials 2020, 13, 1563. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Zhao, X.; Wen, S.; Li, H.; Wang, Y. Effects of cobalt nanoparticles addition on shear strength, wettability and interfacial intermetallic growth of Sn−3.0Ag−0.5Cu solder during thermal cycling. Mater. Sci. Forum. 2015, 815, 97–102. [Google Scholar] [CrossRef]
- Yakymovych, A.; Sklyarchuk, V.; Plevachuk, Y.; Sokoliuk, B. Viscosity and electrical conductivity of the liquid Sn-3.8Ag-0.7Cu alloy with minor Co admixtures. JMEPEG 2016, 25, 4437–4443. [Google Scholar] [CrossRef] [Green Version]
- Yakymovych, A.; Kaptay, G.; Flandorfer, H.; Bernardi, J.; Schwarz, S.; Ipser, H. The nano heat effect of replacing macro-particles by nano-particles in drop calorimetry: The case of core/shell metal/oxide nano-particles. RSC Adv. 2018, 8, 8856. [Google Scholar] [CrossRef] [Green Version]
- Gain, A.; Zhang, L. Effects of Ni nanoparticles addition on the microstructure, electrical and mechanical properties of Sn-Ag-Cu alloy. Materialia 2019, 5, 100234. [Google Scholar] [CrossRef]
- Yakymovych, A.; Švec, P.; Orovcik, L. Nanocomposite SAC solders: The effect of adding Ni and Ni-Sn nanoparticles on morphology and mechanical properties of Sn-3.0Ag-0.5Cu solders. J. Electron. Mater. 2018, 47, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Gan, G.-S.; Gan, L.-Q.; Guo, J.-Z.; Xia, D.-Q.; Zhang, C.; Yang, D.; Wu, Y.; Liu, C. Ultrasonic-assisted soldering of low-Ag SAC lead-free solder paste at low-temperature. Mater. Trans. 2018, 59, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Yakymovych, A.; Plevachuk, Y.; Orovcik, L.; Švec, P., Sr. Nanocomposite SAC solders: The effect of heat treatment on the morphology of Sn-3.0Ag-0.5Cu/Cu solder joints reinforced with Ni and Ni-Sn nanoparticles. Appl. Nanosci. 2022, 12, 977–982. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C.; Yung, K.C.; Sharif, A.; Ali, L. Effect of nano Ni additions on the structure and properties of Sn-9Zn and Sn-8Sn-3Bi solder in ball grid array packages. In Proceedings of the 2nd Electronics System-Integration Technology Conference, Greenwich, UK, 1–4 September 2008; pp. 1291–1294. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L.C. Interfacial microstructure, wettability and material properties of nickel (Ni) nanoparticle doped tin–bismuth–silver (Sn–Bi–Ag) solder on copper (Cu) substrate. J. Mater. Sci. Mater. Electron. 2016, 27, 3982–3994. [Google Scholar] [CrossRef]
- Yakymovych, A.; Plevachuk, Y.; Sklyarchuk, V.; Sokoliuk, B.; Galya, T.; Ipser, H. Microstructure and electro-physical properties of Sn-3.0Ag-0.5Cu nanocomposite solder reinforced with Ni nanoparticles in the melting-solidification temperature range. J. Phase Equilibria Diffus. 2017, 38, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Yakymovych, A.; Weber, H.; Kaban, I.; Ipser, H. Dynamic viscosity of a liquid Sn 3.0Ag 0.5Cu alloy with Ni nanoparticles. J. Mol. Liq. 2018, 268, 176–180. [Google Scholar] [CrossRef]
- Cromer, D.T.; Waber, J.T. Scattering factors computed from relativistic Dirac-Slater wave functions. Acta Crystallogr. 1965, 18, 104–109. [Google Scholar] [CrossRef]
- Kroghmoe, J. A method for converting experimental X-ray intensities to an absolute scale. Acta Crystallogr. 1965, 9, 951–953. [Google Scholar] [CrossRef]
- Wilson, J.R. The structure of liquid metals and alloys. Metall. Rev. 1965, 10, 381–590. [Google Scholar] [CrossRef]
- Calderín, L.; González, D.J.; González, L.E.; López, J.M. Structural, dynamic, and electronic properties of liquid tin: An ab initio molecular dynamics study. J. Chem. Phys. 2008, 129, 194506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waseda, Y. The Structure of Non Crystalline Materials; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Yakymovych, A.; Mudry, S.; Shtablavyi, I.; Ipser, H. Effect of nano Co reinforcements on the structure of the Sn-3.0Ag-0.5Cu solder in liquid and after reflow solid states. Mater. Chem. Phys. 2018, 181, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-W.; Chiu, C.-N.; Hsieh, K.-C. Phase equilibria of the Sn-Ag-Cu-Ni quaternary system at 210 °C. J. Electron. Mater. 2007, 36, 197–206. [Google Scholar] [CrossRef]
- Schmetterer, C.; Flandorfer, H.; Richter, K.W.; Saeed, U.; Kauffman, M.; Roussel, P.; Ipser, H. A new investigation of the system Ni–Sn. Intermetallics 2007, 15, 869–884. [Google Scholar] [CrossRef]
- Vuorinen, V.; Yu, H.; Laurila, T.; Kivilahti, J.K. Formation of intermetallic compounds between liquid Sn and various CuNix metallizations. J. Electron. Mater. 2008, 37, 792–805. [Google Scholar] [CrossRef] [Green Version]
- Schmetterer, C.; Flandorfer, H.; Luef, C.; Kodentsov, A.; Ipser, H. Cu-Ni-Sn: A key system for lead-free soldering. J. Electron. Mater. 2009, 38, 10–24. [Google Scholar] [CrossRef]
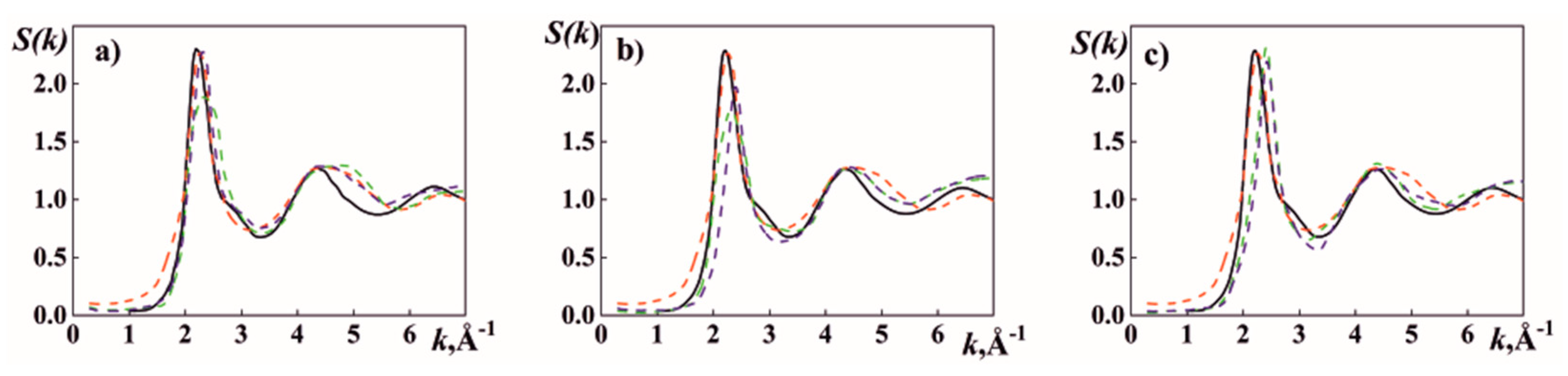

| Composition, wt.% | T, K | r1, nm | r2, nm | r2/r1 | Zsym | |
|---|---|---|---|---|---|---|
| SAC305 | Ni | |||||
| 99.5 | 0.5 | 593 | 3.04 | 5.80 | 1.91 | 7.3 |
| 653 | 3.15 | 5.92 | 1.88 | 8.7 | ||
| 99.0 | 1.0 | 683 | 3.20 | 5.91 | 1.85 | 8.8 |
| 743 | 3.19 | 5.93 | 1.86 | 8.8 | ||
| 98.0 | 2.0 | 793 | 3.21 | 5.89 | 1.83 | 8.6 |
| 853 | 3.16 | 5.93 | 1.88 | 8.7 | ||
| Composition, wt.% | Composition, at.% | T°m, K | T°s, K | (T°m−T°s), K | Source | ||
|---|---|---|---|---|---|---|---|
| SAC305 | Ni | SAC305 | Ni | ||||
| 100.0 | 0.0 | 100.0 | 0.0 | 489.3 ± 0.5 | 475.3 ± 0.6 | 14.0 ± 1.1 | [27] |
| 99.5 | 0.5 | 99.0 | 1.0 | 488.5 | 485.6 ± 1.0 | 2.9 ± 1.0 | exp. |
| 99.0 | 1.0 | 98.0 | 2.0 | 488.5 | 482.1 ± 3.5 | 6.4 ± 3.5 | exp. |
| 98.0 | 2.0 | 96.0 | 4.0 | 486.5 | 480.1 ± 1.5 | 6.4 ± 1.5 | exp. |
| Sample | Sn Phase | Ag-Sn Phase | (Cu,Ni)Sn Phases | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sn at. % | Ag at. % | Sn at. % | Ni at. % | Cu at. % | Sn at. % | ||||
| BA1 | βSn | 100 | Ag3Sn | 74 | 26 | (Cu,Ni)6Sn5 | 3–9 | 41–47 | 48–50 |
| BA2 | βSn | 100 | Ag3Sn | 76 | 24 | (Cu,Ni)6Sn5 (Ni,Cu)3Sn4 | 12–17 30–40 | 33–37 | 47–53 60–70 |
| BA3 | βSn | 100 | Ag3Sn | 74 | 26 | (Cu,Ni)6Sn5 (Ni,Cu)3Sn4 | 13–18 25–26 | 34–37 14–15 | 48–50 59–60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakymovych, A.; Shtablavyi, I. Effect of Nanosized Ni Reinforcements on the Structure of the Sn-3.0Ag-0.5Cu Alloy in Liquid and After-Reflow Solid States. Metals 2023, 13, 1093. https://doi.org/10.3390/met13061093
Yakymovych A, Shtablavyi I. Effect of Nanosized Ni Reinforcements on the Structure of the Sn-3.0Ag-0.5Cu Alloy in Liquid and After-Reflow Solid States. Metals. 2023; 13(6):1093. https://doi.org/10.3390/met13061093
Chicago/Turabian StyleYakymovych, Andriy, and Ihor Shtablavyi. 2023. "Effect of Nanosized Ni Reinforcements on the Structure of the Sn-3.0Ag-0.5Cu Alloy in Liquid and After-Reflow Solid States" Metals 13, no. 6: 1093. https://doi.org/10.3390/met13061093






