Phase-Field Simulation and Dendrite Evolution Analysis of Solidification Process for Cu-W Alloy Contact Materials under Arc Ablation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phase-Field Model of Cu-W Alloy
2.2. Cu-W Alloy Phase-Field Model Calculation Method
3. Dendritic Morphology during the Solidification Process of Cu-W Alloy
3.1. Parameters of Cu-W Alloy Solidification Computation
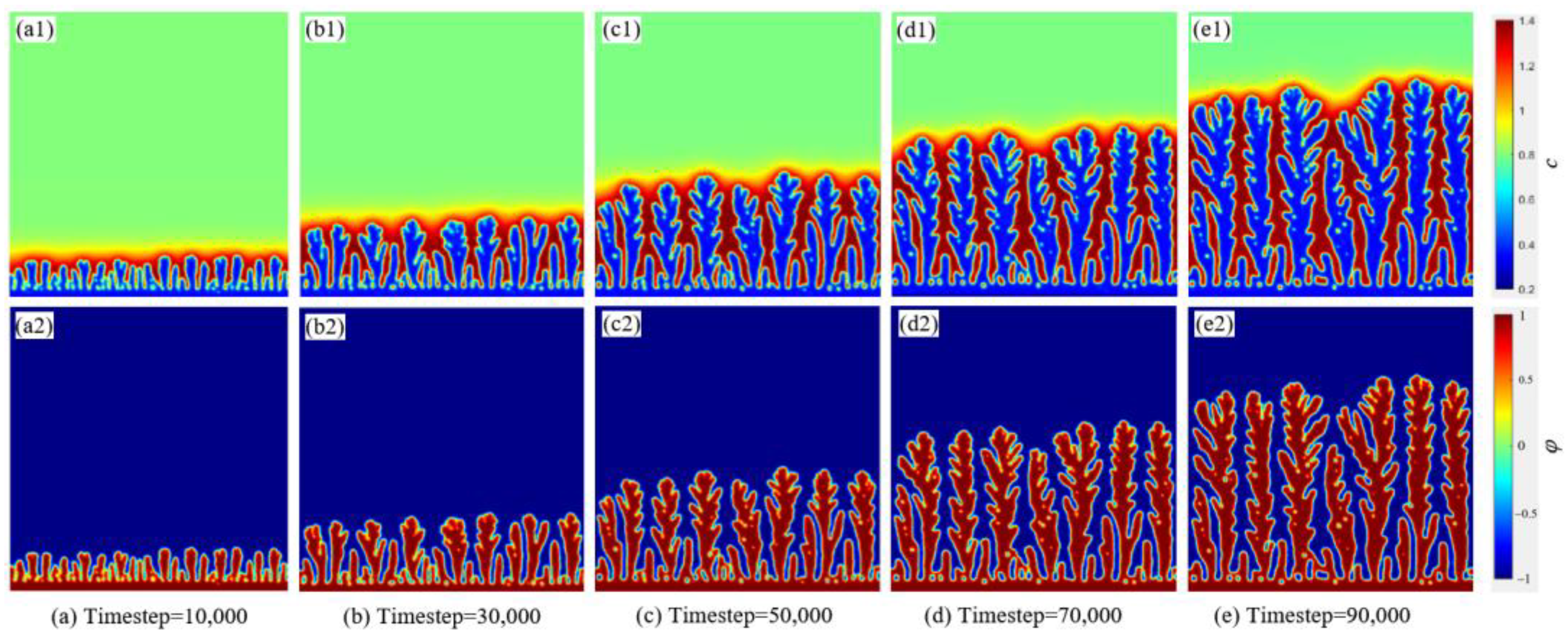
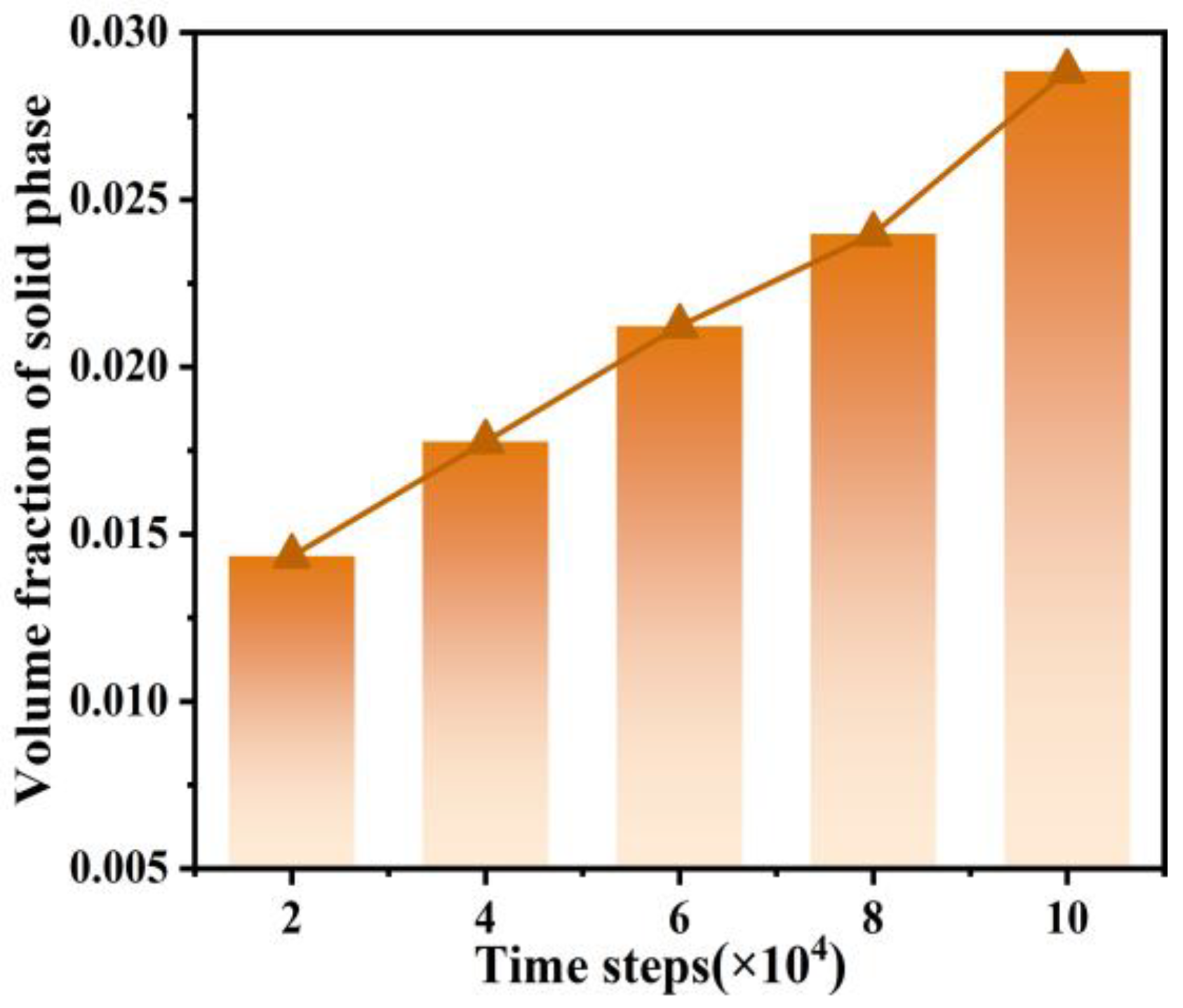
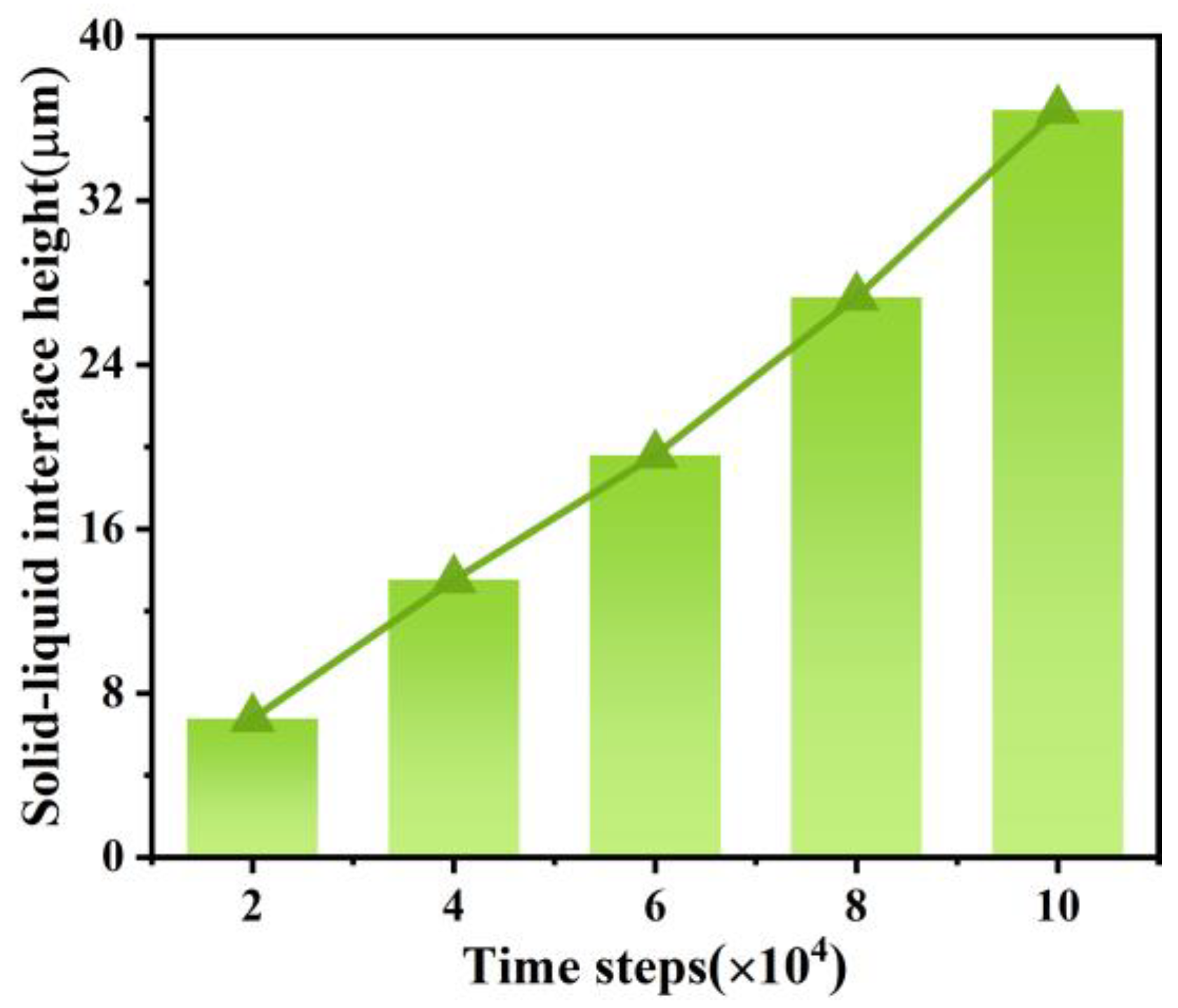
3.2. The Dendritic Morphology at Different Timesteps
4. Analysis of Influencing Factors on Dendritic Morphology of Cu-W Alloy
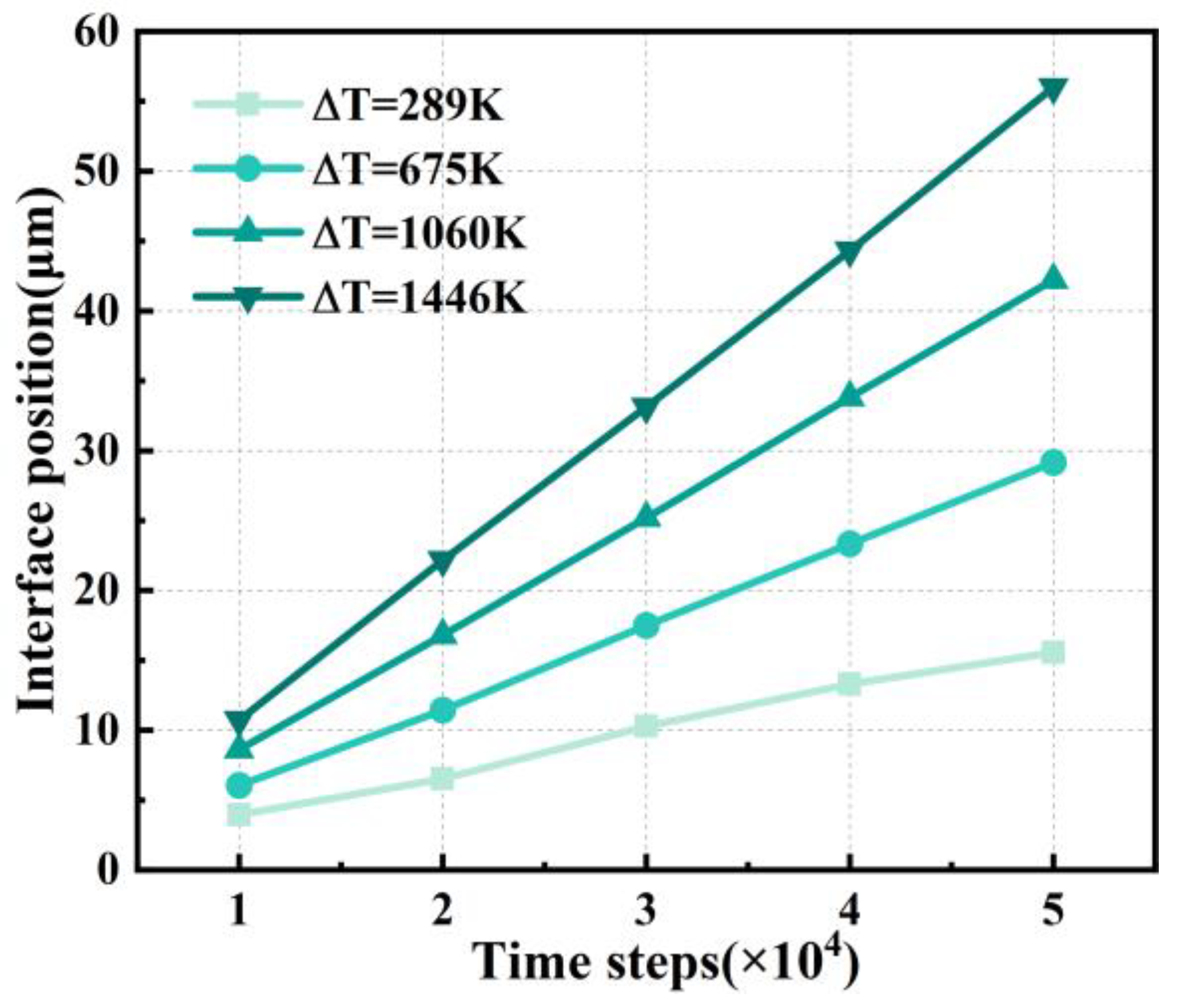
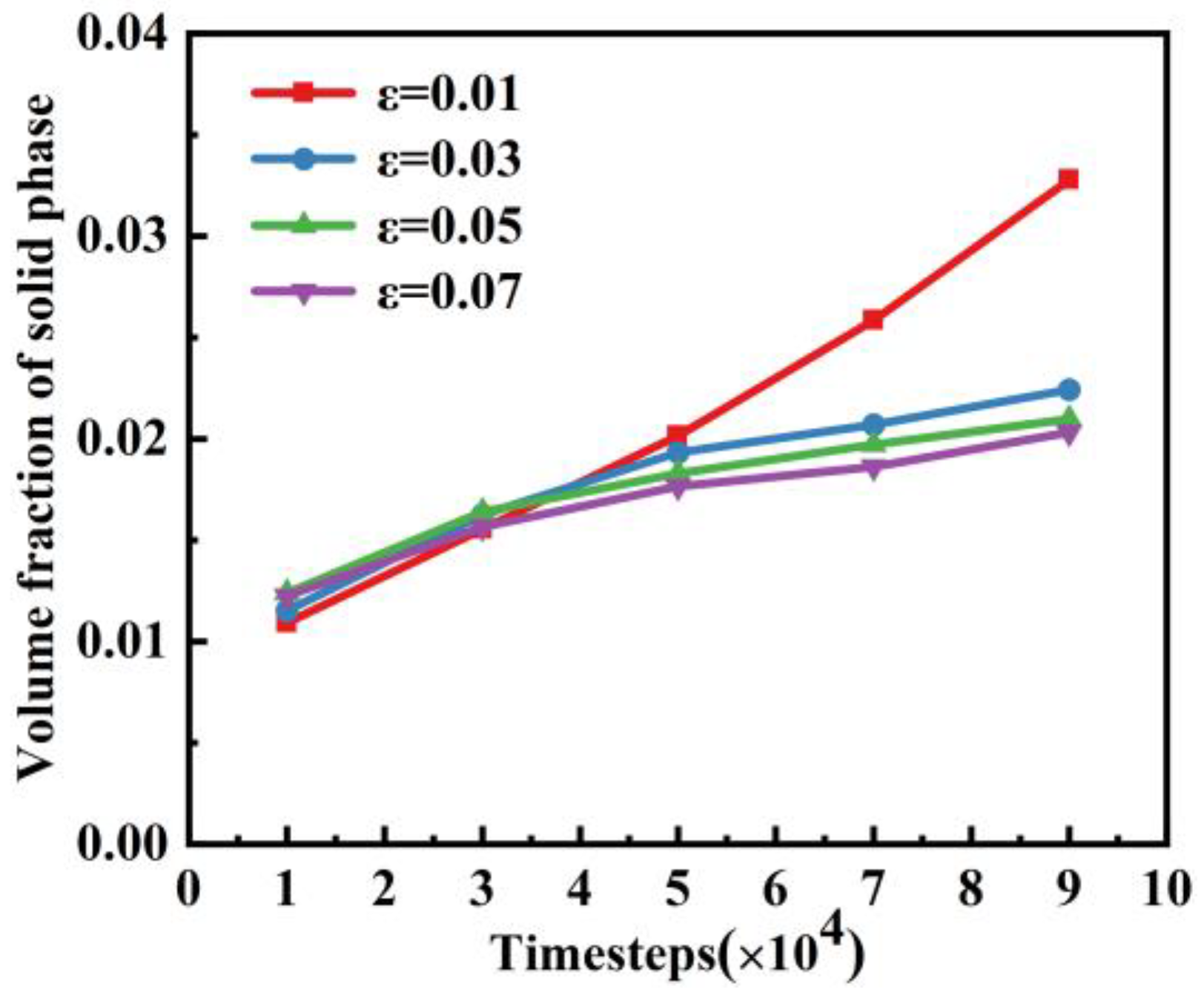
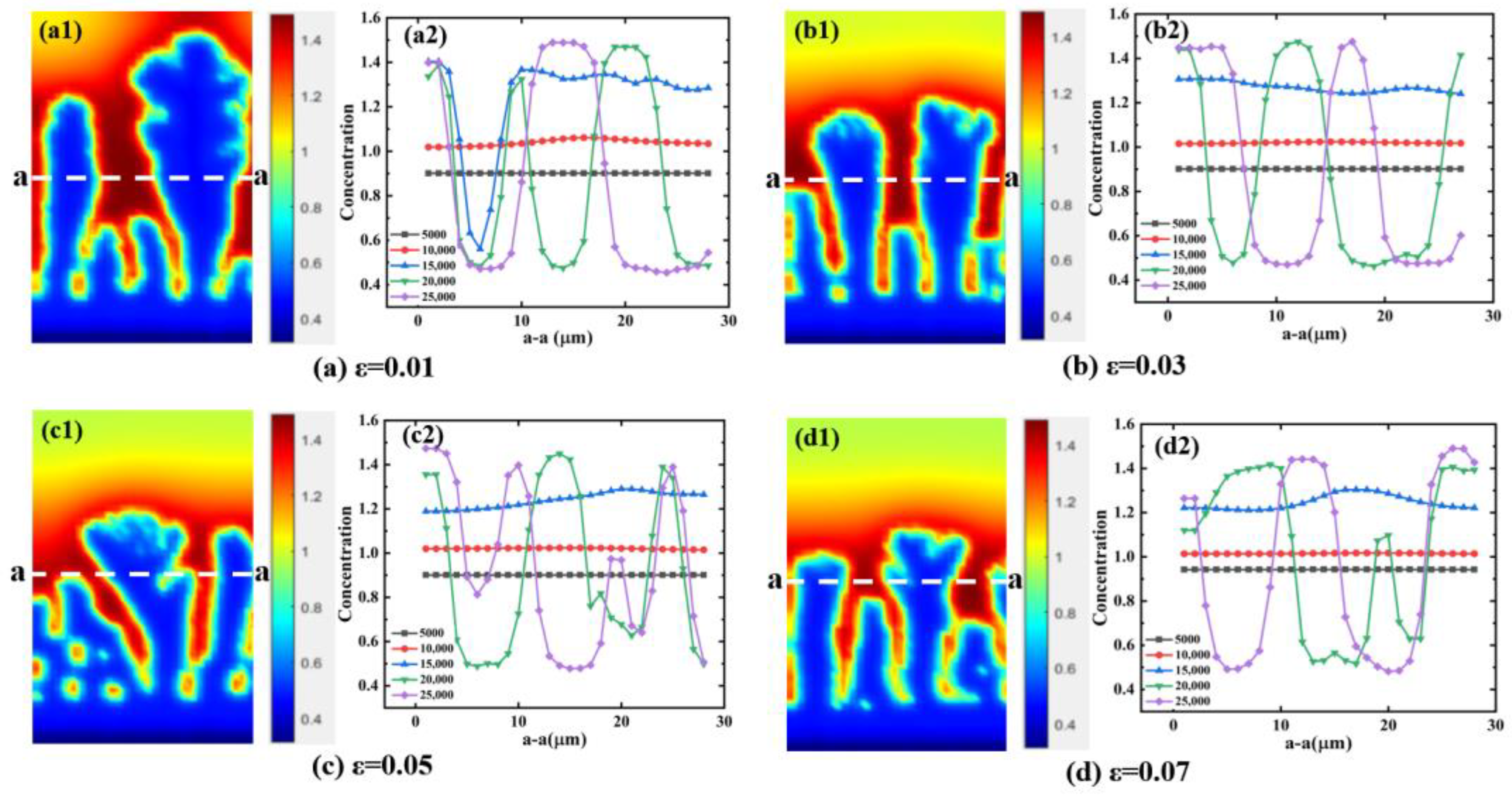
4.1. Effect of Supercooling on Dendritic Morphology
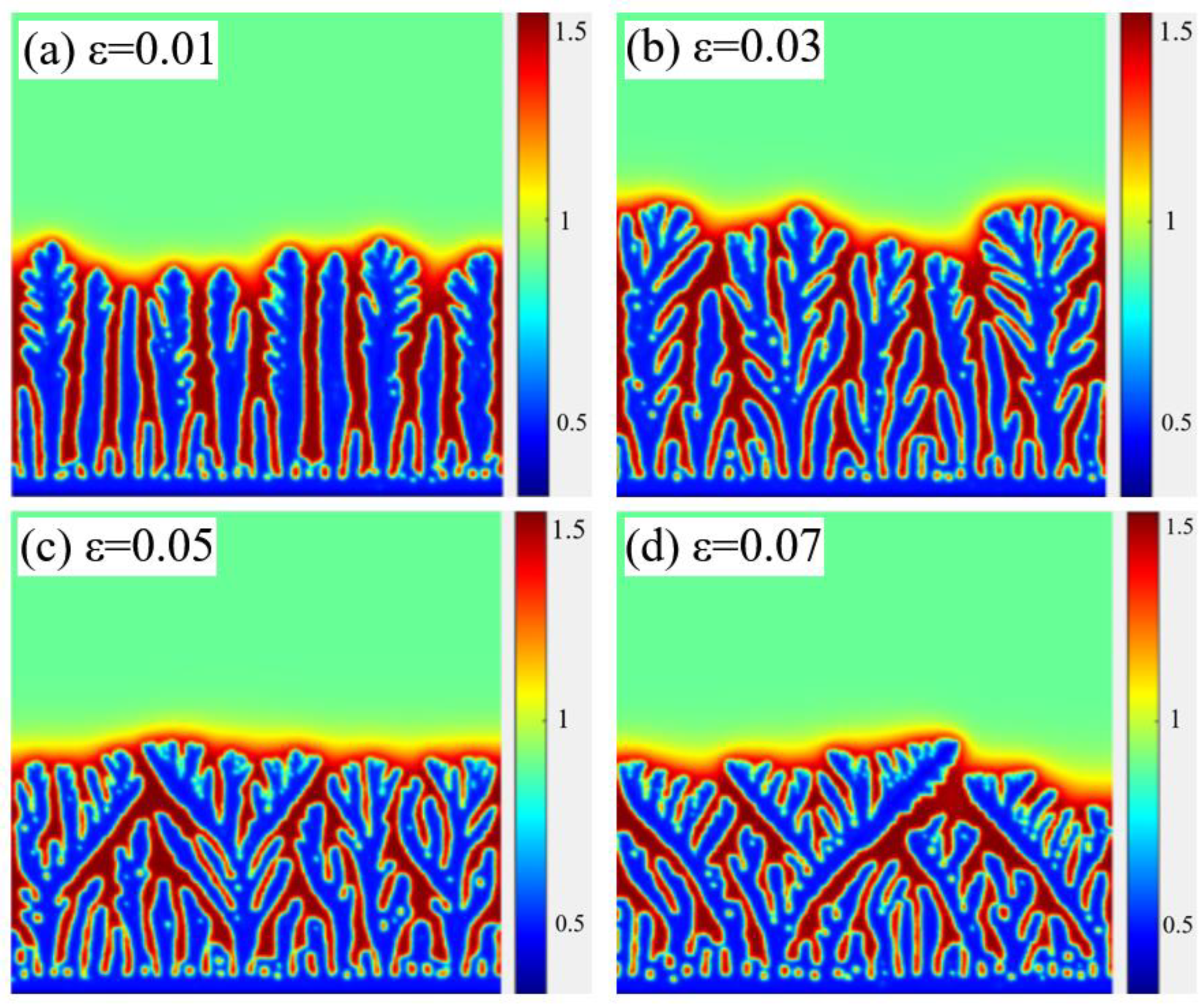
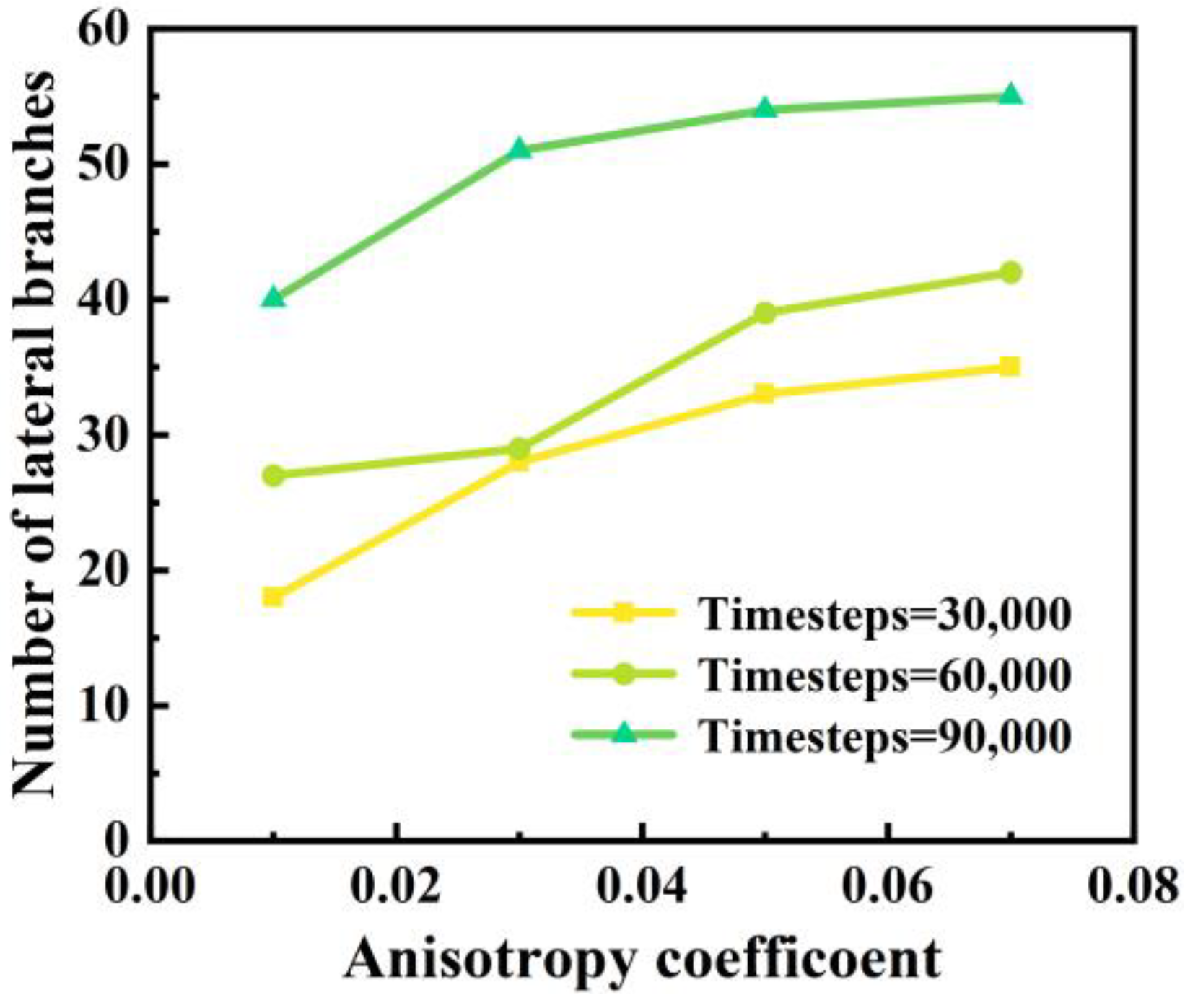
4.2. Influence of Anisotropy Coefficient on Dendritic Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, Z.Y.; Li, Q.M.; Li, J.K.; Wang, M.X.; Ren, H.W.; Zou, L. Phase field model for electric-thermal coupled discharge breakdown of polyimide nanocomposites under high frequency electrical stress. Plasma Sci. Technol. 2024, 26, 025505. [Google Scholar] [CrossRef]
- Li, C.; Hu, J.; Lin, C.; Zhang, B.; Zhang, G.; He, J. Surface charge migration and dc surface flashover of surface-modified epoxy-based insulators. J. Phys. D Appl. Phys. 2017, 50, 065301. [Google Scholar] [CrossRef]
- Long, Z.; Li, J.F.; Yin, L.M.; Zhang, H.H.; Xu, M.R.; Yao, Z.X. Phase field simulation of intermetallic compound evolution at the interface of copper–tin coating under thermal–mechanical–electrical diffusion coupling. J. Electron. Mater. 2023, 52, 3321. [Google Scholar]
- Jiang, Y.; Xiu, S.; Yang, J.; Tan, T.; Liu, Z.; Chu, B. Investigation on the arc root characteristics of vacuum arc between the iron-core cup-shaped transverse magnetic field contacts. Phys. Plasmas 2022, 29, 043505. [Google Scholar] [CrossRef]
- Biswas, S.; Liu, D.; Aagesen, L.K.; Jiang, W. Solidification and grain formation in alloys: A 2D application of the grand-potential-based phase-field approach model. Model. Simul. Mater. Sci. Eng. 2022, 30, 025013. [Google Scholar] [CrossRef]
- Li, Y.R.Y.; Fu, C.C.; Wan, B.; Yan, X.J.; Zhang, W.F.; Li, W. Phase-field modelling of lead-free solder joint void growth under thermal-electrical coupled stress. J. Electron. Mater. 2022, 51, 259. [Google Scholar] [CrossRef]
- Liu, K.; Yang, F.; Wang, S.; Gao, B.; Xu, C. The research on the heat source characteristics and the equivalent heat source of the arc in gaps. Int. J. Heat Mass Transf. 2018, 124, 177. [Google Scholar] [CrossRef]
- Khakpour, A.; Uhrlandt, D.; Methling, R.-P.; Gortschakow, S.; Franke, S.; Imani, M.T.; Weltmann, K.-D. Impact of temperature changing on voltage and power of an electric arc. Electr. Power Syst. Res. 2017, 143, 73. [Google Scholar] [CrossRef]
- Geng, H.; Li, J.; Xiong, J.; Lin, X. Optimisation of interpass temperature and heat input for wire and arc additive manufacturing 5A06 aluminium alloy. Sci. Technol. Weld. Join. 2017, 22, 472. [Google Scholar] [CrossRef]
- Guo, C.; Li, J.; Wang, Z.; Wang, J. Non-uniplanar competitive growth of columnar dendritic grains during directional solidification in quasi-2D and 3D configurations. Mater. Des. 2018, 151, 141. [Google Scholar] [CrossRef]
- Tan, J.H.K.; Sing, S.L.; Yeong, W.Y. Microstructure modelling for metallic additive manufacturing: A review. Virtual Phys. Prototyp. 2020, 15, 87. [Google Scholar] [CrossRef]
- Karma, A. Phase-field formulation for quantitative modeling of alloy solidification. Phys. Rev. Lett. 2001, 87, 115701. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.Z.; Chen, Y.; Cao, Y.F.; Kang, X.H.; Li, D.Z. Fast simulations of a large number of crystals growth in centimeter-scale during alloy solidification via nonlinearly preconditioned quantitative phase-field formula. Comp. Mater. Sci. 2018, 147, 338. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, K.; Hou, H.; Chen, L.Q. Role of interfacial energy anisotropy in dendrite orientation in Al-Zn alloys: A phase field study. Mater. Des. 2022, 216, 110555. [Google Scholar] [CrossRef]
- Liu, Z.H.; Feng, Y.; Jiang, N.Y.; Zhou, Z.J.; Wang, F.; Li, X.C.; Yu, M. Arc erosion behavior of Cu/Ti3SiC2 cathodes in c-C4F8 gas as a substitute for SF6 gas. J. Electron. Mater. 2023, 52, 7818. [Google Scholar] [CrossRef]
- Shao, L.; Chen, B.; Yang, W.; He, J.; Tan, J. Numerical modeling of ice crystal dendritic growth with humidity by using an anisotropic phase field model. Cryst. Growth Des. 2024, 24, 1400. [Google Scholar] [CrossRef]
- Zhao, D.W.; Li, J.F. Phase-field modeling of the effect of liquid-solid interface anisotropies on free dendritic growth. Acta Phys. Sin. 2009, 58, 7094. [Google Scholar] [CrossRef]
- Echebarria, B.; Folch, R.; Karma, A.; Plapp, M. Quantitative phase-field model of alloy solidification. Phys. Rev. E 2004, 70, 061604. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Warren, J.A.; Beckermann, C.; Karma, A. Phase-field simulation of solidification. Annu. Rev. Mater. Res. 2002, 32, 163. [Google Scholar] [CrossRef]
- Li, M.E.; Yang, G.C.; Zhou, Y.H. Phase field modeling of directional solidification of a binary alloy at high velocities. Acta Phys. Sin. 2005, 54, 454. [Google Scholar]
- Danilov, D.; Nestler, B. Phase-field modelling of solute trapping during rapid solidification of a Si–As alloy. Acta Mater. 2006, 54, 4659. [Google Scholar] [CrossRef]
- Provatas, N.; Elder, K. Phase-Field Methods in Materials Science and Engineering, 1st ed.; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Meng, Y.; Shen, Y.; Chen, C.; Li, Y.; Feng, X. Effects of Cu content and mechanical alloying parameters on the preparation of W–Cu composite coatings on copper substrate. J. Alloys Compd. 2014, 75, 585. [Google Scholar] [CrossRef]
- SGTE 2022 Alloy Phase Diagrams. Available online: https://www.Crct.Polymtl.Ca/Fact/Documentation/#Opennewwindow (accessed on 10 September 2024).
- Long, W.Y.; Xia, C.; Xiong, B.W.; Fang, L.G. Phase-field simulation of dendritic growth in a binary alloy with thermodynamics data. Chin. Phys. B 2008, 17, 1078. [Google Scholar]
- Li, D.R.; Cai, Y.X.; Liu, Z.Y.; Wang, E.D. Modelling of physical properties of W-Cu alloy and calculation of theoretical values. China Tungsten Ind. 2011, 26, 003504. [Google Scholar]
- Deng, C.; Jiang, M.L.; Zhou, S.F. Microstructure and properties of pure tungsten and tungsten copper composites by laser additive manufacturing. Spec. Cast. Nonferrous Alloys 2022, 42, 804. [Google Scholar]
- Wang, L.; Wei, Y.; Yu, F.; Zhang, Q.; Peng, Q. Phase-field simulation of dendrite growth under forced flow conditions in an Al–Cu welding molten pool. Cryst. Res. Technol. 2016, 51, 602. [Google Scholar] [CrossRef]
- Duan, P.P.; Xing, H.; Chen, Z.; Hao, G.H.; Wang, B.H.; Jin, K.X. Phase-field modeling of free dendritic growth of magnesium based alloy. Acta Phys. Sin. 2015, 64, 060201. [Google Scholar] [CrossRef]
- Fallah, V.; Amoorezaei, M.; Provatas, N.; Corbin, S.F.; Khajepour, A. Phase-field simulation of solidification morphology in laser power deposition of Ti-Nb alloys. Acta Mater. 2012, 60, 1663. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liang, Y.; Fei, Y.; Zhang, H. Effect of electrical contact mode on the arc-erosion behavior of titanium diboride-nickel co-reinforced and nickel-enhanced silver-based electrical contact materials. J. Electron. Mater. 2022, 51, 1137. [Google Scholar] [CrossRef]
- Wang, Y.B.; Peng, L.M.; Ji, Y.Z.; Chen, X.X.; Wang, C.L.; Wu, Y.J.; Fu, Y.N.; Chen, L.-Q. The effect of cooling rates on the dendritic morphology transition of Mg-6Gd alloy by in situ X-ray radiography. J. Mater. Sci Technol. 2018, 34, 1142. [Google Scholar] [CrossRef]



| Material Parameters | Cu | W | CuW80 |
|---|---|---|---|
| Melting point (K) | 1357 | 3695 | 2815 |
| Density (g/cm3) | 8.96 | 19.35 | 15.15 |
| Specific heat capacity (J/(kg·K)) | 0.13 | 0.39 | 0.19 |
| Latent heat (J/kg) | 1.34 × 105 | 4.01 × 105 | 3.47 × 105 |
| Gibbs–Thomson (K·m) | 1.3 × 10−7 | 8.29 × 10−7 | 6.89 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Mu, J.; Zhao, S.; Li, J.; Yang, Y.; Han, Z.; Xing, Z.; Li, Q. Phase-Field Simulation and Dendrite Evolution Analysis of Solidification Process for Cu-W Alloy Contact Materials under Arc Ablation. Metals 2024, 14, 1100. https://doi.org/10.3390/met14101100
Ren H, Mu J, Zhao S, Li J, Yang Y, Han Z, Xing Z, Li Q. Phase-Field Simulation and Dendrite Evolution Analysis of Solidification Process for Cu-W Alloy Contact Materials under Arc Ablation. Metals. 2024; 14(10):1100. https://doi.org/10.3390/met14101100
Chicago/Turabian StyleRen, Hanwen, Jian Mu, Siyang Zhao, Junke Li, Yateng Yang, Zhiyun Han, Zexi Xing, and Qingmin Li. 2024. "Phase-Field Simulation and Dendrite Evolution Analysis of Solidification Process for Cu-W Alloy Contact Materials under Arc Ablation" Metals 14, no. 10: 1100. https://doi.org/10.3390/met14101100







