Effect of Yttrium and Yttria Addition in Self-Passivating WCr SMART Material for First-Wall Application in a Fusion Power Plant
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructures
3.2. Oxidation Tests
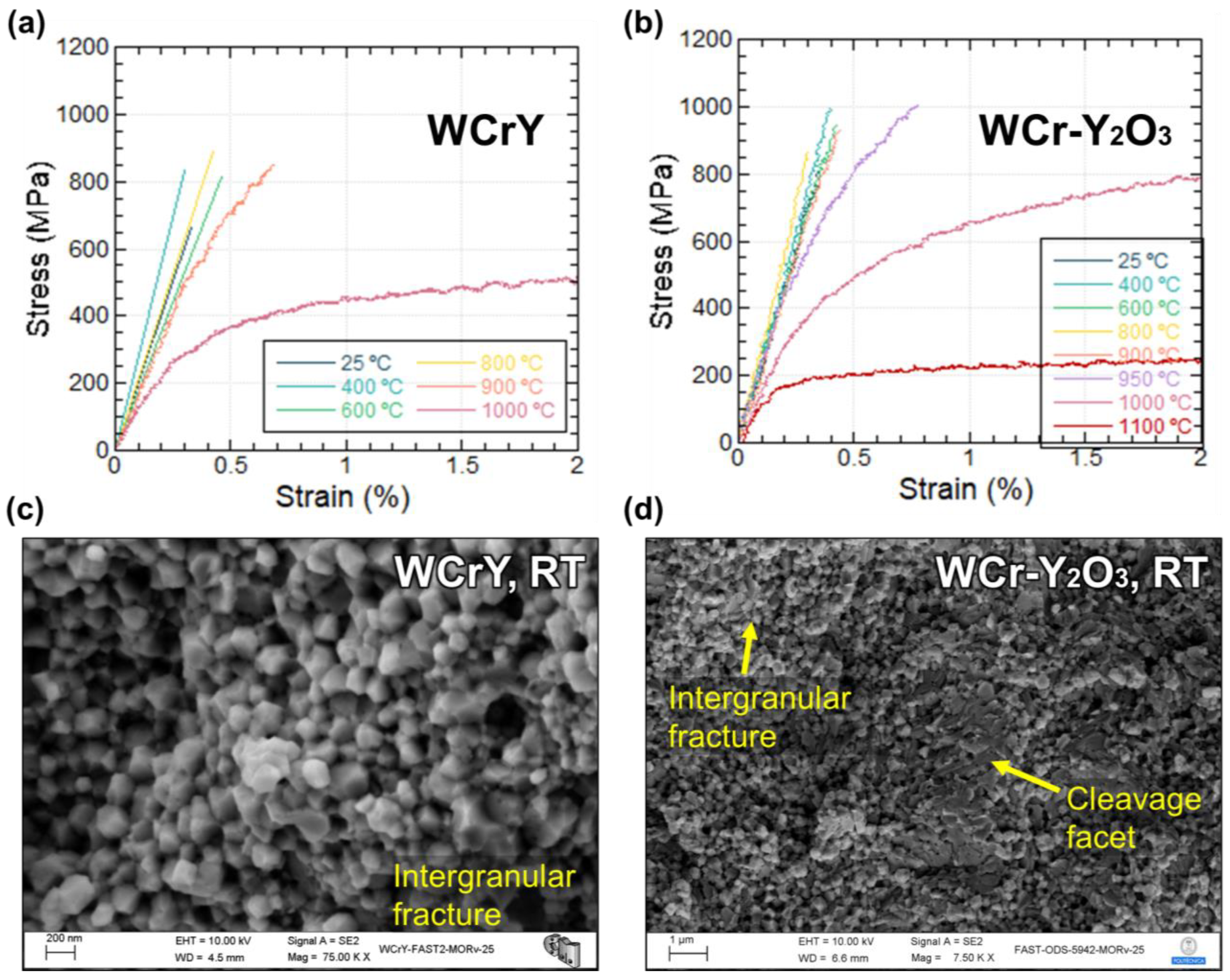
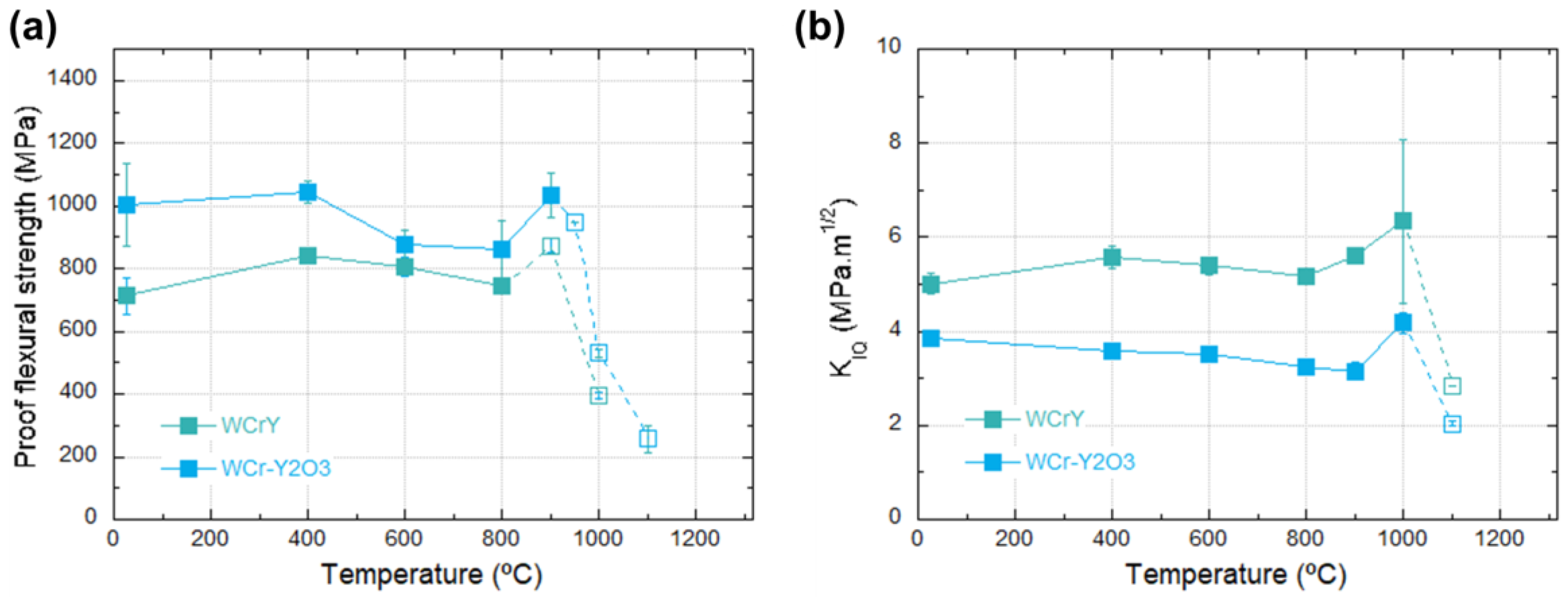
3.3. Three-Point Bending Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smid, I.; Akiba, M.; Vieider, G.; Plöchl, L. Development of tungsten armor and bonding to copper for plasma-interactive components. J. Nucl. Mater. 1998, 258–263, 160–172. [Google Scholar] [CrossRef]
- Maisonnier, D.; Cook, I.; Sardain, P.; Andreani, R.; Di Pace, L.; Forrest, R.; Giancarli, L.; Hermsmeyer, S.; Norajitra, P.; Taylor, N.; et al. A Conceptual Study of Commercial Fusion Power Plants. Final Report of the European Fusion Power Plant Conceptual Study (PPCS); EFDA report number EFDA (05)-27/4.10; EFDA: Addis Ababa, Ethiopia, 2005; Volume 1.
- Wegener, T.; Klein, F.; Litnovsky, A.; Rasinski, M.; Brinkmann, J.; Koch, F.; Linsmeier, C. Development of yttrium-containing self-passivating tungsten alloys for future fusion power plants. Nucl. Mater. Energy 2016, 9, 394–398. [Google Scholar] [CrossRef]
- Litnovsky, A.; Klein, F.; Tan, X.; Ertmer, J.; Coenen, J.W.; Linsmeier, C.; Gonzalez-Julian, J.; Bram, M.; Povstugar, I.; Morgan, T.; et al. Advanced Self-Passivating Alloys for an Application under Extreme Conditions. Metals 2021, 11, 1255. [Google Scholar] [CrossRef]
- Koch, F.; Bolt, H. Self passivating W-based alloys as plasma facing material for nuclear fusion. Phys. Scr. 2007, T128, 100–105. [Google Scholar] [CrossRef]
- Zhu, H.; Tan, X.; Tu, Q.; Mao, Y.; Shu, Z.; Chen, J.; Luo, L.; Litnovsky, A.; Coenen, J.W.; Linsmeier, C.; et al. Effect of Pressure on Densification and Microstructure of W-Cr-Y-Zr Alloy during SPS Consolidated at 1000 °C. Metals 2022, 12, 1437. [Google Scholar] [CrossRef]
- Xiong, Z.; Ma, W.; Deng, Z.; Dong, D. Strong yet ductile self-passivating W-10Cr alloy by laser powder bed fusion. Int. J. Refract. Met. Hard Mater. 2023, 116, 106365. [Google Scholar] [CrossRef]
- Wang, W.; Tan, X.; Yang, S.; Mao, Y.; Luo, L.; Zhu, X.; Litnovsky, A.; Coenen, J.; Linsmeier, C.; Wu, Y. The influence of powder characteristics on densification behavior and microstructure evolution of W-Cr-Zr alloy consolidated by field-assisted sintering technology. Int. J. Refract. Met. Hard Mater. 2022, 108, 105939. [Google Scholar] [CrossRef]
- Wang, W.; Tan, X.; Yang, S.; Luo, L.; Zhu, X.; Mao, Y.; Litnovsky, A.; Coenen, J.; Linsmeier, C.; Wu, Y. On grain growth and phase precipitation behaviors during W-Cr-Zr alloy densification using field-assisted sintering technology. Int. J. Refract. Met. Hard Mater. 2021, 98, 105552. [Google Scholar] [CrossRef]
- Wang, Y.; Harutyunyan, Z.; Gasparyan, Y.; Ogorodnikova, O.; Sinelnikov, D.; Efimov, N.; Tan, X.; Umerenkova, A.; Grishaev, M. Annealing effect on deuterium retention in W-Cr-Y alloy. J. Nucl. Mater. 2024, 593, 154975. [Google Scholar] [CrossRef]
- Harutyunyan, Z.; Gasparyan, Y.; Efimov, V.; Litnovsky, A.; Klein, F.; Pisarev, A.; Coenen, J.W.; Linsmeier, C. Analysis of trapping sites for deuterium in W–Cr–Y SMART alloy. Vacuum 2022, 199, 110956. [Google Scholar] [CrossRef]
- Harutyunyan, Z.; Ogorodnikova, O.; Gasparyan, Y.; Umerenkova, A.; Wang, Y.; Sal, E.; García-Rosales, C. Deuterium retention in W-Cr-Y alloy: Impact of the manufacturing method and helium presence. J. Nucl. Mater. 2023, 578, 154353. [Google Scholar] [CrossRef]
- Terentyev, D.; Jenus, P.; Sal, E.; Zinovev, A.; Chang, C.-C.; Garcia-Rosales, C.; Kocen, M.; Novak, S.; Van Renterghem, W. Development of irradiation tolerant tungsten alloys for high temperature nuclear applications. Nucl. Fusion 2022, 62, 086035. [Google Scholar] [CrossRef]
- Veverka, J.; Vilémová, M.; Lukáč, F.; Kądzielawa, A.P.; Legut, D.; Vontorová, J.; Kozlík, J.; Chráska, T. Decreasing the W-Cr solid solution decomposition rate: Theory, modelling and experimental verification. J. Nucl. Mater. 2023, 576, 154288. [Google Scholar] [CrossRef]
- Qian, Y.; Gilbert, M.R.; Dezerald, L.; Nguyen-Manh, D.; Cereceda, D. Ab initio study of tungsten-based alloys under fusion power-plant conditions. J. Nucl. Mater. 2023, 581, 154422. [Google Scholar] [CrossRef]
- Kirillova, V.; Popov, N.; Gurova, J.; Bachurina, D.; Tan, X.; Fedotov, I.; Suchkov, A. Brazing SMART tungsten alloys to RAFM steels by Titanium-Zirconium-Beryllium brazing alloy. Fusion Eng. Des. 2024, 201, 114297. [Google Scholar] [CrossRef]
- Efimov, N.; Sinelnikov, D.; Kolodko, D.; Grishaev, M.; Nikitin, I. On the reconstruction of LEIS spectra after distortion by an electrostatic energy analyzer. Appl. Surf. Sci. 2024, 676, 161006. [Google Scholar] [CrossRef]
- Efimov, N.; Sinelnikov, D.; Grishaev, M.; Nikitin, I.; Wang, Y.; Harutyunyan, Z.; Gasparyan, Y. On the possibility of quantitative W-Cr-Y analysis by grazing ion-surface scattering spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2024, 546, 165177. [Google Scholar] [CrossRef]
- Calvo, A.; García-Rosales, C.; Ordás, N.; Iturriza, I.; Schlueter, K.; Koch, F.; Pintsuk, G.; Tejado, E.; Pastor, J.Y. Self-passivating W-Cr-Y alloys: Characterization and testing. Fusion Eng. Des. 2017, 124, 1118–1121. [Google Scholar] [CrossRef]
- Chen, S.; Xue, L.; Yin, S.; Yan, Y.; Zhou, Q. Microstructures and Antioxidation of W Self-Passivating Alloys: Synergistic Effect of Yttrium and Milling Time. Metals 2024, 14, 194. [Google Scholar] [CrossRef]
- Ye, C.; Chen, S.; Liu, W.; Xue, L.; Yin, S.; Yan, Y. Effects of Yttrium on High Temperature Oxidation Resistance of W-Si-Y Self-Passivating Alloys. Metals 2022, 12, 2040. [Google Scholar] [CrossRef]
- Hou, P.Y. 1.10—Oxidation of Metals and Alloys. In Shreir’s Corrosion; Cottis, B., Ed.; Elsevier: Oxford, UK, 2010; pp. 195–239. [Google Scholar]
- Fritscher, K. The Reactive Element Effect. Metall. Mater. Trans. A 2023, 54, 64–74. [Google Scholar] [CrossRef]
- Stringer, J.; Wilcox, B.A.; Jaffee, R.I. The high-temperature oxidation of nickel-20 wt. % chromium alloys containing dispersed oxide phases. Oxid. Met. 1972, 5, 11–47. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Holzbrecher, H.; Briefs, K.G.; Beske, H. Differences in growth mechanisms of oxide scales formed on ODS and conventional wrought alloys. Oxid. Met. 1989, 32, 67–88. [Google Scholar] [CrossRef]
- Whittle, D.P.; Stringer, J. Improvements in high temperature oxidation resistance by additions of reactive elements or oxide dispersions. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1980, 295, 309–329. [Google Scholar]
- Klein, F.; Wegener, T.; Litnovsky, A.; Rasinski, M.; Tan, X.; Gonzalez-Julian, J.; Schmitz, J.; Bram, M.; Coenen, J.; Linsmeier, C. Oxidation resistance of bulk plasma-facing tungsten alloys. Nucl. Mater. Energy 2018, 15, 226–231. [Google Scholar] [CrossRef]
- Calvo, A.; Schlueter, K.; Tejado, E.; Pintsuk, G.; Ordás, N.; Iturriza, I.; Neu, R.; Pastor, J.; García-Rosales, C. Self-passivating tungsten alloys of the system W-Cr-Y for high temperature applications. Int. J. Refract. Met. Hard Mater. 2018, 73, 29–37. [Google Scholar] [CrossRef]
- Wang, W.; Tan, X.; Liu, J.; Chen, X.; Wu, M.; Luo, L.; Zhu, X.; Chen, H.; Mao, Y.; Litnovsky, A.; et al. The influence of heating rate on W-Cr-Zr alloy densification process and microstructure evolution during spark plasma sintering. Powder Technol. 2020, 370, 9–18. [Google Scholar] [CrossRef]
- Calvo, A.; García-Rosales, C.; Koch, F.; Ordás, N.; Iturriza, I.; Greuner, H.; Pintsuk, G.; Sarbu, C. Manufacturing and testing of self-passivating tungsten alloys of different composition. Nucl. Mater. Energy 2016, 9, 422–429. [Google Scholar] [CrossRef]
- Vilémová, M.; Illková, K.; Lukáč, F.; Matějíček, J.; Klečka, J.; Leitner, J. Microstructure and phase stability of W-Cr alloy prepared by spark plasma sintering. Fusion Eng. Des. 2018, 127, 173–178. [Google Scholar] [CrossRef]
- Pint, B.A.; Wright, I.G. Oxidation Behavior of ODS Fe–Cr Alloys. Oxid. Met. 2005, 63, 193–213. [Google Scholar] [CrossRef]
- Quadakkers, W. Oxidation of ODS alloys. J. Phys. IV 1993, 3, C9-177–C9-186. [Google Scholar] [CrossRef]
- Ramanarayanan, T.A.; Ayer, R.; Petkovic-Luton, R.; Leta, D.P. The influence of yttrium on oxide scale growth and adherence. Oxid. Met. 1988, 29, 445–472. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, Z.; Yu, L.; Liu, Y. Achieving high strength and ductility in ODS-W alloy by employing oxide@W core-shell nanopowder as precursor. Nat. Commun. 2021, 12, 5052. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Liu, X.; Zhao, Z.; Luo, L.; Cheng, J.; Zan, X.; Wang, Z.; Xu, Q.; Wu, Y. Excellent performance of W–Y2O3 composite via powder process improvement and Y2O3 refinement. Mater. Des. 2021, 212, 110249. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Pang, B.; Xi, X.; Nie, Z. Structure evolution of Y2O3 and consequent effects on mechanical properties of W–Y2O3 alloy prepared by ball milling and SPS. Mater. Sci. Eng. A 2022, 832, 142448. [Google Scholar] [CrossRef]
- Li, L.; Dong, Z.; Ma, Z.; Liu, C.; Yu, L.; Liu, Y. Ultrahigh strength and toughness in W-Y2O3 alloy with bimodal and lamellar structures. Mater. Res. Lett. 2023, 11, 439–445. [Google Scholar] [CrossRef]
- Sobieraj, D.; Wróbel, J.S.; Gilbert, M.R.; Litnovsky, A.; Klein, F.; Kurzydłowski, K.J.; Nguyen-Manh, D. Composition Stability and Cr-Rich Phase Formation in W-Cr-Y and W-Cr-Ti Smart Alloys. Metals 2021, 11, 743. [Google Scholar] [CrossRef]
- Sobieraj, D.; Wróbel, J.S.; Gilbert, M.R.; Kurzydłowski, K.J.; Nguyen-Manh, D. Co-segregation of Y and Zr in W-Cr-Y-Zr alloys: First-principles modeling at finite temperature and application to SMART materials. J. Alloy. Met. Syst. 2023, 2, 100011. [Google Scholar] [CrossRef]
- Sublet, J.-C.; Eastwood, J.; Morgan, J.; Gilbert, M.; Fleming, M.; Arter, W. FISPACT-II: An advanced simulation system for activation, transmutation and material modelling. Nucl. Data Sheets 2017, 139, 77–137. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Guinea, G.V.; Pastor, J.Y.; Planas, J.; Elices, M. Stress intensity factor, compliance and CMOD for a general three-point-bend beam. Int. J. Fract. 1998, 89, 103–116. [Google Scholar] [CrossRef]
- Pastor, J.Y.; Guinea, G.; Planas, J.; Elices, M. Nueva expresión del factor de intensidad de tensiones para la probeta de flexión en tres puntos. An. Mecánica Fract. 1995, 12, 85–90. [Google Scholar]
- Dou, P.; Qiu, L.; Jiang, S.; Kimura, A. Crystal and metal/oxide interface structures of nanoparticles in Fe–16Cr–0.1Ti–0.35Y2O3 ODS steel. J. Nucl. Mater. 2019, 523, 320–332. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Karpinos, D.M.; Listovnichaya, S.P.; Balakhnina, V.N.; Okunevskii, Y.N. Reaction between thin yttrium oxide and chromium films in the presence of oxygen. Sov. Powder Met. Met. Ceram. 1979, 18, 668–671. [Google Scholar] [CrossRef]
- Pasebani, S.; Dutt, A.K.; Burns, J.; Charit, I.; Mishra, R.S. Oxide dispersion strengthened nickel based alloys via spark plasma sintering. Mater. Sci. Eng. A 2015, 630, 155–169. [Google Scholar] [CrossRef]
- de Castro, V.; Marquis, E.; Lozano-Perez, S.; Pareja, R.; Jenkins, M. Stability of nanoscale secondary phases in an oxide dispersion strengthened Fe–12Cr alloy. Acta Mater. 2011, 59, 3927–3936. [Google Scholar] [CrossRef]
- Tang, C.; Pan, F.; Qu, X.; Jia, C.; Duan, B.; He, X. Spark plasma sintering cobalt base superalloy strengthened by Y–Cr–O compound through high-energy milling. J. Mech. Work. Technol. 2008, 204, 111–116. [Google Scholar] [CrossRef]
- Mao, Z.; Xiong, L.; Liu, S. The formation of the complex oxide in Ni-based alloy powder during mechanical milling and heat treatment. J. Alloys Compd. 2021, 879, 160333. [Google Scholar] [CrossRef]
- Chevalier, S.; Larpin, J. Formation of perovskite type phases during the high temperature oxidation of stainless steels coated with reactive element oxides. Acta Mater. 2002, 50, 3107–3116. [Google Scholar] [CrossRef]
- Zhang, H.; Gorley, M.J.; Chong, K.B.; Fitzpatrick, M.E.; Roberts, S.G.; Grant, P.S. An in situ powder neutron diffraction study of nano-precipitate formation during processing of oxide-dispersion-strengthened ferritic steels. J. Alloys Compd. 2014, 582, 769–773. [Google Scholar] [CrossRef]
- Kim, B.; Jang, J.; Kim, T.K.; Ahn, J.H. Formation of Nano-Sized Y2O3 Dispersoids in Mechanically Alloyed Ni–(Cr, Y2O3, Y) Alloys During Heat-Treatments. J. Nanosci. Nanotechnol. 2012, 12, 5510–5513. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M.; Cheng, K.; Zhang, L.; Du, Y. Thermodynamic modeling of the chromium-yttrium-oxygen system. Calphad 2019, 64, 1–10. [Google Scholar] [CrossRef]
- Robino, C.V. Representation of mixed reactive gases on free energy (Ellingharn-Richardson) diagrams. Met. Mater. Trans. B 1996, 27, 65–69. [Google Scholar] [CrossRef]
- Flower, H.; Gould, P.; Moon, D.; Tuson, A. The oxidation of chromium. In Microscopy of Oxidation; CRC Press: Boca Raton, FL, USA, 2023; pp. 98–112. [Google Scholar]
- Pujilaksono, B.; Jonsson, T.; Halvarsson, M.; Panas, I.; Svensson, J.E.; Johansson, L.G. Paralinear Oxidation of Chromium in O2 + H2O Environment at 600–700 °C. Oxid. Met. 2008, 70, 163–188. [Google Scholar] [CrossRef]
- Schmid, B.; Aas, N.; Grong, Ø.; Ødegård, R. High-temperature oxidation of nickel and chromium studied with an in-situ environmental scanning electron microscope. Scanning 2001, 23, 255–266. [Google Scholar] [CrossRef]
- Klein, F.; Litnovsky, A.; Wegener, T.; Tan, X.; Gonzalez-Julian, J.; Rasinski, M.; Schmitz, J.; Linsmeier, C.; Bram, M.; Coenen, J.W. Sublimation of advanced tungsten alloys under DEMO relevant accidental conditions. Fusion Eng. Des. 2019, 146, 1198–1202. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Liang, J.; Zhou, X. High-Temperature Oxidation Behavior and Oxide Scale Structure of Yttrium-Modified Ni–16Mo–7Cr–4Fe Superalloy at 1273 K. Oxid. Met. 2019, 92, 67–88. [Google Scholar] [CrossRef]
- Seybolt, A. High temperature oxidation of chromium containing Y2O3. Corros. Sci. 1966, 6, 263–269. [Google Scholar] [CrossRef]
- Hou, P.Y.; Stringer, J. The influence of ion-implanted yttrium on the selective oxidation of chromium in Co-25 wt.% Cr. Oxid. Met. 1988, 29, 45–73. [Google Scholar] [CrossRef]
- Przybylski, K.; Yurek, G.J. The Influence of Implanted Yttrium on the Microstructures of Chromia Scales Formed on a Co-45 Weight Percent Cr Alloy. J. Electrochem. Soc. 1988, 135, 517–523. [Google Scholar] [CrossRef]
- Molin, S.; Persson, Å.; Skafte, T.; Smitshuysen, A.; Jensen, S.; Andersen, K.; Xu, H.; Chen, M.; Hendriksen, P. Effective yttrium based coating for steel interconnects of solid oxide cells: Corrosion evaluation in steam-hydrogen atmosphere. J. Power Sources 2019, 440, 226814. [Google Scholar] [CrossRef]
- Pillis, M.F.; Correa, O.V.; Ramanathan, L.V. High temperature oxidation behavior of yttrium dioxide coated Fe-20Cr alloy. Mater. Res. 2016, 19, 611–617. [Google Scholar] [CrossRef]
- Stott, F.H.; Wood, G.C.; Stringer, J. The influence of alloying elements on the development and maintenance of protective scales. Oxid. Met. 1995, 44, 113–145. [Google Scholar] [CrossRef]
- Saito, Y.; Önay, B.; Maruyama, T. The reactive element effect (REE) in oxidation of alloys. J. Phys. Iv 1993, 3, C9-217–C9-230. [Google Scholar] [CrossRef]
- Liu, R.; Xie, Z.; Fang, Q.; Zhang, T.; Wang, X.; Hao, T.; Liu, C.; Dai, Y. Nanostructured yttria dispersion-strengthened tungsten synthesized by sol–gel method. J. Alloys Compd. 2015, 657, 73–80. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, G.J.; Jiang, F.; Ding, X.D.; Sun, Y.J.; Sun, J.; Ma, E. Nanostructured high-strength molybdenum alloys with unprecedented tensile ductility. Nat. Mater. 2013, 12, 344–350. [Google Scholar] [CrossRef]
- Gurland, J.; Plateau, J. The Mechanism of Ductile Rupture of Metals Containing Inclusions; Brown Univ., Providence; Institut de Recherches de la Siderugie: St.-Germain-en-Laye, France, 1963. [Google Scholar]
- Klimiankou, M.; Lindau, R.; Möslang, A. HRTEM Study of yttrium oxide particles in ODS steels for fusion reactor application. J. Cryst. Growth 2003, 249, 381–387. [Google Scholar] [CrossRef]
- Lindau, R.; Möslang, A.; Schirra, M.; Schlossmacher, P.; Klimenkov, M. Mechanical and microstructural properties of a hipped RAFM ODS-steel. J. Nucl. Mater. 2002, 307–311, 769–772. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, S.I.; Ryu, H.J.; Hong, S.H. Effect of two-stage sintering process on microstructure and mechanical properties of ODS tungsten heavy alloy. Mater. Sci. Eng. A 2007, 458, 323–329. [Google Scholar] [CrossRef]
- Litnovsky, A.; Chen, J.; Bram, M.; Gonzalez-Julian, J.; Zoz, H.; Benz, H.U.; Huber, J.; Pintsuk, G.; Coenen, J.W.; Linsmeier, C. SMART materials for DEMO: Towards industrial production. Fusion Eng. Des. 2024, 203, 114423. [Google Scholar] [CrossRef]



| Composition | dg (nm) | dp (nm) | ρN (m−2) | a (Å) | HV 0.5 | |
|---|---|---|---|---|---|---|
| As-Milled Powder | As-Sintered Ingot | |||||
| WCrY | 183 ± 18 | 33 ± 17 | 2.2 × 1013 | 3.098 | 3.099 | 1207 ± 9 |
| WCr-Y2O3 | 200 ± 12 | 42 ± 21 | 2.0 × 1013 | 3.097 | 3.102 | 1135 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Tejado, E.; Rasiński, M.; Litnovsky, A.; Nguyen-Manh, D.; Prestat, E.; Whitfield, T.; Pastor, J.Y.; Bram, M.; Coenen, J.W.; et al. Effect of Yttrium and Yttria Addition in Self-Passivating WCr SMART Material for First-Wall Application in a Fusion Power Plant. Metals 2024, 14, 1092. https://doi.org/10.3390/met14091092
Chen J, Tejado E, Rasiński M, Litnovsky A, Nguyen-Manh D, Prestat E, Whitfield T, Pastor JY, Bram M, Coenen JW, et al. Effect of Yttrium and Yttria Addition in Self-Passivating WCr SMART Material for First-Wall Application in a Fusion Power Plant. Metals. 2024; 14(9):1092. https://doi.org/10.3390/met14091092
Chicago/Turabian StyleChen, Jie, Elena Tejado, Marcin Rasiński, Andrey Litnovsky, Duc Nguyen-Manh, Eric Prestat, Tamsin Whitfield, Jose Ygnacio Pastor, Martin Bram, Jan Willem Coenen, and et al. 2024. "Effect of Yttrium and Yttria Addition in Self-Passivating WCr SMART Material for First-Wall Application in a Fusion Power Plant" Metals 14, no. 9: 1092. https://doi.org/10.3390/met14091092












