Assessing Apparent Equilibrium Concentrations in Cementation of Trace Pd, Pt, Au, and Rh from Nitrate Solutions Using Mg, Al, Fe, and Zn
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermodynamics of Cementation Reactions
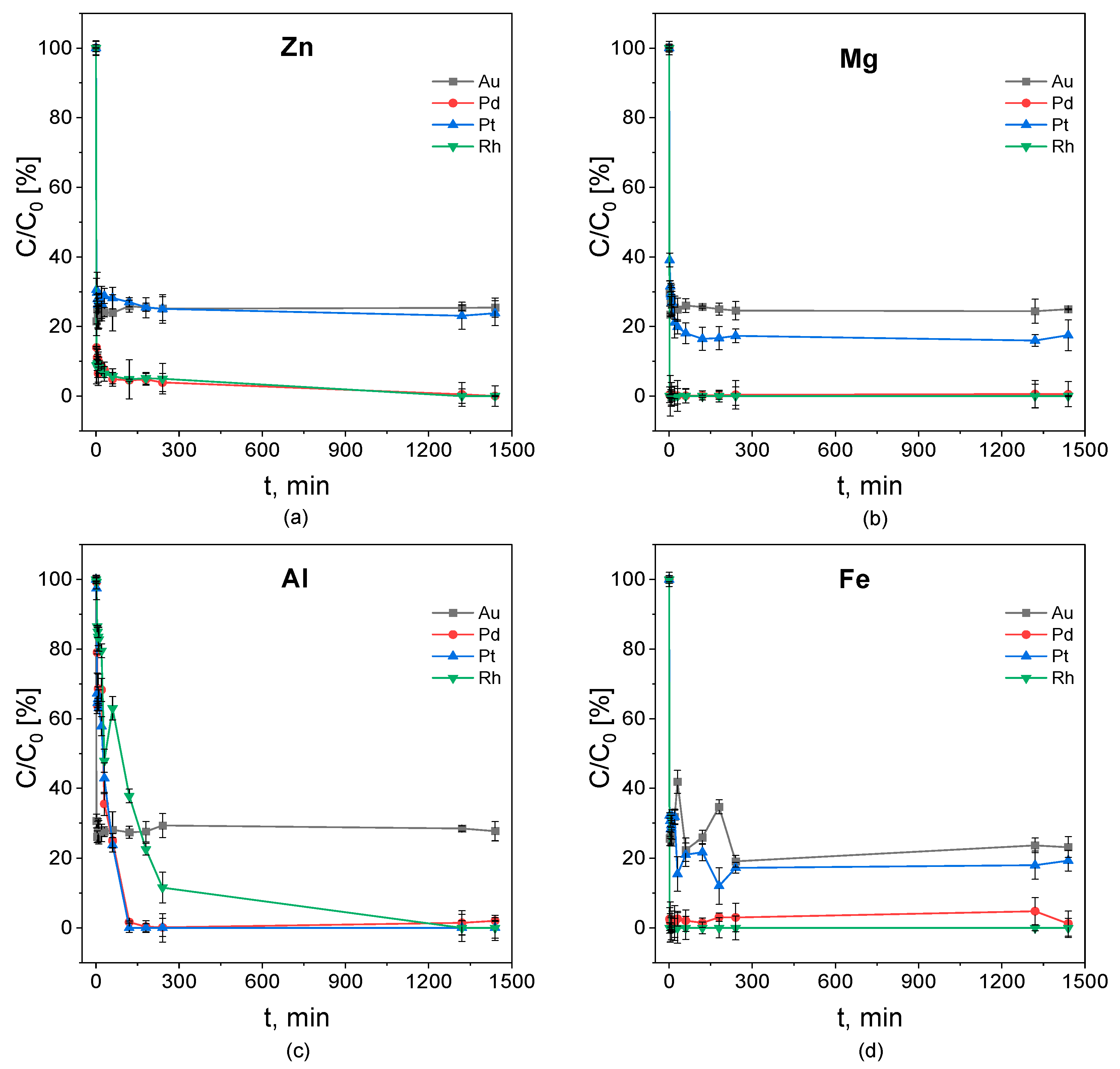
3.2. Cementation Kinetics Studies
3.3. Apparent Equilibrium of the Cementation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guha, B.; Bandyopadhyay, G. Gold Price Forecasting Using ARIMA Model. J. Adv. Manag. J. 2016, 4, 117–121. [Google Scholar] [CrossRef]
- Umicore. Available online: https://pmm.umicore.com/en/prices/ (accessed on 19 July 2024).
- Frimmel, H.E. Earth’s continental crustal gold endowment. Earth Planet. Sci. Lett. 2008, 267, 45–55. [Google Scholar] [CrossRef]
- Jadhav, U. A review of recovery of metals from industrial waste. J. Mod. Manag. Entrep. 2012, 54, 159–167. [Google Scholar]
- Padamata, S.K.; Yasinskiy, A.; Polyakov, P.; Pavlov, E.; Varyukhin, D. Recovery of Noble Metals from Spent Catalysts: A Review. Metall. Mater. Trans. B 2020, 51, 2413–2435. [Google Scholar] [CrossRef]
- Temur, H.; Yartaçı, A.; Kocakerim, M.M. A Study On The Optimum Conditions Of The Cementation Of Copper İn Chlorination Solution Of Chalcopyrite Concentrate By Iron Scraps. Balıkesir Üniversitesi Fen Bilim. Enstitüsü Derg. 2006, 8, 63–73. [Google Scholar]
- Mpinga, C.N.; Bradshaw, S.M.; Akdogan, G.; Snyders, C.A.; Eksteen, J.J. Evaluation of the Merrill–Crowe process for the simultaneous removal of platinum, palladium and gold from cyanide leach solutions. Hydrometallurgy 2014, 142, 36–46. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, B.-S.; Kim, E.-Y.; Kim, S.-K.; Ryu, J.-W.; Lee, J.-c. Recovery of Platinum Group Metals from the Leach Solution of Spent Automotive Catalysts by Cementation. J. Korean Inst. Resour. Recycl. 2011, 20, 36–45. [Google Scholar] [CrossRef]
- Mahapatra, R.; Srikant, S.; Rao, R.; Mohanty, B. Recovery of basic valuable metals and alloys from E-waste using microwave heating followed by leaching and cementation process. Sādhanā 2019, 44, 209. [Google Scholar] [CrossRef]
- Aboody, H.; Taheri Najafabadi, A.; Mohammadi, S.; Hashemnezhad, S.E. Valorization of spent catalyst of sponge iron production industries into high-purity metallic nickel via a novel three-step method of leaching, cementation and purification. Process Saf. Environ. Prot. 2024, 188, 1145–1159. [Google Scholar] [CrossRef]
- Barzyk, W.; Kowal, A.; Pomianowski, A. Noble metal (Ag, Au) cementation on non-stoichiometric cuprous sulphide grains. Colloids Surf. A Physicochem. Eng. Asp. 2002, 208, 321–335. [Google Scholar] [CrossRef]
- Patcharawit, T.; Kansomket, C.; Kritsarikun, W.; Taseela, K.; Paernaphan, C.; Laphosin, T.; Tannukit, T.; Khumkoa, S. Recovery of pure silver from spent silver electroplating solutions via electrochemical process and zinc cementation. J. Met. Mater. Miner. 2023, 33, 14–20. [Google Scholar] [CrossRef]
- Ranchev, M.; Angelov, T.; Yankova, T.; Valchanova, I.; Grigorova, I. Silver Recovery from Acidic Thiourea Solutions by Cementation and Precipitation Techniques. In Proceedings of the ISWA World Congress, Novi Sad, Serbia, 19–21 September 2016. [Google Scholar]
- Goc, K.; Kluczka, J.; Benke, G.; Malarz, J.; Pianowska, K.; Leszczyńska-Sejda, K. Application of Ion Exchange for Recovery of Noble Metals. Minerals 2021, 11, 1188. [Google Scholar] [CrossRef]
- Rabah, M.; Hewaidy, I.; Farghaly, F. Recovery of Molybdenum and Cobalt Powders from Spent Hydrogenation Catalyst. Powder Metall. 1997, 40, 283–288. [Google Scholar] [CrossRef]
- Stefanowicz, T.; Osińska, M.; Napieralska-Zagozda, S. Copper Recovery by the Cementation Method. Hydrometallurgy 1997, 47, 69–90. [Google Scholar] [CrossRef]
- Sulka, G.D.; Jaskuła, M. Influence of the sulphuric acid concentration on the kinetics and mechanism of silver ion cementation on copper. Hydrometallurgy 2005, 77, 131–137. [Google Scholar] [CrossRef]
- Martinez, G. Kinetic Aspects of Gold and Silver Recovery in Cementation with Zinc Power and Electrocoagulation Iron Process. Adv. Chem. Eng. Sci. 2012, 2, 342–349. [Google Scholar] [CrossRef]
- Pérez-Labra, M.; Romero-Serrano, J.A.; Reyes-Pérez, M.; Barrientos-Hernández, F.R.; Hernández-Ramírez, A.; Juarez-Tapia, J.C.; Reyes-Cruz, V.E. Kinetic Aspects and Thermo chemical Analysis of Silver Cementation from Residual X Ray Fixer by Cementation on Zinc. RA J. Appl. Res. 2017, 3, 1100–1107. [Google Scholar]
- Hsu, Y.J.; Tran, T. Selective removal of gold from copper-gold cyanide liquors by cementation using zinc. Miner. Eng. 1996, 9, 1–13. [Google Scholar] [CrossRef]
- Stanković, V.; Serbula, S.; Jančeva, B. Cementation of copper onto brass particles in a packed bed. J. Min. Metall. Sect. B Metall. 2004, 40, 21–39. [Google Scholar] [CrossRef]
- Cao, Y.; Duby, P. Cobalt cementation with ferromanganese. Hydrometallurgy 2001, 61, 195–205. [Google Scholar] [CrossRef]
- Tzaneva, B.; Petrova, T.; Hristov, J.; Fachikov, L. Electrochemical investigation of cementation process. Bulg. Chem. Commun. 2016, 48, 91–95. [Google Scholar]
- Anacleto, A.L.; Carvalho, J.R. Mercury cementation from chloride solutions using iron, zinc and aluminium. Miner. Eng. 1996, 9, 385–397. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.-J.; Seo, J.-H.; Cho, J.-S.; Cho, K.-H.; Lee, J. Effect of Ultrasound Irradiation during Cementation Process for Recovery of Iridium. Resour. Recycl. 2021, 30, 61–67. [Google Scholar] [CrossRef]
- Sulka, G.D.; Jaskuła, M. Effect of sulphuric acid and copper sulphate concentrations on the morphology of silver deposit in the cementation process. Electrochim. Acta 2006, 51, 6111–6119. [Google Scholar] [CrossRef]
- Kuntyi, O.I.; Zozulya, G.I.; Kurilets, O.G. Cementation of gold by magnesium in cyanide solutions. Russ. J. Non-Ferr. Met. 2007, 48, 413–417. [Google Scholar] [CrossRef]
- Elshazly, H. Intensification of the rate of heavy metal removal from industrial effluents by cementation on a reciprocating array of vertical parallel plates, Alex. Alex. Eng. J. 2005, 44, 789–895. [Google Scholar]
- Kim, J.; Kim, R.; Han, K.N. Advances in Hydrometallurgical Gold Recovery through Cementation, Adsorption, Ion Exchange and Solvent Extraction. Minerals 2024, 14, 607. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, S.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway. Metals 2021, 11, 248. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S.; Choi, S.H. Separation of Ag(I) by Ion Exchange and Cementation from a Raffinate Containing Ag(I), Ni(II) and Zn(II) and Traces of Cu(II) and Sn(II). Processes 2018, 6, 112. [Google Scholar] [CrossRef]
- Gros, F.; Baup, S.; Aurousseau, M. Copper cementation on zinc and iron mixtures: Part 1: Results on rotating disc electrode. Hydrometallurgy 2011, 106, 127–133. [Google Scholar] [CrossRef]
- Makhloufi, L.; Saidani, B.; Hammache, H. Removal of lead ions from acidic aqueous solutions by cementation on iron. Water Res. 2000, 34, 2517–2524. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, W.; Li, J.; Wang, X.; Liu, L.; Wang, J. A critical review on the recycling of copper and precious metals from waste printed circuit boards using hydrometallurgy. Front. Environ. Sci. Eng. 2017, 11, 8. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Q.; Cao, J.; Li, H.; Bian, Z. Precious metal recovery. Joule 2021, 5, 3097–3115. [Google Scholar] [CrossRef]
- Zupanc, A.; Install, J.; Jereb, M.; Repo, T. Sustainable and Selective Modern Methods of Noble Metal Recycling. Angew. Chem. Int. Ed. 2023, 62, e202214453. [Google Scholar] [CrossRef]
- Kumari, R.; Samadder, S.R. A critical review of the pre-processing and metals recovery methods from e-wastes. J. Environ. Manag. 2022, 320, 115887. [Google Scholar] [CrossRef]
- Bigum, M.; Brogaard, L.; Christensen, T.H. Metal recovery from high-grade WEEE: A life cycle assessment. J. Hazard. Mater. 2012, 207–208, 8–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Xie, H.; Zeng, X.; Li, J. Current Status on Leaching Precious Metals from Waste Printed Circuit Boards. Procedia Environ. Sci. 2012, 16, 560–568. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling the Platinum Group Metals: A European Perspective. Platin. Met. Rev. 2012, 56, 29–35. [Google Scholar] [CrossRef]
- Rzelewska, M.; Regel-Rosocka, M. Wastes generated by automotive industry—Spent automotive catalysts. Phys. Sci. Rev. 2018, 3. [Google Scholar] [CrossRef]
- Karavasteva, M. Kinetics and deposit morphology of gold cemented on magnesium, aluminum, zinc, iron and copper from ammonium thiosulfate–ammonia solutions. Hydrometallurgy 2010, 104, 119–122. [Google Scholar] [CrossRef]
- Goc, K.; Kluczka, J.; Benke, G.; Malarz, J.; Pianowska, K.; Leszczyńska-Sejda, K. Precipitation of Precious Metals Concentrates from Post-Elution Solutions from Ion-Exchange Processes. Minerals 2024, 14, 625. [Google Scholar] [CrossRef]
- Zoleta, J.; Jeon, S.; Kuze, A.; Okada, N.; Park, I.; Ito, M.; Elakneswaran, Y.; Hiroyoshi, N. Selective Cementation of Gold Using an Iron Oxide and Zero-Valent Aluminum Galvanic System from Gold–Copper Ammoniacal Thiosulfate Solutions. Metals 2023, 13, 1289. [Google Scholar] [CrossRef]
- Arima, H.; Fujita, T.; Yen, W.-T. Gold Cementation from Ammonium Thiosulfate Solution by Zinc, Copper and Aluminium Powders. Mater. Trans. 2002, 43, 485–493. [Google Scholar] [CrossRef]
- ISO 9001:2008; Quality Management Systems—Requirements. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 17025:2005; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2005.
- Lide, D.R. CRC Handbook of Chemistry and Physics, 89th ed.; Taylor & Francis: New York, NY, USA, 2008. [Google Scholar]
- Sadananda, K.; Yang, J.H.; Iyyer, N.; Phan, N.; Rahman, A. Sacrificial Zn–Ni coatings by electroplating and hydrogen embrittlement of high-strength steels. Corros. Rev. 2021, 39, 487–517. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 87th ed Editor-in-Chief: David R. Lide (National Institute of Standards and Technology). CRC Press/Taylor and Francis Group: Boca Raton, FL. 2006. 2608 pp. $139.95. ISBN 0-8493-0487-3. J. Am. Chem. Soc. 2007, 129, 724. [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 93rd ed.; Haynes, W.M., Ed.; 2012–2013/Editor-in-Chief; CRC: Boca Raton, FL, USA, 2012. [Google Scholar]
- Atkins, P.W. Physical Chemistry; Macmillan Higher Education: New York, NY, USA, 1997. [Google Scholar]
- Yin, X.; Hsieh, K.; Wang, X.; Wu, Z.; Ye, Q.; Bao, H.; Deng, Y.; Chen, H.; Luo, P.; Liu, H.; et al. Enhancing Generic Reaction Yield Prediction through Reaction Condition-Based Contrastive Learning. Research 2024, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.; Ciribelli, B.; Colmati, F. Nernst equation applied to electrochemical systems and centenary of his Nobel Prize in chemistry. Int. J. Innov. Educ. Res. 2020, 8, 670–683. [Google Scholar] [CrossRef]
- Lee, J. Gold Cementation on Copper in Thiosulfate Solution: Kinetic, Electrochemical, and Morphological Studies. Ph.D. Thesis, University of Arizona Press, Tuscon, Arizona, 2003. [Google Scholar]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution; Taylor & Francis: New York, NY, USA, 1985. [Google Scholar]
- Kazmi, M.; Irfan, M.; Zhou, L.; Yuan, S.; Fatima, H.; Tian, L.-Y.; Ye, Y.-L.; Lu, Q.-S.; Lu, X.-Y.; Yang, S.-Z.; et al. Electron donors and mediators in the thermodynamics and kinetics of CO2 bioreduction. Renew. Sustain. Energy Rev. 2022, 156, 111997. [Google Scholar] [CrossRef]
- Ziouane, Y.; Leturcq, G. New Modeling of Nitric Acid Dissociation Function of Acidity and Temperature. ACS Omega 2018, 3, 6566–6576. [Google Scholar] [CrossRef]
- Aberasturi, D.; Pinedo, R.; Ruiz de Larramendi, I.; Larramendi, J.I.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Baharun, N.; Ling, O.P.; Rezaei Ardani, M.; Ariffin, K.S.; Yaraghi, A.; Abdullah, N.S.; Putra, T.A.R.; Ismail, S. Effect of hydrogen peroxide and lead(II) nitrate on gold cyanide leaching of Malaysian mesothermal deposit gold ore. Physicochem. Probl. Miner. Process. 2020, 56, 905–918. [Google Scholar] [CrossRef]
- Aakyiir, M. Effects of Potassium Nitrate (Saltpetre) on Gold Cyanidation. Ghana J. Technol. 2017, 2, 77–81. [Google Scholar]
- Deschenes, G.; Lastra, R.; Brown, J.R.; Jin, S.; May, O.; Ghali, E. Effect of lead nitrate on cyanidation of gold ores: Progress on the study of the mechanisms. Miner. Eng. 2000, 13, 1263–1279. [Google Scholar] [CrossRef]
- Yoo, K.; Park, Y.; Choi, S.; Park, I. Improvement of Copper Metal Leaching in Sulfuric Acid Solution by Simultaneous Use of Oxygen and Cupric Ions. Metals 2020, 10, 721. [Google Scholar] [CrossRef]
- Hine, F.; Yamakawa, K. Mechanism of oxidation of cuprous ion in hydrochloric acid solution by oxygen. Electrochim. Acta 1970, 15, 769–781. [Google Scholar] [CrossRef]
- Kim, D.-H.; Hwang, S.; Cho, J.-J.; Yu, S.; Kim, S.; Jeon, J.; Ahn, K.H.; Lee, C.; Song, H.-K.; Lee, H. Toward Fast Operation of Lithium Batteries: Ion Activity as the Factor To Determine the Concentration Polarization. ACS Energy Lett. 2019, 4, 1265–1270. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Veríssimo, L.M.P.; Sobral, A.J.F.N.; Lobo, V.M.M.; Esteso, M.A. Effect of the mean distance of closest approach of ions on the diffusion coefficient calculations in aqueous solutions of silver salts. Comptes Rendus. Chim. 2013, 16, 469–475. [Google Scholar] [CrossRef]
- Li, D.; Liu, D. Crystal Structure of [p-C6H4(CH2ImMe)2][PtCl6]. Anal. Sci. 2003, 19, 1089–1090. [Google Scholar] [CrossRef]
- Hargittai, M.; Schulz, A.; Réffy, B.; Kolonits, M. Molecular Structure, Bonding, and Jahn−Teller Effect in Gold Chlorides: Quantum Chemical Study of AuCl3, Au2Cl6, AuCl4-, AuCl, and Au2Cl2 and Electron Diffraction Study of Au2Cl6. J. Am. Chem. Soc. 2001, 123, 1449–1458. [Google Scholar] [CrossRef]
- Caminiti, R.; Carbone, M.; Sadun, C. Palladium (II) and platinum (II) aqueous solutions. Evidence for the solvation of the [PdCl4]2− and [PtCl4]2− ions. J. Mol. Liq. 1998, 75, 149–158. [Google Scholar] [CrossRef]
- Yusenko, K.V.; Sukhikh, A.; Kraus, W.; Gromilov, S.A. Synthesis and Crystal Chemistry of Octahedral Rhodium(III) Chloroamines. Molecules 2020, 25, 768. [Google Scholar] [CrossRef]
- Velikonja, A.; Kralj-Iglič, V.; Iglič, A. On Asymmetric Shape of Electric Double Layer Capacitance Curve. Int. J. Electrochem. Sci. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Koch, K.R.; Burger, M.R.; Kramer, J.; Westra, A.N. 195Pt NMR and DFT computational methods as tools towards the understanding of speciation and hydration/solvation of [PtX6]2− (X = Cl−, Br−) anions in solution. Dalton Trans. 2006, 2006, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgis, G.M.; Maribo-Mogensen, B.; Thomsen, K. The Debye-Hückel theory and its importance in modeling electrolyte solutions. Fluid Phase Equilibria 2018, 462, 130–152. [Google Scholar] [CrossRef]
- Kim, N.; Han, K. Dissolution kinetic model for metal powders—A case of varying surface area. Miner. Metall. Process. 2006, 23, 111–120. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann Ltd.: Oxford, UK, 1997. [Google Scholar]
- Kayanuma, Y.; Okabe, T.H.; Mitsuda, Y.; Maeda, M. New recovery process for rhodium using metal vapor. J. Alloys Compd. 2004, 365, 211–220. [Google Scholar] [CrossRef]
- Shyam, T.S.; Dhruve, H. Comparative Analysis of Methods Employed in Rhodium Recovery. J. Chem. Rev. 2019, 1, 282–286. [Google Scholar] [CrossRef]
- Harjanto, S.; Cao, Y.; Shibayama, A.; Naitoh, I.; Nanami, T.; Kasahara, K.; Okumura, Y.; Liu, K.; Fujita, T. Leaching of Pt, Pd and Rh from Automotive Catalyst Residue in Various Chloride Based Solutions. Mater. Trans.—Mater Trans. 2006, 47, 129–135. [Google Scholar] [CrossRef]
- Elomaa, H.; Seisko, S.; Junnila, T.; Sirviö, T.; Wilson, B.; Aromaa, J.; Lundström, M. The Effect of the Redox Potential of Aqua Regia and Temperature on the Au, Cu, and Fe Dissolution from WPCBs. Recycling 2017, 2, 14. [Google Scholar] [CrossRef]
- Ali, K. Gold Extraction with Chlorination. 2019. Available online: https://www.researchgate.net/publication/335635899_GOLD_EXTRACTION_WITH_CHLORINATION?channel=doi&linkId=5d7133414585151ee49f5fa8&showFulltext=true (accessed on 19 August 2024).
- Kazakova, N.; Lucheva, B.; Iliev, P. A study on the cementation process of non-ferrous metals from a brine leaching solution. Communities Collect. 2020, 55, 223–227. [Google Scholar]
- Demirkıran, N.; Ekmekyapar, A.; Kunkul, A.; Baysar, A. A Kinetic Study of Copper Cementation with Zinc in Aqueous Solutions. Int. J. Miner. Process. 2007, 82, 80–85. [Google Scholar] [CrossRef]
- Tossell, J.A. The speciation of gold in aqueous solution: A theoretical study. Geochim. Et Cosmochim. Acta 1996, 60, 17–29. [Google Scholar] [CrossRef]
- Mironov, I. Properties of Gold(III) Hydroxide and Aquahydroxogold(III) Complexes in Aqueous Solution. Russ. J. Inorg. Chem. 2005, 50, 1115. [Google Scholar]
- Colombo, C.; Oates, C.J.; Monhemius, J.; Plant, J. Complexation of platinum, palladium and rhodium with inorganic ligands in the environment. Geochem.-Explor. Environ. Anal. 2008, 8, 91–101. [Google Scholar] [CrossRef]
- Wojnicki, M.; Krawontka, A.; Wojtaszek, K.; Skibińska, K.; Csapó, E.; Pędzich, Z.; Podborska, A.; Kwolek, P. The Mechanism of Adsorption of Rh(III) Bromide Complex Ions on Activated Carbon. Molecules 2021, 26, 3862. [Google Scholar] [CrossRef]
- Lippert, B.; Sanz Miguel, P.J. More of a misunderstanding than a real mismatch? Platinum and its affinity for aqua, hydroxido, and oxido ligands. Coord. Chem. Rev. 2016, 327–328, 333–348. [Google Scholar] [CrossRef]
- Wojnicki, M.; Fitzner, K.; Luty-BŁOcho, M. Kinetic studies of gold recovery from dilute aqueous solutions using Fe2+ chloride ions. Trans. Nonferrous Met. Soc. China 2015, 25, 2027–2036. [Google Scholar] [CrossRef]
- Wojnicki, M.; Pacławski, K.; Rudnik, E.; Fitzner, K. Kinetics of palladium(II) chloride complex reduction in aqueous solutions using dimethylamineborane. Hydrometallurgy 2011, 110, 56–61. [Google Scholar] [CrossRef]
- Walton, R. Zinc cementation. In Developments in Mineral Processing; Adams, M.D., Wills, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 589–601. [Google Scholar]
- Angelov, A.; Groudev, S. Treatment of gold-bearing solutions by cementation with metallic zinc. Min. Miner. Process. 2002, 44–45, 117–121. [Google Scholar]
- Barakat, M.A.; Mahmoud, M.H.H.; Mahrous, Y.S. Recovery and separation of palladium from spent catalyst. Appl. Catal. A Gen. 2006, 301, 182–186. [Google Scholar] [CrossRef]
- Ding, Y.; Zheng, H.; Li, J.; Zhang, S.; Liu, B.; Ekberg, C. An Efficient Leaching of Palladium from Spent Catalysts through Oxidation with Fe(III). Materials 2019, 12, 1205. [Google Scholar] [CrossRef]
- Coetzee, R.; Dorfling, C.; Bradshaw, S.M. Precipitation of Ru, Rh and Ir with iron ions from synthetic nickel sulphate leach solutions. Hydrometallurgy 2018, 175, 79–92. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Florence, T.; Batley, G.; Benes, P. Chemical Speciation In Natural Waters. Crit. Rev. Anal. Chem. 2008, 9, 219–296. [Google Scholar] [CrossRef]
- Equilibrium Constants for Hydrolysis and Associated Equilibria in Critical Compilations: Platinum. Available online: https://www.cost-nectar.eu/docs/wg1_pt/Pt.pdf (accessed on 20 August 2024).
- Czapla-Masztafiak, J.; Kubas, A.; Kayser, Y.; Fernandes, D.L.A.; Kwiatek, W.M.; Lipiec, E.; Deacon, G.B.; Al-Jorani, K.; Wood, B.R.; Szlachetko, J.; et al. Mechanism of hydrolysis of a platinum(IV) complex discovered by atomic telemetry. J. Inorg. Biochem. 2018, 187, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Equilibrium Constants for Hydrolysis and Associated Equilibria in critical Compilations Gold(III). Available online: https://www.cost-nectar.eu/docs/wg1_pt/AuIII.pdf (accessed on 20 August 2024).
- Robb, W. Kinetics and mechanisms of reactions of gold(III) complexes. I. The equilibrium hydrolysis of tetrachlorogold(III) in acid medium. Inorg. Chem. 1967, 6, 382–386. [Google Scholar] [CrossRef]
- Vlassopoulos, D.; Wood, S.A. Gold speciation in natural waters: I. Solubility and hydrolysis reactions of gold in aqueous solution. Geochim. et Cosmochim. Acta 1990, 54, 3–12. [Google Scholar] [CrossRef]
- Milić, N.B.; Bugarčić, Ž.D. Hydrolysis of the palladium(II) ion in a sodium chloride medium. Transit. Met. Chem. 1984, 9, 173–176. [Google Scholar] [CrossRef]
- Boily, J.-F.F.; Seward, T.M.; Charnock, J.M. The hydrolysis and precipitation of Pd(II) in 06 mol kg-1 NaCl: A potentiometric, spectrophotometric, and EXAFS study. Geochim. Et Cosmochim. Acta 2007, 71, 4834–4845. [Google Scholar] [CrossRef]
- Panunzi, A.; Basolo, F. Mechanism of base hydrolysis of some rhodium(III) complexes. Inorganica Chim. Acta 1967, 1, 223–227. [Google Scholar] [CrossRef]
- Equilibrium Constants for Hydrolysis and Associated Equilibria in Critical Compilations: Rhodium. Available online: https://cost-nectar.eu/docs/wg1_pt/Rh.pdf (accessed on 20 August 2024).
- Đurović, M.D.; Bugarčić, Ž.D.; van Eldik, R. Stability and reactivity of gold compounds—From fundamental aspects to applications. Coord. Chem. Rev. 2017, 338, 186–206. [Google Scholar] [CrossRef]
- Chiban, M.; Soudani, A.; Zerbet, M.; Sinan, F. Removal of nitrate ions by using low-cost adsorbents: Equilibrium isotherm, kinetics and thermodynamic study. Nitrate Occur. Charact. Health Consid. 2012, 4, 31–48. [Google Scholar]
- Reyter, D. Electrochemical Reduction of Nitrate. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K.-i., Savinell, R.F., Eds.; Springer: New York, NY, 2014; pp. 585–593. [Google Scholar]
- Polatides, C.; Dortsiou, M.; Kyriacou, G. Electrochemical removal of nitrate ion from aqueous solution by pulsing potential electrolysis. Electrochim. Acta 2005, 50, 5237–5241. [Google Scholar] [CrossRef]
- Metals Daily. Available online: https://www.metalsdaily.com/data/lmegold/ (accessed on 15 July 2024).
- LME. Available online: https://www.lme.com/ (accessed on 15 July 2024).
- Dimitrijević, S.; Trujić, V.; Ivanović, A.; Dimitrijević, S.; Mirić, M. Recycling of Precious Metals from E-scrap. Iran. J. Chem. Chem. Eng. 2013, 32, 17–23. [Google Scholar] [CrossRef]
- Raiguel, S.; Gijsemans, L.; Van den Bossche, A.; Onghena, B.; Binnemans, K. Solvent Extraction of Gold(III) with Diethyl Carbonate. ACS Sustain. Chem. Eng. 2020, 8, 13713–13723. [Google Scholar] [CrossRef]
- Wojnicki, M.; Pacławski, K. Kinetics of the adsorption of gold(III) chloride complex ions onto activated carbon. Arch. Metall. Mater. 2009, 54, 853–860. [Google Scholar]

| pH | Nitrate Ions Concentration [M] | |
|---|---|---|
| Cementing metal Mg, Al, Zn, Fe | 1.5 | 0 |
| 2 | 0 | |
| 3 | 0 | |
| 2 | 0.5 | |
| 2 | 1 |
| Cementing Metal | Standard Potential [V] | Keq | ΔG [kJ/mol] |
|---|---|---|---|
| Mg/Mg2+ [49] | −2.37 | 1.929 × 10114 | −563.92 |
| Al/Al3+ [50] | −1.66 | 6.377 × 1098 | −652.28 |
| Zn/Zn2+ [51] | −0.76 | 1.121 × 1045 | −257.12 |
| Fe/Fe2+ [52] | −0.44 | 1.930 × 1035 | −201.39 |
| Cementing Metal | Ceq,Pt [M] | Ceq,Au [M] | Ceq,Pd [M] | Ceq,Rh [M] |
|---|---|---|---|---|
| Mg | 7.40 × 10−220 | 4.37 × 10−178 | 3.71 × 10−105 | 5.09 × 10−150 |
| Al | 3.17 × 10−169 | 3.89 × 10−140 | 7.91 × 10−80 | 8.97 × 10−112 |
| Zn | 5.34 × 10−111 | 1.92 × 10−96 | 9.98 × 10−51 | 2.24 × 10−68 |
| Fe | 2.31 × 10−89 | 3.25 × 10−80 | 6.56 × 10−40 | 3.78 × 10−52 |
| Cementing Metal | pH Before | Nitrate Ions Concentration [M] | Pt [ppm] | Au [ppm] | Pd [ppm] | Rh [ppm] | pH After |
|---|---|---|---|---|---|---|---|
| Mg | 1.5 | 0 | 0 | 0 | 0 | 0 | 8.7 |
| 2 | 0 | 0 | 0 | 0 | 0 | 9.3 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 9.6 | |
| 2 | 0.5 | 0.69 | 0.61 | 0.02 | 0 | 8.9 | |
| 2 | 1 | 0.96 | 0.73 | 0 | 0 | 9.0 | |
| Al | 1.5 | 0 | 0 | 0 | 0.05 | 0.02 | 4.1 |
| 2 | 0 | 0 | 0 | 0.06 | 0.02 | 5.9 | |
| 3 | 0 | 0 | 0.46 | 0.28 | 0.07 | 6.1 | |
| 2 | 0.5 | 0 | 0.45 | 0.10 | 0 | 6.1 | |
| 2 | 1 | 0 | 0.54 | 0 | 0 | 6.0 | |
| Zn | 1.5 | 0 | 0 | 0 | 0 | 0 | 5.5 |
| 2 | 0 | 0 | 0 | 0 | 0 | 5.8 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 6.1 | |
| 2 | 0.5 | 0.80 | 0.52 | 0 | 0 | 5.4 | |
| 2 | 1 | 1.15 | 0.67 | 0 | 0 | 5.5 | |
| Fe | 1.5 | 0 | 0 | 0 | 0.19 | 0 | 6.8 |
| 2 | 0 | 0 | 0 | 0.07 | 0 | 6.8 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 7.2 | |
| 2 | 0.5 | 0.80 | 0.48 | 0.17 | 0 | 6.7 | |
| 2 | 1 | 0.97 | 0.56 | 0.27 | 0 | 6.5 |
| Ionic Strength [M] | ||
|---|---|---|
| 0 M NO3− | 0.5 M NO3− | 1 M NO3− |
| 0.010425 | 0.510425 | 1.010425 |
| Chloride Complex | Activity Coefficients | ||
|---|---|---|---|
| 0 M NO3− | 0.5 M NO3− | 1 M NO3− | |
| [PtCl6]2− | 0.6260 | 0.0548 | 0.0207 |
| [AuCl4]− | 0.8895 | 0.4837 | 0.3793 |
| [PdCl4]2− | 0.6260 | 0.0548 | 0.0207 |
| [RhCl6]3− | 0.3486 | 0.0015 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtaszek, K.; Pach, A.; Michalek, T.; Dudek, K.; Wojnicki, M. Assessing Apparent Equilibrium Concentrations in Cementation of Trace Pd, Pt, Au, and Rh from Nitrate Solutions Using Mg, Al, Fe, and Zn. Metals 2024, 14, 990. https://doi.org/10.3390/met14090990
Wojtaszek K, Pach A, Michalek T, Dudek K, Wojnicki M. Assessing Apparent Equilibrium Concentrations in Cementation of Trace Pd, Pt, Au, and Rh from Nitrate Solutions Using Mg, Al, Fe, and Zn. Metals. 2024; 14(9):990. https://doi.org/10.3390/met14090990
Chicago/Turabian StyleWojtaszek, Konrad, Adrianna Pach, Tomasz Michalek, Kamil Dudek, and Marek Wojnicki. 2024. "Assessing Apparent Equilibrium Concentrations in Cementation of Trace Pd, Pt, Au, and Rh from Nitrate Solutions Using Mg, Al, Fe, and Zn" Metals 14, no. 9: 990. https://doi.org/10.3390/met14090990






