Efficient Gallium Recovery from Aqueous Solutions Using Polyacrylonitrile Nanofibers Loaded with D2EHPA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
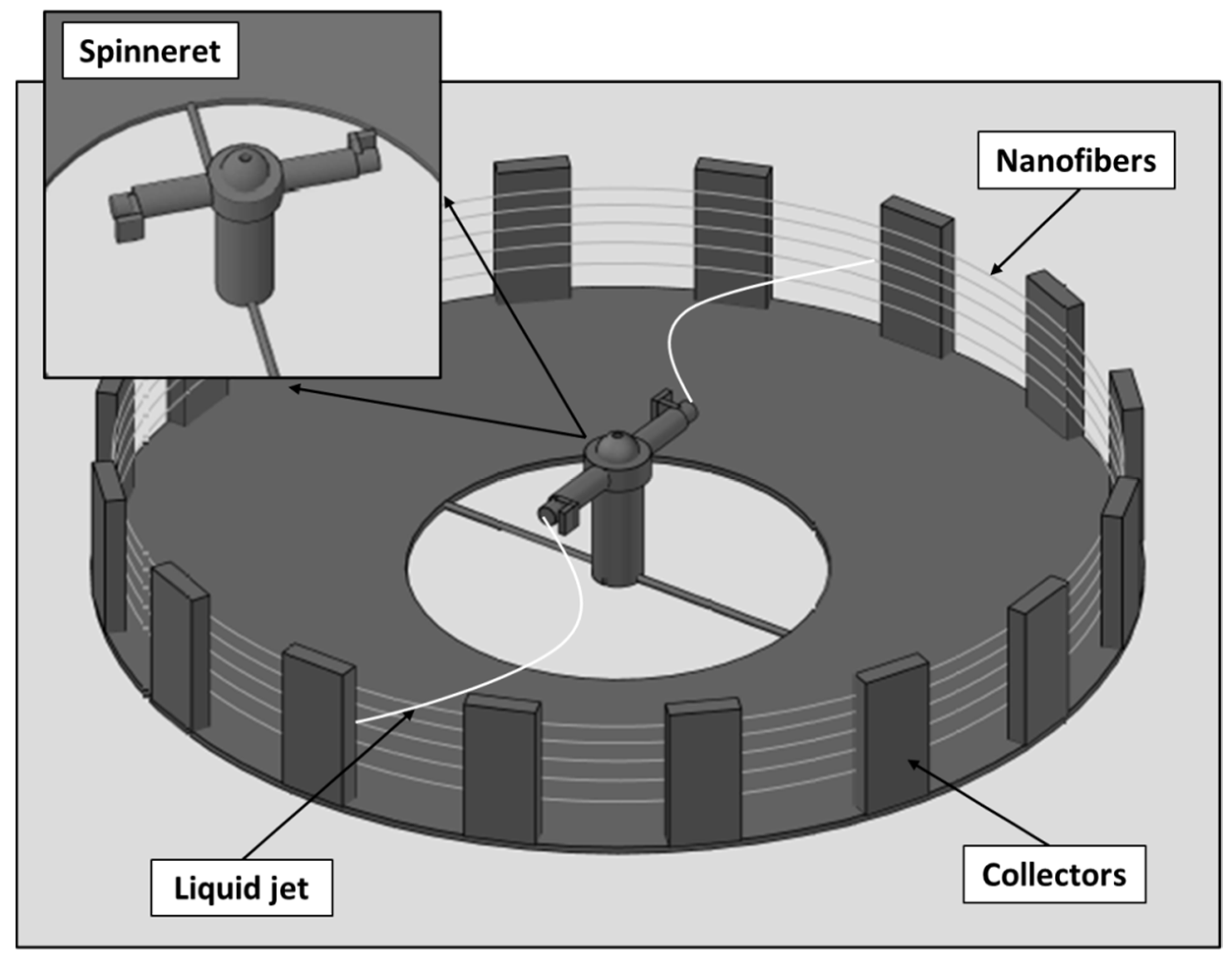
2.2. Preparation of the Nanofibers
2.3. Characterization of the Nanofibers
2.4. Adsorption Experiments
2.5. Kinetics Studies
2.6. Equilibrium Studies
2.7. Thermodynamics Parameters Estimation
2.8. Modeling and Parameter Estimation
2.9. Desorption and Regeneration of Adsorbent
3. Results
3.1. Materials Characterization
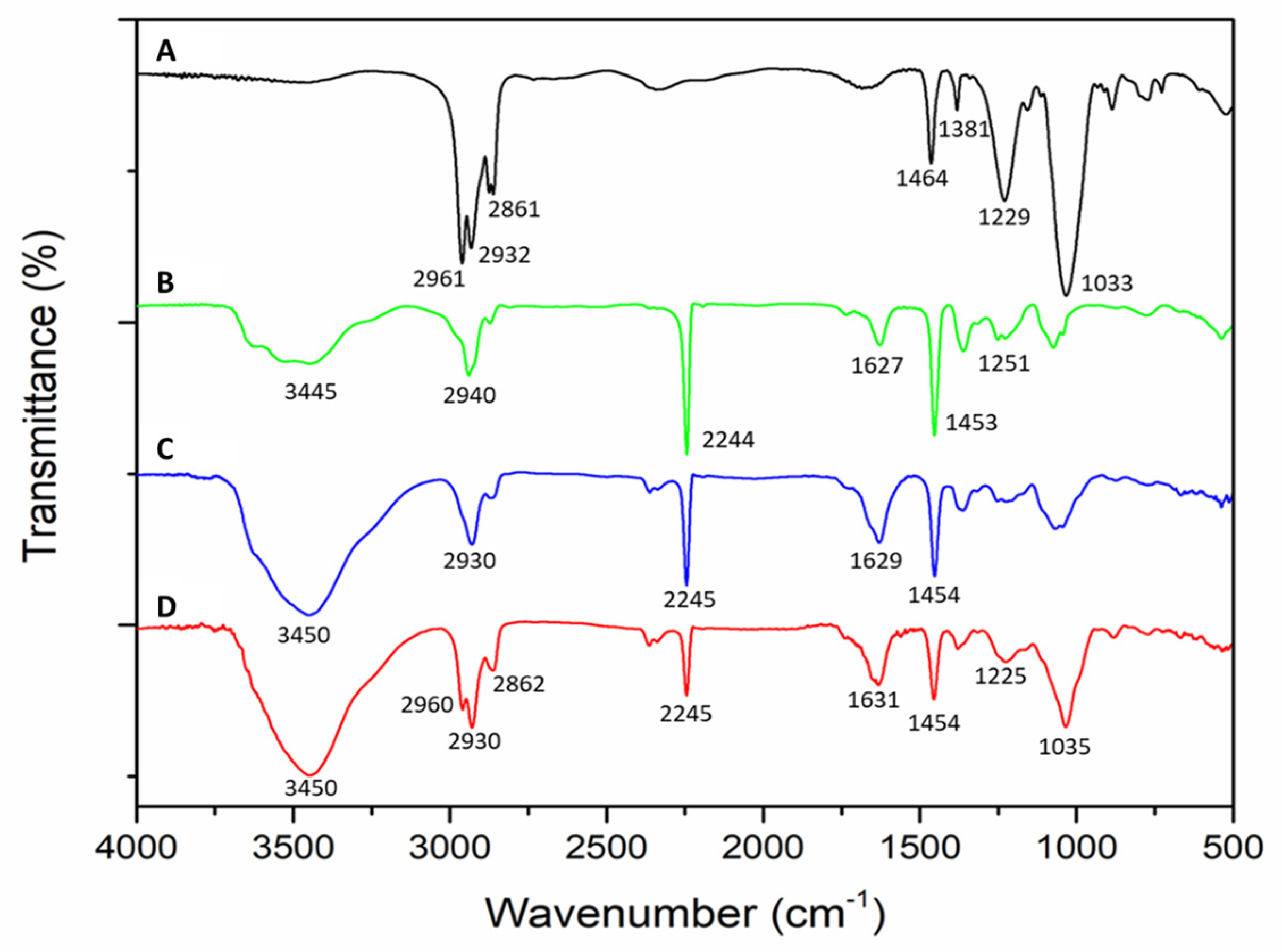
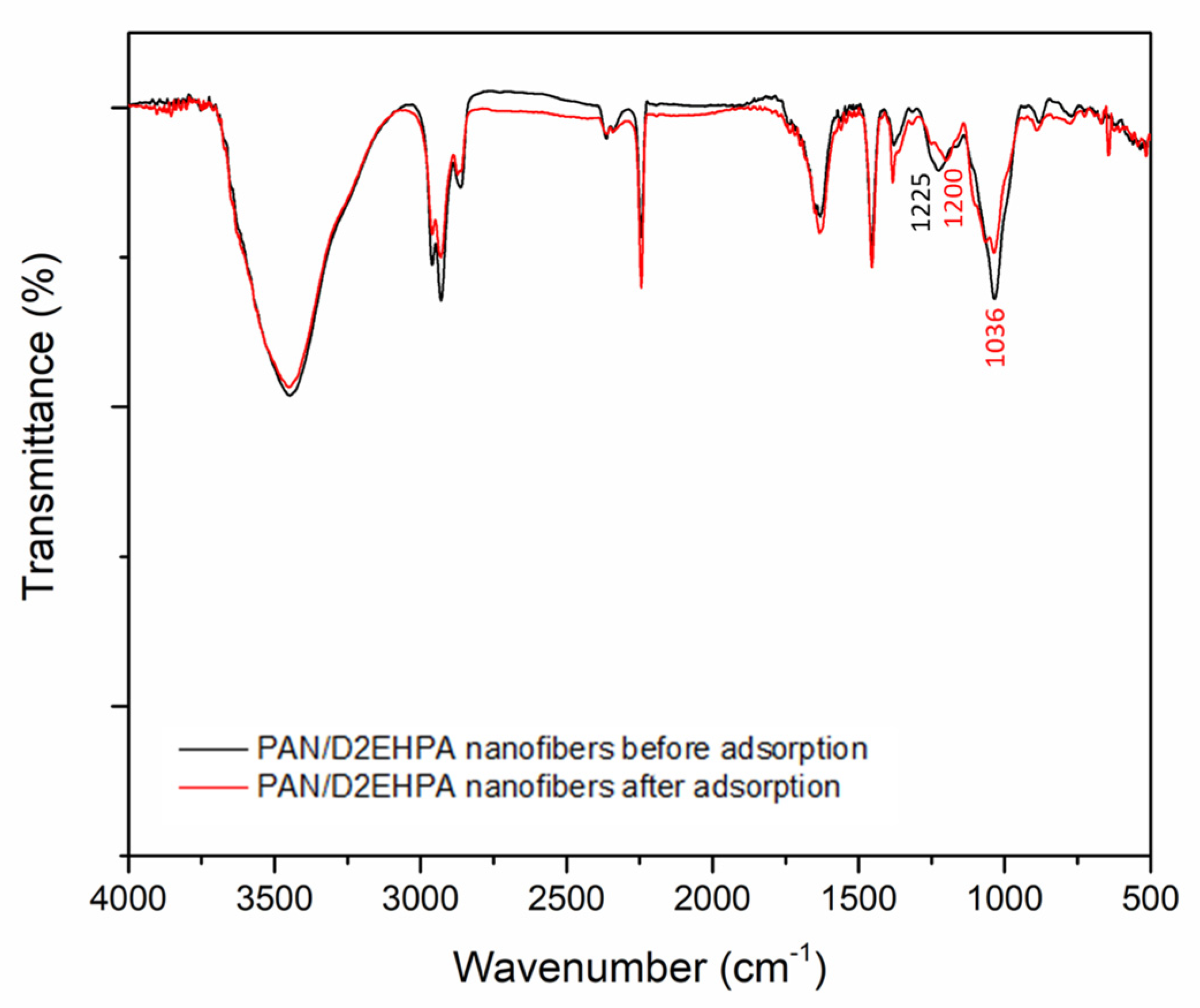
3.1.1. Infrared Spectroscopy (FT-IR) Analysis
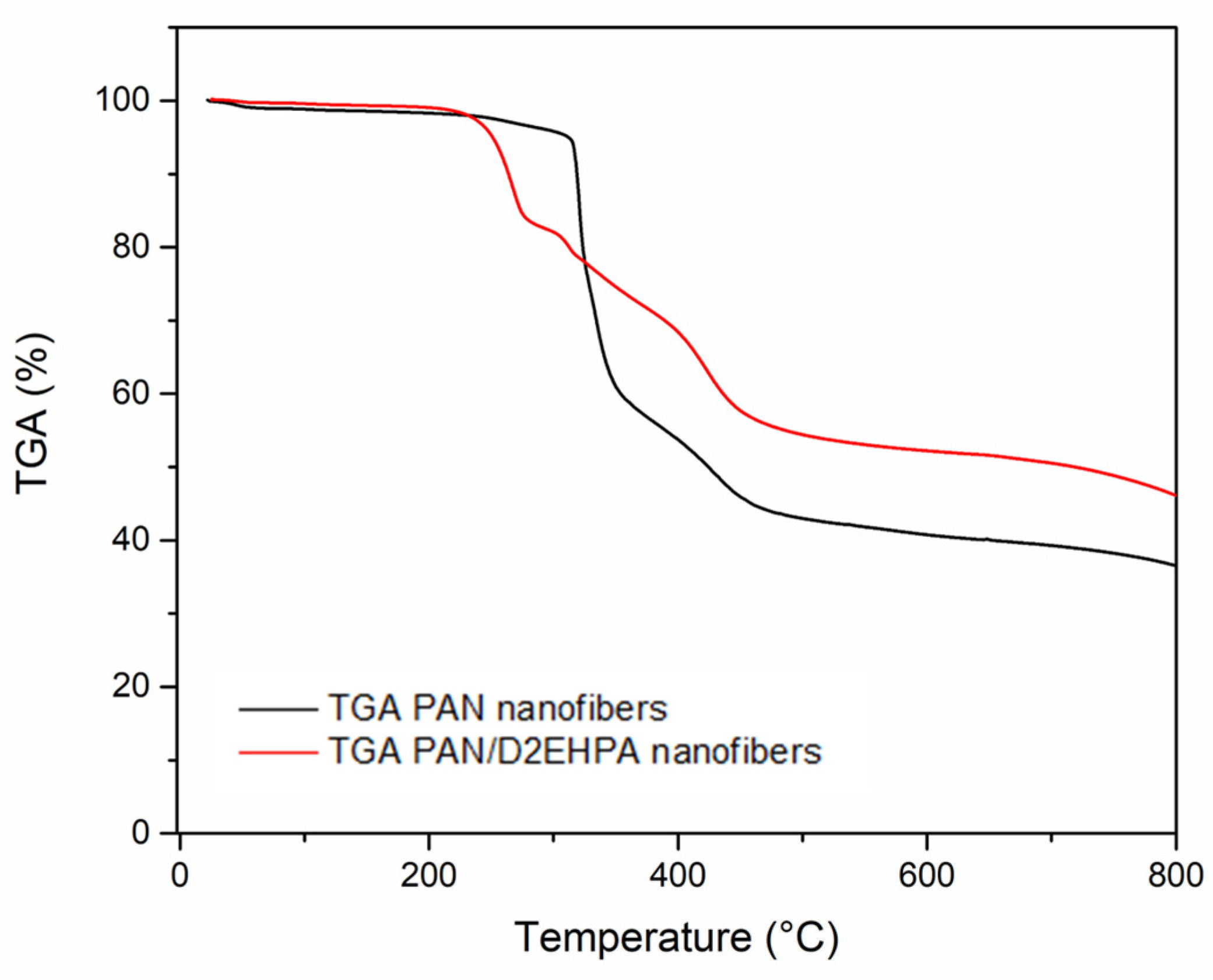
3.1.2. Thermogravimetric Analysis (TGA)
3.1.3. Scanning Electron Microscopy (SEM)
3.2. Adsorption of Gallium
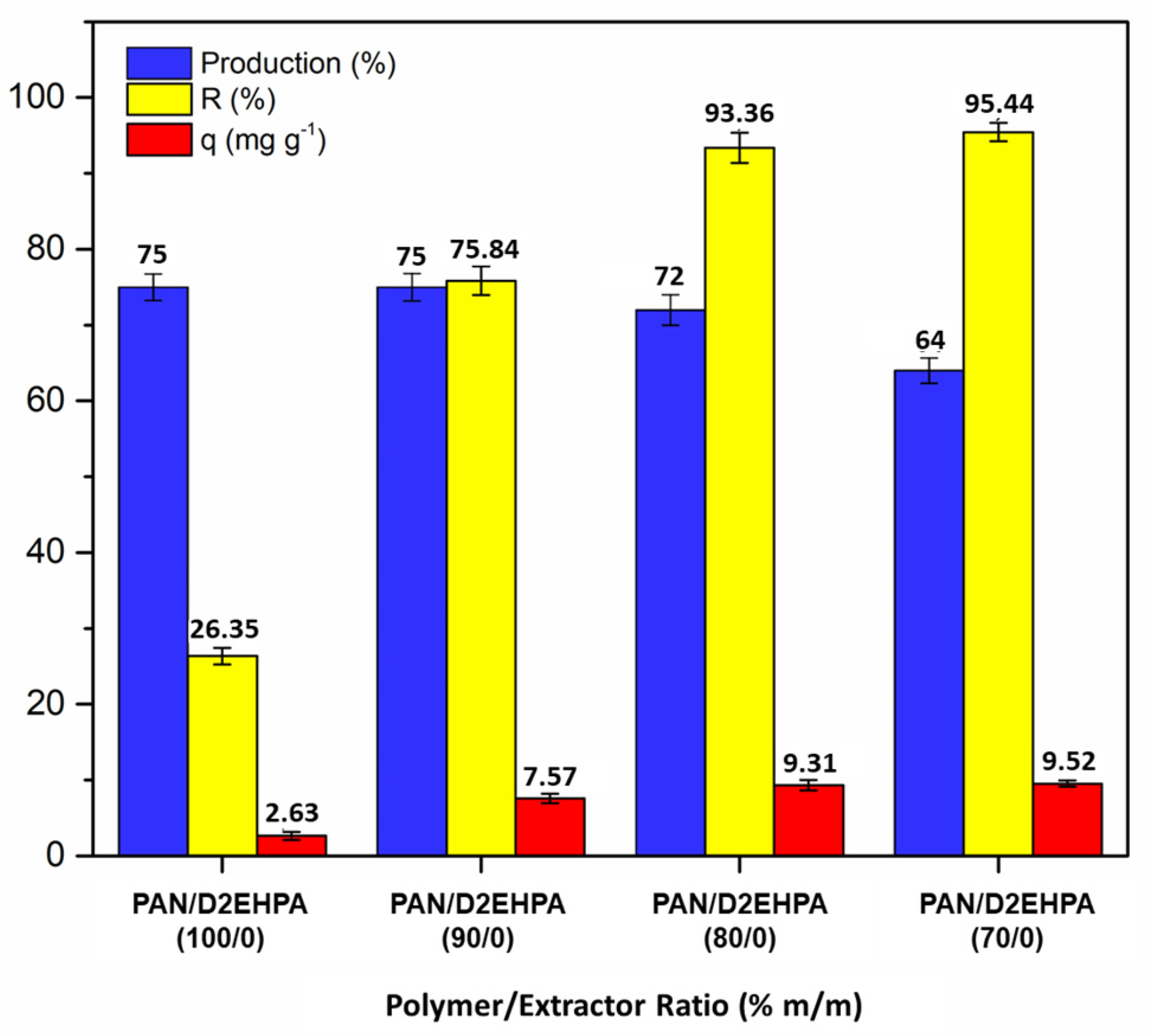
3.2.1. Effect of Polymer/Extractant Ratio
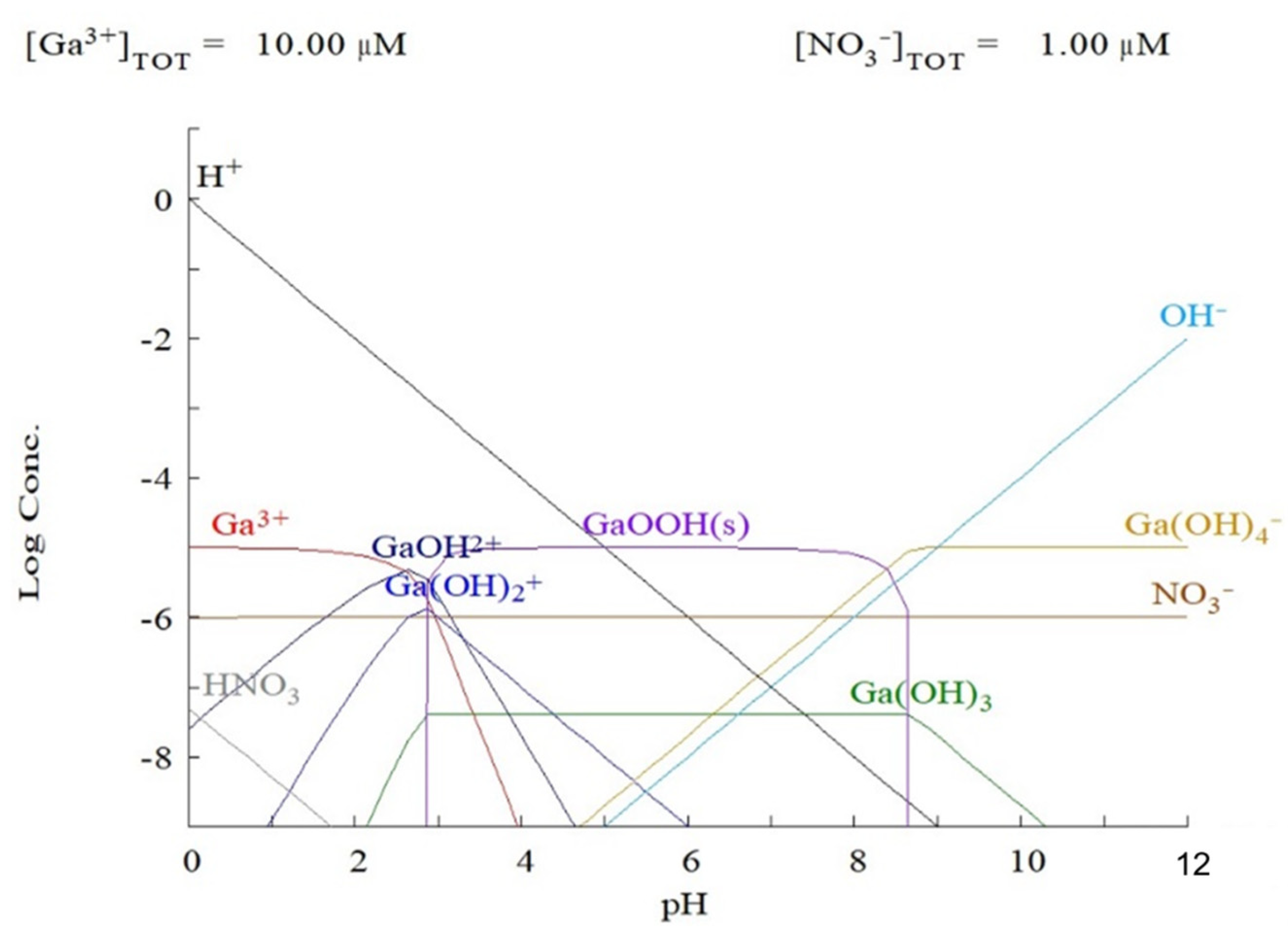
3.2.2. Effect of pH
3.2.3. Adsorption Kinetics: Model Selection and Fitting
3.2.4. Adsorption Equilibrium: Model Selection and Fitting
3.2.5. Thermodynamics Studies
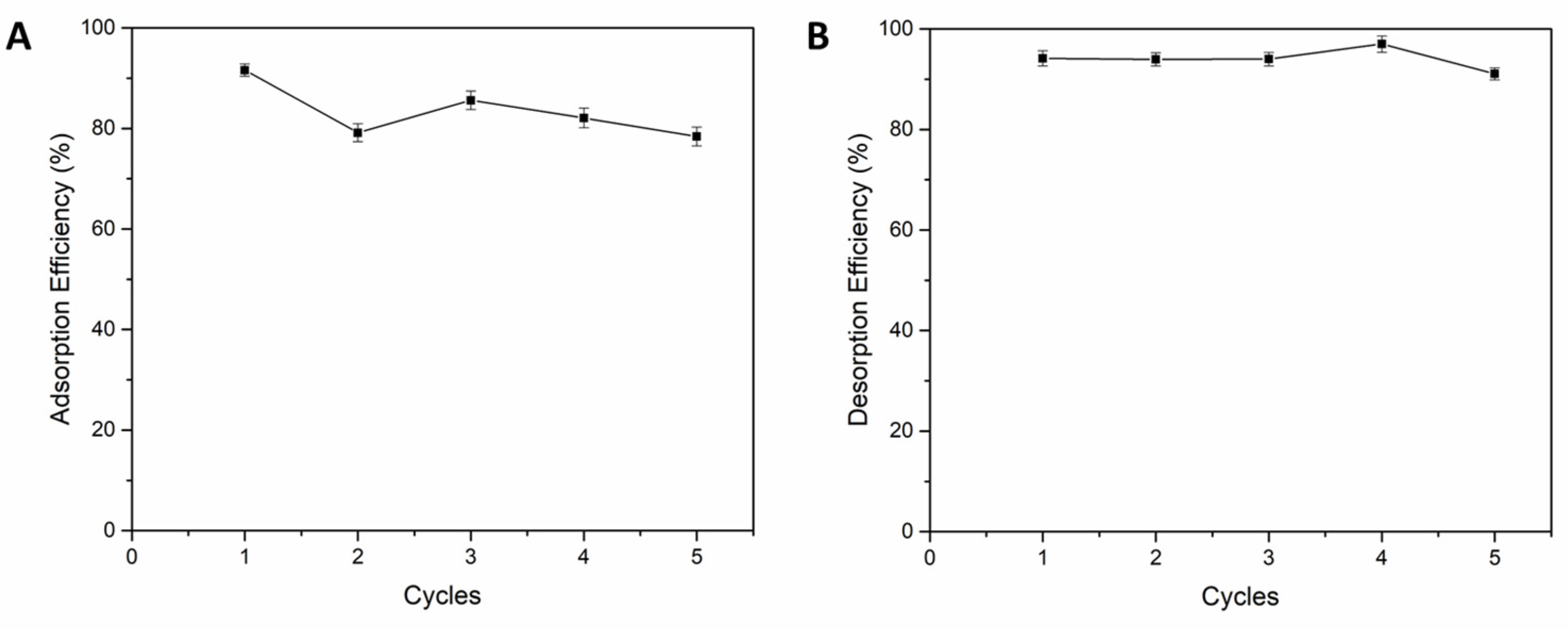
3.2.6. Desorption and Regeneration of Adsorbent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gladyshev, S.; Akcil, A.; Abdulvaliev, R.; Tastanov, Y.; Beisembekova, K.; Temirova, S. Kinetic study of gallium electrochemical reduction in alkaline solution. Hydrometallurgy 2013, 140, 95–101. [Google Scholar] [CrossRef]
- Gupta, B.; Mudhar, N.; Begum, Z.; Singh, I. Extraction and recovery of Ga (III) from waste material using Cyanex 923. Hydrometallurgy 2007, 87, 8–26. [Google Scholar] [CrossRef]
- Chen, W.-S.; Wang, Y.-C.; Chiu, K.-L. The separation and recovery of indium, gallium, and zinc from spent GZO(IGZO) targets. J. Environ. Chem. Eng. 2017, 5, 381–390. [Google Scholar] [CrossRef]
- Zhao, F.; Zou, Y.; Lv, X.; Liang, H.; Jia, Q.; Ning, W. Synthesis of CoFe2O4−Zeolite Materials and Application to the Adsorption of Gallium and Indium. J. Chem. Eng. Data 2015, 60, 1338–1344. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Hu, Y.; Yang, X.; Yao, S. Novel surface molecularly imprinted material modified multi-walled carbon nanotubes as solid-phase extraction sorbent for selective extraction gallium ion from fly ash. Talanta 2010, 82, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, L.; Liang, M.; Chen, Y.; Zou, H. Cross-linked polyvinyl amidoxime fiber: A highly selective and recyclable adsorbent of gallium from Bayer liquor. Iran. Polym. J. 2018, 27, 589–597. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Li, A.; Long, Q.; Huang, F.; Xiao, L.; Li, X.; Wang, J.; Xiao, Q.; et al. Recovery of gallium from Bayer red mud through acidic-leaching-ion exchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Mahamuni, S.V.; Wadgaornkar, P.P.; Anuse, M.A. Liquid–liquid extraction and recovery of gallium (III) from acid media with 2-octylaminopyridine in chloroform: Analysis of bauxite ore. J. Serbian Chem. Soc. 2010, 75, 1099–1113. [Google Scholar] [CrossRef]
- Zeng, C.; Gonzalez-Alvarez, A.; Orenstein, E.; Field, J.A.; Shadman, F.; Sierra-Alvarez, R. Ecotoxicity assessment of ionic As (III), As (V), In (III) and Ga (III) species potentially released from novel III-V semiconductor materials. Ecotoxicol. Environ. Saf. 2017, 140, 30–36. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Song, Y.; Lou, Z.; Shan, W.; Xiong, Y. Novel chitosan-based ions imprinted bio-adsorbent for enhanced adsorption of gallium (III) in acidic solution. J. Mol. Liq. 2020, 320, 114413. [Google Scholar] [CrossRef]
- Sun, W.; Qi, M.; Cheng, S.; Li, C.; Dong, B.; Wang, L. Gallium and gallium compounds: New insights into the ‘‘Trojan horse” strategy in medical applications. Mat. Des. 2023, 227, 111704. [Google Scholar] [CrossRef]
- Syu, C.-H.; Chen, P.-W.; Huang, C.-C.; Lee, D.-Y. Accumulation of gallium (Ga) and indium (In) in rice grains in Ga- and In-contaminated paddy soils. Environ. Pollut. 2020, 261, 114189. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.F.; Wang, S.L.; Yeh, K.C. Effect of Gallium Exposure in Arabidopsis thaliana is Similar to Aluminum Stress. Environ. Sci. Technol. 2017, 51, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Syu, C.-H.; Chien, P.-C.; Huang, C.-C.; Jiang, P.-Y.; Juang, K.-W.; Lee, D.-Y. The growth and uptake of Ga and In of rice (Oryza sative L.) seedlings as affected by Ga and In concentrations in hydroponic cultures. Ecotoxicol. Environ. Saf. 2017, 135, 32–39. [Google Scholar] [CrossRef]
- Batley, G.E.; Campbell, P.G.C. Metal contaminants of emerging concern in aquatic systems. Environ. Chem. 2022, 19, 23–40. [Google Scholar] [CrossRef]
- Yang, J.-L. Comparative acute toxicity of gallium(III), antimony(III), indium(III), cadmium(II), and copper(II) on freshwater swamp shrimp (Macrobrachium nipponense). Biol. Res. 2014, 13, 47. [Google Scholar] [CrossRef]
- Bu-Olayan, A.H.; Thomas, B.V. Bourgeoning impact of the technology critical elements in the marine environment. Environ. Pollut. 2020, 265, 115064. [Google Scholar] [CrossRef]
- Ahmed, I.M.; El-nadi, Y.A.; El-hefny, N.E. Extraction of gallium (III) from hydrochloric acid by Cyanex 923 and Cyanex 925. Hydrometallurgy 2013, 131–132, 24–28. [Google Scholar] [CrossRef]
- Liu, J.S.; Chen, H.; Chen, X.Y.; Guo, Z.L.; Hu, Y.C.; Liu, C.P.; Sun, Y.Z. Extraction and separation of In (III), Ga (III) and Zn (II) from sulfate solution using extraction resin. Hydrometallurgy 2006, 82, 137–143. [Google Scholar] [CrossRef]
- Lee, C.H.; Lin, H.Y.; Cadogan, E.I.; Popuri, S.R.; Chang, C.Y. Biosorption Performance of Biodegradable Polymer Powders for the Removal of Gallium (III) ions from Aqueous Solution. Pol. J. Chem. Technol. 2015, 17, 124–132. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; Jaafar, W.Z.; Alomar, M.K.; Fayaed, S.S.; Mohd, N.S.; Hin, L.S.; El-Shafie, A. Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.S.; Costa, M.T.; Almeida, R.L.; Abud, A.K.S.; Soletti, J.I.; Dotto, G.L.; Tanabe, E.H.; Sellaoui, L.; Carvalho, S.H.V.; et al. Adsorption of methylene blue on agroindustrial wastes: Experimental investigation and phenomenological modelling. Prog. Biophys. Mol. Biol. 2018, 141, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Chen, B.-Y.; Zhou, S.; Chang, C.-T. Removal of Cr (VI) using polyacrylonitrile chloride composite nanofibers. J. Taiwan Inst. Chem. Eng. 2017, 70, 401–410. [Google Scholar] [CrossRef]
- Dastbaz, A.; Keshtkar, A.R. Adsorption of Th4+, U6+, Cd2+ and Ni2+ from aqueous solution by anovel modified polyacrylonitrile composite nanofiber adsorbent prepared by electrospinning. Appl. Surf. Sci. 2010, 293, 336–344. [Google Scholar] [CrossRef]
- Haddad, M.; Alharbi, H. Enhancement of heavy metal ion adsorption using electrospun polyacrylonitrile nanofibers loaded with ZnO nanoparticles. J. Appl. Polym. Sci. 2018, 136, 1–11. [Google Scholar] [CrossRef]
- Kummer, G.; Schonhart, C.; Fernandes, M.G.; Dotto, G.L.; Missio, A.L.; Bertuol, D.A.; Tanabe, E.H. Development of Nanofibers Composed of Chitosan/Nylon 6 and Tannin/Nylon 6 for Effective Adsorption of Cr (VI). J. Polym. Environ. 2018, 26, 4073–4084. [Google Scholar] [CrossRef]
- Martins, T.R.; Costa, P.S.; Bertuol, D.A.; Aguiar, M.L.; Tanabe, E.H. Development of Recycled Expanded Polystyrene Nanofibers Modified by Chitosan for the Removal of Lead (II) from Water. Metals 2022, 12, 1334. [Google Scholar] [CrossRef]
- Alnaqbi, M.A.; Greish, Y.E.; Mohsin, M.; Elumalai, E.J.; Blooshi, A. Morphological variations of micro-nanofibrous sorbents prepared by electrospinning and their effects on the sorption of crude oil. J. Environ. Chem. Eng. 2016, 4, 1850–1861. [Google Scholar] [CrossRef]
- Riahi, D.N. Modeling and computation of nonlinear rotating polymeric jets during forcespinning process. Int. J. Non-Linear Mech. 2017, 92, 1–7. [Google Scholar] [CrossRef]
- Zander, N.E. Formation of melt and solution spun polycaprolactone fibers by centrifugal spinning. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Alabduljabbar, F.A.; Haider, S.; Alghyamah, A.A.; Haider, A.; Khan, R.; Almasry, W.A.; Patel, R.; Mujtaba, I.M.; Ali, F.A.A. Ethanol amine functionalized electrospun nanofibers membrane for the treatment of dyes polluted wastewater. Appl. Nanosci. 2022, 12, 3153–3166. [Google Scholar] [CrossRef]
- Padron, S.; Fuentes, A.; Caruntu, D.; Lozano, K. Experimental study of nanofiber production through force spinning. J. Appl. Phys. 2013, 113, 024318. [Google Scholar] [CrossRef]
- Salussoglia, A.I.P.; Tanabe, E.H.; Aguiar, M.L. Evaluation of a vacuum collection system in the preparation of PAN fibers by forcespinning for application in ultrafine particle filtration. J. Appl. Polym. Sci. 2020, 137, 49334. [Google Scholar] [CrossRef]
- Nakamura, T.; Sakai, A.; Nishihama, S.; Yoshizuka, K. Solvent Extraction of Indium, Gallium, and Zinc Ions with Acidic Organophosphates having Bulky Alkyl Groups. Solvent Extr. Ion Exch. 2009, 27, 501–512. [Google Scholar] [CrossRef]
- Yadav, K.K.; Singh, D.K.; Anitha, M.; Varshney, L.; Singh, S. Studies on separation of rare earths from aqueous media by polyethersulfone beads containing D2EHPA as extractant. Sep. Purif. Technol. 2013, 118, 350–358. [Google Scholar] [CrossRef]
- Cadore, J.S.; Bertuol, D.A.; Tanabe, E.H. Recovery of indium from LCD screens using solid-phase extraction onto nanofibers modified with Di-(2-ethylhexyl) phosphoric acid (DEHPA). Process Saf. Environ. Prot. 2019, 127, 141–150. [Google Scholar] [CrossRef]
- Silva, F.N.; Bassaco, M.M.; Bertuol, D.A.; Tanabe, E.H. An eco-friendly approach for metals extraction using polymeric nanofibers modified with di-(2 ethylhexyl) phosphoric acid (DEHPA). J. Clean. Prod. 2019, 210, 786–794. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Zhang, S.; Xu, G.; Fu, K.; Lee, H.; Zhang, X. Parameter study and characterization for polyacrylonitrile nanofibers fabricated via centrifugal spinning process. Eur. Polym. J. 2013, 49, 3834–3845. [Google Scholar] [CrossRef]
- Agubra, V.A.; De la Garza, D.; Gallegos, L.; Alcoutlabi, M. ForceSpinning of polyacrylonitrile for mass production of lithium-ion battery separators. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Segala, B.N.; Bertuol, D.A.; Tanabe, E.H. Production of polyacrylonitrile nanofibres modified with Cyanex 272 for recovery of gallium from solution. Environ. Technol. 2022, 43, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Chai, Y.; Hua, Z.; Xiao, Y.; Yang, Y. Adsorption Performances and Mechanisms of Amidoxime Resin toward Gallium (III) and Vanadium (V) from Bayer Liquor. ACS Sustain. Chem. Eng. 2016, 4, 53–59. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Z.; Yue, W.; Zhenning, L.; Weijun, S.; Ying, X.; Yu, F. Preparation of a biomass adsorbent for gallium (III) based on corn stalk modified by iminodiacetic acid. J. Taiwan Inst. Chem. Eng. 2018, 91, 291–298. [Google Scholar] [CrossRef]
- Qin, J.; Liu, G.; Fan, H.; Tan, W. The hydrophobic mechanism of di(2-ethylhexyl) phosphoric acid to hemimorphite flotation. Colloids Surf. A. 2018, 545, 68–77. [Google Scholar] [CrossRef]
- Elias, A.; Didi, M.A.; Villemin, D.; Semaoune, T.; Ouattas, S. Synthesis of mono-and dialkylphosphates by the reactions of hydroxycompounds with the phosphorus pentaoxide under microwave irradiation. Phosphorus Sulfur Silicon Relat. Elem. 2010, 179, 2599–2607. [Google Scholar] [CrossRef]
- Martín, D.M.; Faccini, M.; García, M.A.; Amantia, D. Highly efficient removal of heavy metal ions from polluted water using ion-selective polyacrylonitrile nanofibers. J. Environ. Chem. Eng. 2018, 6, 236–245. [Google Scholar] [CrossRef]
- Karbownik, I.; Rac-Rumijowska, O.; Fiedot-Toboła, M.; Rybicki, T.; Teterycz, H. The Preparation and Characterization of Polyacrylonitrile-Polyaniline (PAN/PANI) Fibers. Materials 2019, 12, 664. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Li, P.; Zhang, W. Effective Cd2+ chelating fiber based on polyacrylonitrile. React. Funct. Polym. 2009, 69, 48–54. [Google Scholar] [CrossRef]
- Zheng, P.; Zhang, W.; Li, C.; Wang, N.; Li, J.; Qin, Z.; An, Q. Efficient bio ethanol recovery by non-contact vapor permeation process using membranes with tailored pore size and hydrophobicity. Chem. Eng. Sci. 2019, 207, 448–455. [Google Scholar] [CrossRef]
- Dutta, M.; Bhattacharjee, S.; De, S. Separation of reactive dyes from textile effluent by hydrolyzed polyacrylonitrile hollow fiber ultrafiltration quantifying the transport of multicomponent species through charged membrane pores. Sep. Purif. Technol. 2020, 234, 116063. [Google Scholar] [CrossRef]
- An, Q.; Sun, W.; Zhao, Q.; Ji, Y.; Gao, C. Study on a novel nanofiltration membrane prepared by interfacial polymerization with zwitterionic amine monomers. J. Mem. Sci. 2013, 431, 171–179. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Bey, S.; Clarizia, G.; Mansouri, L.; Benamor, M. Gas permeation behavior of CTA polymer inclusion membrane (PIM) containing an acidic carrier for metal recovery (DEHPA). Sep. Purif. Technol. 2011, 80, 38–44. [Google Scholar] [CrossRef]
- Satpathy, S.; Mishra, S. Extractive separation studies of Ga (III) and Ni (II) in the presence of lactic acid using DEHPA in petrofin. Sep. Purif. Technol. 2017, 179, 513–522. [Google Scholar] [CrossRef]
- Singh, K.K.; Pathak, S.K.; Kumar, M.; Mahtele, A.K.; Tripathi, S.C.; Bajaj, P.N. Study of uranium sorption using D2EHPA-impregnated polymeric beads. J. Appl. Polym. Sci. 2013, 130, 3355–3364. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Chen, J. Preparation of Cyanex 272-containing silica composite by sol–gel method for neodymium adsorption. J. Radioanal. Nucl. Chem. 2013, 295, 1667–1672. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Bhargava, P. Influence of spinning parameters on synthesis of alumina fibers by centrifugal spinning. Ceram. Int. 2018, 44, 11644–11649. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; White, C.J.B.; Ning, X.; Nie, H.; Zhu, L. Studies on electrospun nylon-6/chitosan complex nanofiber interactions. Electrochim. Acta 2009, 54, 5739–5745. [Google Scholar] [CrossRef]
- Li, W.; Tang, Y.; Zeng, Y.; Tong, Z.; Liang, D.; Cui, W. Adsorption behavior of Cr (VI) ions on tannin-immobilized activated clay. Chem. Eng. J. 2012, 193–194, 88–95. [Google Scholar] [CrossRef]
- Chegrouche, S.; Bensmaili, A. Removal of Ga (III) from aqueous solution by adsorption on activated bentonite using a factorial design. Water Res. 2002, 36, 2898–2904. [Google Scholar] [CrossRef]
- Chou, W.L.; Wang, C.T.; Huang, Y.H. Removal of gallium ions from aqueous solutions using tea waste by adsorption. Fresenius Environ. Bull. 2010, 19, 2848–2856. [Google Scholar]
- Zhang, L.; Zhu, Y.; Li, H.; Liu, N.; Liu, X.; Guo, X. Kinetic and thermodynamic studies of adsorption of gallium (III) on nano-TiO2. Rare Met. 2010, 29, 16–20. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic parameters of liquid-phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G. Adsorption, Ion Exchange and Catalysis: Design of Operations and Environmental Applications, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2006; p. 602. [Google Scholar]




| Model | Temperature (°C) | |||
|---|---|---|---|---|
| 25 | 35 | 45 | 55 | |
| PFO | ||||
| q1 (mg g−1) | 9.1583 ± 0.2065 | 9.5110 ± 0.1374 | 10.0030 ± 0.0925 | 10.3919 ± 0.08546 |
| k1 (min−1) | 0.1222 ± 0.0112 | 0.3699 ± 0.0340 | 0.3713 ± 0.0219 | 0.3415 ± 0.0171 |
| R2 | 0.9796 | 0.9847 | 0.9937 | 0.9952 |
| 0.9773 | 0.9830 | 0.9930 | 0.9947 | |
| ARE (%) | 5.49 | 3.24 | 2.18 | 1.96 |
| MSE | 0.19 | 0.13 | 0.06 | 0.05 |
| PSO | ||||
| q2 (mg g−1) | 9.8419 ± 0.1799 | 9.8698 ± 0.1173 | 10.3803 ± 0.1077 | 10.8444 ± 0.09912 |
| k2 (g mg−1 min−1) | 0.0189 ± 0.0020 | 0.0779 ± 0.0095 | 0.0813 ± 0.0109 | 0.0604 ± 0.0052 |
| R2 | 0.9899 | 0.9926 | 0.9944 | 0.9958 |
| 0.9887 | 0.9918 | 0.9938 | 0.9953 | |
| ARE (%) | 4.51 | 2.42 | 1.85 | 1.77 |
| MSE | 0.09 | 0.06 | 0.05 | 0.04 |
| Elovich | ||||
| a (mg g−1 min−1) | 0.7536 ± 0.1027 | 1.8346 ± 0.4432 | 1.7258 ± 0.4089 | 1.4505 ± 0.3184 |
| b (g mg−1) | 13.5961 ± 9.1638 | 2.95 × 105 ± 1.1080 | 2.61 × 105 ± 9.54 × 105 | 3.50 × 104 ± 1.02 × 105 |
| R2 | 0.9414 | 0.9624 | 0.9630 | 0.9590 |
| 0.9348 | 0.9582 | 0.9589 | 0.9544 | |
| ARE (%) | 10.08 | 6.02 | 5.73 | 5.95 |
| MSE | 0.55 | 0.31 | 0.04 | 0.05 |
| Model | Temperature (°C) | |||
|---|---|---|---|---|
| 25 | 35 | 45 | 55 | |
| Langmuir | ||||
| qm (mg g−1) | 26.0781 ± 2.0044 | 28.2560 ± 1.7170 | 29.8030 ± 1.3662 | 30.0122 ± 1.4174 |
| kL (L mg−1) | 0.4702 ± 0.2701 | 0.5307 ± 0.2039 | 2.0421 ± 0.6571 | 2.6579 ± 0.9394 |
| R2 | 0.7742 | 0.8511 | 0.8902 | 0.8814 |
| 0.7291 | 0.8213 | 0.8682 | 0.8577 | |
| ARE (%) | 14.66 | 9.23 | 9.95 | 7.88 |
| MSE | 9.01 | 7.07 | 6.00 | 6.72 |
| Freundlich | ||||
| kF (mg g−1) (mg L−1)−1/nF | 10.9141 ± 1.2778 | 12.2427 ± 1.5929 | 16.8224 ± 1.8976 | 17.1351 ± 1.6963 |
| 1/nF | 0.2156 | 0.2083 | 0.1519 | 0.1501 |
| R2 | 0.9371 | 0.9109 | 0.8761 | 0.9051 |
| 0.9245 | 0.8931 | 0.8513 | 0.8861 | |
| ARE (%) | 6.73 | 10.47 | 12.36 | 10.02 |
| MSE | 2.51 | 4.23 | 6.77 | 5.38 |
| BET | ||||
| qm (mg g−1) | 19.2934 ± 1.0124 | 22.4484 ± 1.6436 | 26.0702 ± 1.6906 | 25.7405 ± 1.1103 |
| kS (L mg−1) | 1.4101 ± 0.4519 | 0.9657 ± 0.3324 | 2.7847 ± 0.8487 | 3.8718 ± 0.8709 |
| kR (L mg−1) | 0.0042 ± 5.44864 × 10−4 | 0.0036 ± 8.47457 × 10−4 | 0.0026 ± 9.3356 × 10−4 | 0.0029 ± 5.75802 × 10−4 |
| R2 | 0.9727 | 0.9591 | 0.9546 | 0.97761 |
| 0.9590 | 0.9387 | 0.9319 | 0.96642 | |
| ARE (%) | 3.74 | 6.30 | 7.13 | 5.10 |
| MSE | 1.09 | 1.94 | 2.48 | 1.27 |
| qexperimental | 30.19 | 31.96 | 32.92 | 33.13 |
| Adsorbent | pH | T (°C) | Ce (mg L−1) | q (mg g−1) | Reference |
|---|---|---|---|---|---|
| P507 extraction resin | 3.0 | 40 | 93.3 | 30.4 | [19] |
| Amidoxime resin (LSC700) | 2.0 | 45 | 290 | 29.24 | [44] |
| Bentonite | 2.5 | 20 | 203.77 | 10.67 | [67] |
| Discarded tea | 3.0 | 25 | 80 | 10.9 | [68] |
| Nano-TiO2 | 3.0 | 40 | 10.0 | 8.00 | [69] |
| Polyacrylonitrile nanofibers with D2EHPA | 2.5 | 45 | 85.0 | 33.13 | This work |
| Temperature (K) | ∆G0 (kJ mol−1) | ∆H0 (kJ mol−1) | ∆S0 (kJ mol−1 K−1) |
|---|---|---|---|
| 298 | −29.46 | 32.82 | 0.21 |
| 308 | −31.55 | ||
| 318 | −34.69 | ||
| 328 | −35.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segala, B.N.; Wenzel, B.M.; Power, N.P.; Krishnamurthy, S.; Bertuol, D.A.; Tanabe, E.H. Efficient Gallium Recovery from Aqueous Solutions Using Polyacrylonitrile Nanofibers Loaded with D2EHPA. Metals 2023, 13, 1545. https://doi.org/10.3390/met13091545
Segala BN, Wenzel BM, Power NP, Krishnamurthy S, Bertuol DA, Tanabe EH. Efficient Gallium Recovery from Aqueous Solutions Using Polyacrylonitrile Nanofibers Loaded with D2EHPA. Metals. 2023; 13(9):1545. https://doi.org/10.3390/met13091545
Chicago/Turabian StyleSegala, Bibiane Nardes, Bruno München Wenzel, Nicholas P. Power, Satheesh Krishnamurthy, Daniel Assumpção Bertuol, and Eduardo Hiromitsu Tanabe. 2023. "Efficient Gallium Recovery from Aqueous Solutions Using Polyacrylonitrile Nanofibers Loaded with D2EHPA" Metals 13, no. 9: 1545. https://doi.org/10.3390/met13091545
APA StyleSegala, B. N., Wenzel, B. M., Power, N. P., Krishnamurthy, S., Bertuol, D. A., & Tanabe, E. H. (2023). Efficient Gallium Recovery from Aqueous Solutions Using Polyacrylonitrile Nanofibers Loaded with D2EHPA. Metals, 13(9), 1545. https://doi.org/10.3390/met13091545







