Recent Progress of Phase Change Materials and Their Applications in Facility Agriculture and Related-Buildings—A Review
Abstract
:1. Introduction
2. Classification of PCMs
2.1. Inorganic PCMs
| PCMs | Phase Transition Temperature (°C) | Phase Change Latent Heat (kJ/kg) | Thermal Conductivity | References | |
|---|---|---|---|---|---|
| Solid | Liquid | ||||
| CaCl2·6H2O | 29.6 | 212 | 1.08 | 0.56 | [14] |
| 29 | 190.8 | 0.54 | [15] | ||
| 28 | 188.34 | 1.09 | 0.54 | [16] | |
| CaCl2·12H2O | 29.8 | 174 | - | - | [15] |
| Na2SO4·10H2O | 32 | 180 | 1.088 | 0.54 | [14] |
| Na2CO3·10H2O | 33 | 247 | - | - | [9] |
| Na2HPO4·12H2O | 40 | 279 | - | - | [6] |
| FeBr3·6H2O | 21 | 105 | - | - | [6] |
| FeCl3·6H2O | 37 | 223 | - | - | [6] |
| KFe(SO4)2·12H2O | 33 | 173 | - | - | [6] |
| KF·4H2O | 19 | 231 | 0.584 | 0.479 | [9] |
| K2HPO4·6H2O | 14 | 109 | - | - | [6] |
| LiNO3·3H2O | 30 | 256 | 1.46 | 1.425 | [9] |
| Mn(NO3)·6H2O | 25.8 | 125.9 | - | - | [17] |
| Mn(NO3)2·4H2O | 37.1 | 115 | - | - | [6] |
2.2. Organic PCMs
| PCM | Phase Transition Temperature (°C) | Phase Change Latent Heat (kJ/kg) | Density (kg/m3) | Specific Heat (kJ/kg·K) | Thermal Conductivity (W/m·K) | Reference | |
|---|---|---|---|---|---|---|---|
| Solid | Liquid | ||||||
| C14 | 5.5 | 228 | 760 (liquid, 20 °C) | 2.20 | - | - | [6] |
| C15 | 10 | 205 | 770 (liquid, 20 °C) | 2.18 | - | - | [6] |
| C16 | 16.7 | 237.1 | 760 (liquid, 20 °C) | 2.30 | - | - | [6,15] |
| C17 | 21.2 | 213 | 776 (liquid, 20 °C) | 2.35 | - | - | [6,15] |
| C18 | 28 | 244 | 774 (liquid, 70 °C) | 2.40 | 0.15 | - | [6,20] |
| C19 | 32 | 222 | 782 (solid) | 2.45 | - | - | [6,9] |
| C20 | 36.7 | 246 | 779 | 2.50 | - | - | [6] |
| C16H34 | 18.25 | 236 | 770 (solid) | 2.30 | - | - | [9] |
| C17H36 | 22.05 | 214 | 775 (solid) | 2.35 | - | - | [9] |
| 20.8–21.7 | 171–172 | 760 (liquid) | 2.35 | - | - | [15] | |
| C18H38 | 28.25 | 244 | 779 (solid) | 2.40 | - | - | [9] |
| 28 | 200 | 774 (liquid) | 2.14 | 0.358 | 0.148 | [15] | |
| CH3COOH | 17 | 192 | 1049 | 2.05 | - | - | [6] |
| 16.7 | 184 | - | - | - | - | [6] | |
| CH3(CH2)10COOH | 42–44 | 178 | - | - | - | 0.147 | [9] |
| CH3(CH2)8COOH | 32 | 152.7 | 878 (liquid, 45 °C) | 1.80 | 0.153 | - | [15] |
| 36 | 152 | 0.88 | 1.80 | - | - | [6] | |
| 30.1 | 153 | 878 (solid) | 1.79 | 0.16 | 0.16 | [9] | |
| 29.62 | 139.77 | 0.89 | 1.79 | - | - | [30] | |
| CH3(CH2)6COOH | 16.1 | 144.2 | 0.910 | 2.30 | - | - | [30] |
| 16 | 148.5 | - | - | - | 0.149 | [9] | |
| CH3(CH2)10COOH | 42.4 | 186.4 | 0.871 | 1.75 | - | - | [30,31] |
| C21H42O2 | 14–18 | 140–142 | - | - | - | - | [9] |
| C22H44O2 | 19 | 140 | 760 (liquid) | 2.0 | - | - | [15] |
| 18–23 | 123–200 | 872 (liquid) | 2.25 | 0.21 | [15] | ||
| C20H38O2 | 27–29 | 122 | - | - | - | - | [17] |
| C12H22O4 | 19 | 140 | - | - | - | - | [9] |
| C21H 42O2S | 21 | 143 | - | - | - | - | [9] |
| C36H60O2 | 21.9 | 201 | - | - | - | - | [9] |
| C20H 40O2S | 26 | 90 | - | - | - | - | [9] |
| C20H38O2 | 27–29 | 122 | - | - | - | - | [9] |
| SN27 | 27 | 192.6 | 1.53 (solid), 1.71 (liquid) | 1.9–2.2 | 1.05 | 0.58 | [9] |
| BioPCM-Q21 | 21 | 225.6 | 235 (s) | 2.0–2.3 | 0.21 | 0.19 | [9] |
| PEG E600H(OC2H2)n·OH | 22 | 127 | 1126 (l) | 2.30 | - | 0.189 | [15] |
| 20–25 | 146 | - | - | - | - | [6] | |
2.3. Composite PCMs
| Composite PCM | Phase Transition Temperature (°C) | Phase Change Latent Heat (kJ/kg) | Density (kg/m3) | Specific Heat (kJ/kg·K) | Thermal Conductivity (W/m·K) | Reference |
|---|---|---|---|---|---|---|
| Na2SO4·10H2O + 80% Na2HPO4·12H2O + 6% KCl | 23.89 | 183.25 | 1900 | 2.10 | 0.143 | [40] |
| CaCl2·6H2O + NH4Cl + KCl | 24.2 | 149.6 | 1500–1700 | 1.5–2.5 | 0.5–0.6 | [37] |
| Na2SO4·10H2O + Na2CO3·10H2O + NaCl | 23 | 156.7 | - | - | - | [41] |
| 40% Na2CO3·10H2O + 60% Na2HPO4·12H2O | 27.3 | 220.2 | - | - | - | [16] |
| 70% Na2HPO4·12H2O + CH3(CH2)8COOH | 33 | 168.8 | - | - | 0.468 (15 °C) | [33] |
| CaCl2·6H2O + SrCl2·6H2O + TiO2 + EG | 10.67 | 88.39 | - | - | 8.831 | [36] |
| C18+C21 | 25.8–26 | 173.93 | - | - | - | [15] |
| C18+C22 | 25.5–27 | 203.80 | - | - | - | [15] |
| CH3(CH2)8COOH + C12H24O2 | 18 | 120 | - | - | 0.143 | [15] |
| 21 | 143 | - | - | - | [15] | |
| 75.2% CH3(CH 2)8COOH + 24.8% C16H32O2 | 22.1 | 153 | - | - | - | [15] |
| CaCl2·6H2O + CaBr2·6H2O | 14.7 | 140 | - | - | - | [15] |
| C14H28O2 + C10H20O2 | 24 | 147.7 | - | - | - | [15] |
| CaCl2 + MgCl2·6H2O | 25 | 95 | - | - | - | [15] |
| 75% CaCl2·6H2O + 25% MgCl2·6H2O | 21.4 | 102.3 | 1590 | - | - | [16] |
| 66% CaCl2·6H2O + 33% MgCl2·6H2O | 25 | 127 | - | - | - | [16] |
| Na2SO4·10H2O-Na2CO3·10H2O + EV | 23.98 | 110.3 | - | - | 0.192 | [35] |
| 67% Ca(NO3)2·4H2O + 33% Mg(NO3)2·6H2O | 30 | 136 | - | - | - | [16] |
| CH3CONH2 + NH2CONH2 | 27 | 163 | - | - | - | [15] |
| RT25-RT30 | 26.6 | 232 | 785 | 2.2 | 0.19 (solid) 0.18 (liquid) | [9] |
| Triethylolethane + urea | 29.8 | 218 | - | - | - | [16] |
| Triethylolethane + water + urea | 13.4 | 160 | - | - | - | [9] |
| Ca(NO3)·4H2O + Mg(NO3)3·6H2O | 30 | 136 | - | - | - | [16] |
| 45% Ca(NO3)2·6H2O + 55% Zn(NO3)2·6H2O | 25 | 130 | - | - | - | [16] |
| CH3COONa·3H2O + NH2CONH2 | 30 | 200.5 | - | - | - | [15] |
3. Encapsulation and Utilization Methods for PCMs
3.1. Macro-Encapsulated PCMs
3.2. Shape-Stabilized PCMs
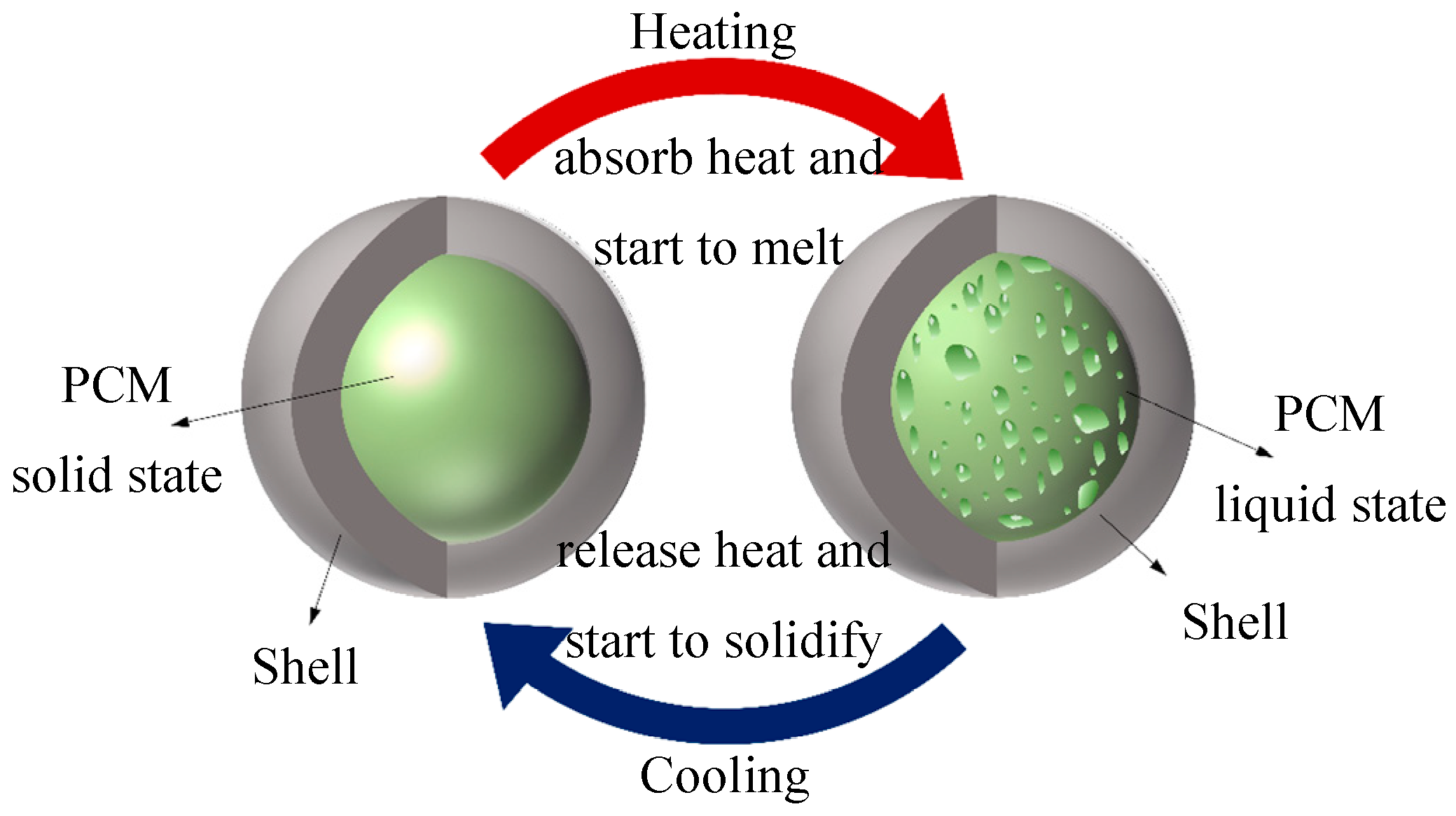
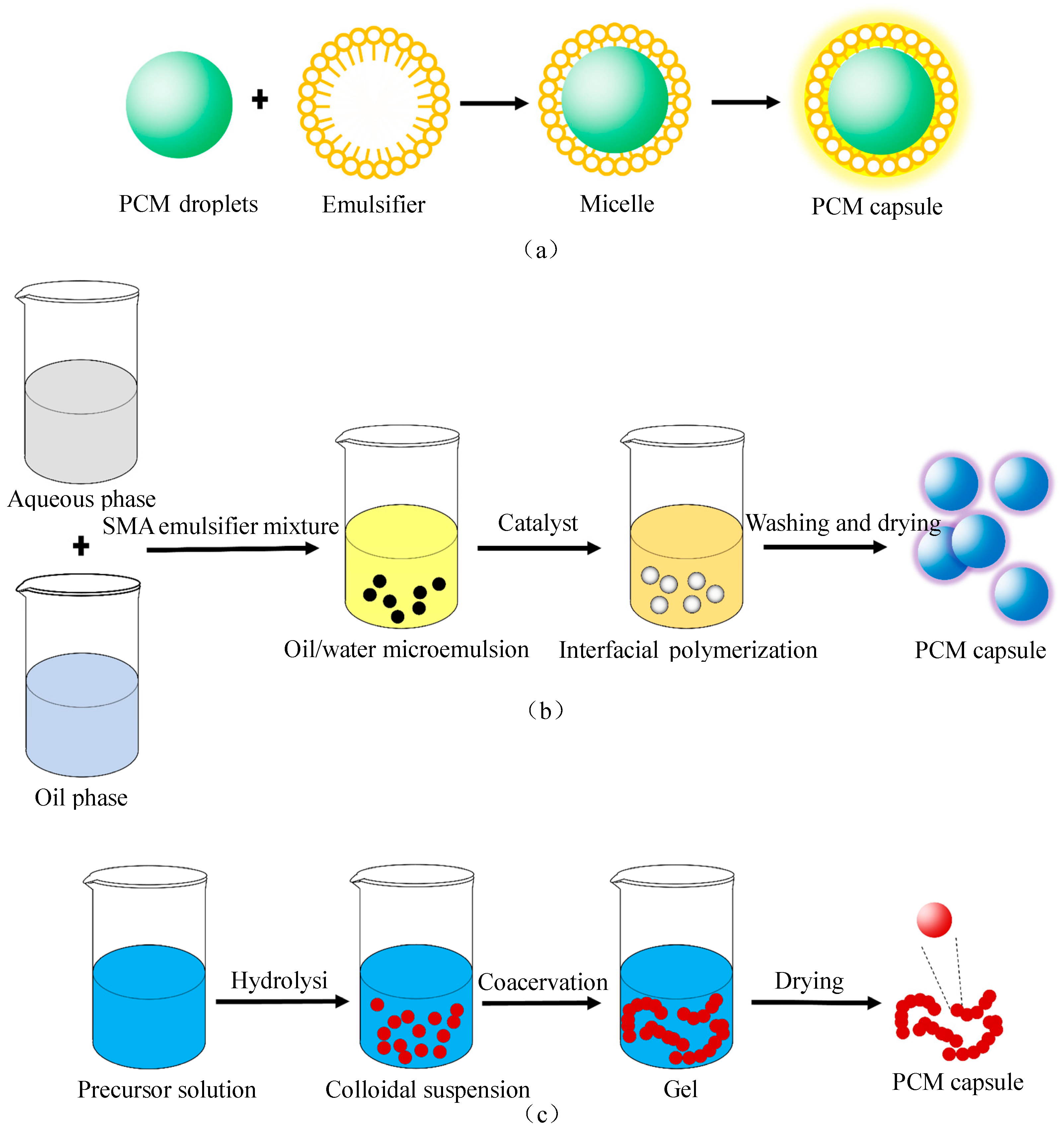
3.3. Phase Change Capsules (PCCs)
| PCM | Preparation Method | Size and Appearance | Heat Property | Reference |
|---|---|---|---|---|
| N-tetradecane–urea and formaldehyde polymerization | In situ polymerization | Particle size is about 100 nm | Melting latent heat of 134.16 kJ/kg, with a high mass fraction of 60% | [67] |
| Paraffin wax and C18–melamine formaldehyde resin | In situ polymerization | Diameter is 4 µm | Phase change enthalpies of paraffin wax and C18 microcapsules are as high as 164.8 and 185.1 J/g, respectively, corresponding to core material contents of 87.0% and 84.3% | [68] |
| 63% capric acid and 37% palmitic acid–melamine formaldehyde | In situ polymerization | Spherical, smooth, and evenly distributed | Encapsulation efficiency is approximately 75.2%; the melting point is 36.1 °C, the melting latent heat is 106.2 kJ/kg | [66] |
| N-eicosane–polyurea | Interfacial polymerization | Good and intact spherical shape | The encapsulation ratio is 82.9%; the melting latent heat is 209.8 kJ/kg | [69] |
| Paraffin wax–SiO2 dioxide | Sol–gel | ME PCM size is about 8–15 µm | The encapsulation rate of paraffin wax is 87.5%; microencapsulated paraffin composite material solidifies at 58.27 °C with a PCLH value of 107.05 kJ/kg, and melts at 58.37 °C, with a PCLH value of 165.68 kJ/kg | [71] |
| Stearic acid–SiO2 dioxide | Sol–gel | PCM size is 20–30 µm | The encapsulation rate is 90.7%; microencapsulated SA melts at 53.5 °C, with a PCLH value of 171.0 kJ/kg, and solidifies at 52.6 °C, with a PCLH value of 162.0 kJ/kg | [72] |
| Eutectic mixture of capric acid and stearic acid–silica | Self-templating method | Regular spherical particles with dense surfaces | The phase transition temperature and PCLH values are 21.4 °C and 91.48 J/g, respectively | [70] |
| Paraffin wax–gelatin/gum arabic | Coacervation and spray drying | Microcapsules are spherical with a relatively uniform structure | Microcapsules have high energy storage and release capabilities (145–240 J/g) | [62] |
4. Applications of PCMs
4.1. Passive PCHS System
4.1.1. Phase Change Walls (PCWs)
4.1.2. Phase Change Heat Storage Units
4.1.3. Challenges and Future Works
4.2. Active PCHS Systems
4.2.1. Solar Heat Source Active PCHS Systems
4.2.2. Geothermal Heat Source Active PCHS Systems
4.2.3. Other Heat Source Active PCHS Systems
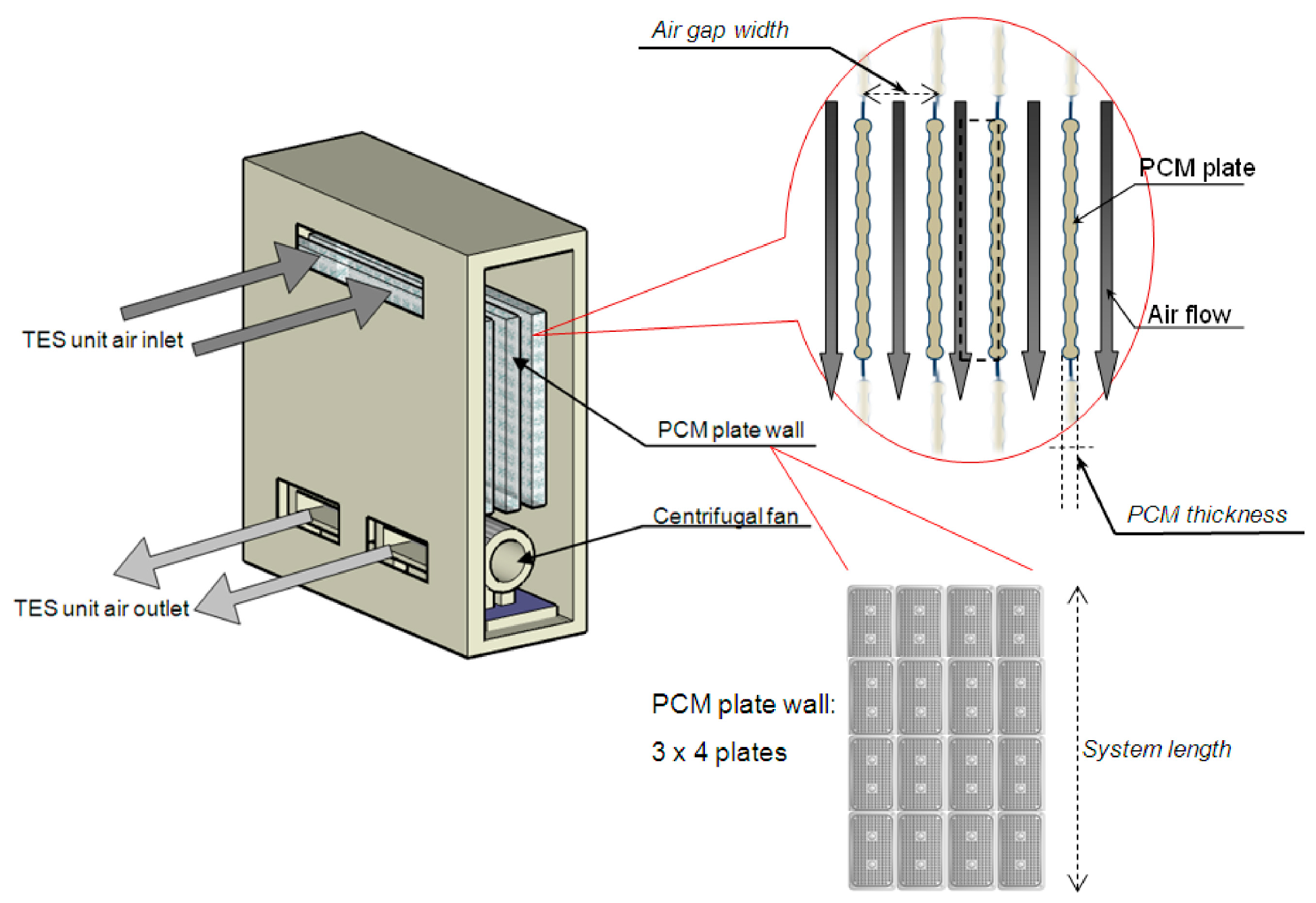
| PCM | Encapsulation and Utilization Method | Application | Crop | Method | Reference |
|---|---|---|---|---|---|
| CaCl2·6H2O | Macro-encapsulated PCM | PCW | Plant | Numerical simulation | [11] |
| Paraffin wax, graphite, high-density polyethylene, cement mortar | Shape-stabilized PCM | PCW | Vegetable | Physical experiment; numerical model; matrix laboratory | [79] |
| Paraffin wax | Macro-encapsulated PCM | PCW | Plant | Physical experiment and numerical simulation | [78] |
| Paraffin wax (RT18H) | Macro-encapsulated PCM | PCHS unit | Tomato | Physical experiment | [42] |
| A mixture of 40% oleic acid and 60% capric acid | Macro-encapsulated PCM | PCHS unit | Zucchini and pepper | Field measurement | [26] |
| Cobalt chloride automatic regulation for sunshade protection to prevent overheating | Macro-encapsulated PCM | PCHS unit | Shade-loving plant | DSC measurement | [112] |
| CaCl2·6H2O | Macro-encapsulated PCM | PCHS unit | Plant | Physical experiment | [12] |
| TH29, PCM 21, PCM 17 | PCW | None | Mathematical model, Java code program | [113] | |
| Butyl stearate | Macro-encapsulated PCM | PCW | Spinach | Model and physical experiment | [27] |
| Sodium acetate hydrate | Macro-encapsulated PCM | Active PCHS systems with other heat sources | Cucumber | Model and physical experiment | [13] |
| Commercial salt hydrate PCM (S19) | Macro-encapsulated PCM | Active PCHS systems with geothermal energy sources | Tomato | Theoretical model | [114] |
| Capsule (AC27) | PCC | Active PCHS systems with solar energy sources | Tomato | Physical experiment | [99] |
| CaCl2·6H2O | PCC | Active PCHS systems with solar energy sources | Plant | Physical experiment | [100] |
| Paraffin wax | Macro-encapsulated PCM | Active PCHS systems with solar energy sources | Pepper | Physical experiment | [29] |
4.2.4. Challenges and Future Works
4.3. Applications of PCMs in Buildings
4.3.1. Passive PCHS Systems in Buildings
4.3.2. Active PCHS System in Buildings
4.3.3. Challenges and Future Works
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, W.; Bu, K.; Zha, L.; Zhang, J.; Lai, D.; Bao, H. Energy Consumption of Plant Factory with Artificial Light: Challenges and Opportunities. arXiv 2024, arXiv:2405.09643. [Google Scholar]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Engler, N.; Krarti, M.J.E. Optimal designs for net zero energy controlled environment agriculture facilities. Energy Build. 2022, 272, 112364. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Sun, M.; Wu, J. Potential of technological innovation to reduce the carbon footprint of urban facility agriculture: A food–energy–water–waste nexus perspective. J. Environ. Manag. 2023, 339, 117806. [Google Scholar] [CrossRef] [PubMed]
- Maraveas, C.; Karavas, C.-S.; Loukatos, D.; Bartzanas, T.; Arvanitis, K.G.; Symeonaki, E. Agricultural greenhouses: Resource management technologies and perspectives for zero greenhouse gas emissions. Agriculture 2023, 13, 1464. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Goel, V.; Saxena, A.; Kumar, M.; Thakur, A.; Sharma, A.; Bianco, V.J.A.T.E. Potential of phase change materials and their effective use in solar thermal applications: A critical review. Appl. Therm. Eng. 2023, 219, 119417. [Google Scholar] [CrossRef]
- Pankaew, P.; Aumporn, O.; Janjai, S.; Pattarapanitchai, S.; Sangsan, M.; Bala, B.K. Performance of a large-scale greenhouse solar dryer integrated with phase change material thermal storage system for drying of chili. Int. J. Green Energy 2020, 17, 632–643. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Hua, W.; Ji, J.; Lv, X. Current status and development of research on phase change materials in agricultural greenhouses: A review. J. Energy Storage 2023, 66, 107104. [Google Scholar] [CrossRef]
- Hadjieva, M.; Stoykov, R.; Filipova, T. Composite salt-hydrate concrete system for building energy storage. Renew. Energy 2000, 19, 111–115. [Google Scholar] [CrossRef]
- Berroug, F.; Lakhal, E.K.; El Omari, M.; Faraji, M.; El Qarnia, H. Thermal performance of a greenhouse with a phase change material north wall. Energy Build. 2011, 43, 3027–3035. [Google Scholar] [CrossRef]
- Benli, H.; Durmuş, A. Performance analysis of a latent heat storage system with phase change material for new designed solar collectors in greenhouse heating. Sol. Energy 2009, 83, 2109–2119. [Google Scholar] [CrossRef]
- Yan, S.-R.; Fazilati, M.A.; Samani, N.; Ghasemi, H.R.; Toghraie, D.; Nguyen, Q.; Karimipour, A. Energy efficiency optimization of the waste heat recovery system with embedded phase change materials in greenhouses: A thermo-economic-environmental study. J. Energy Storage 2020, 30, 101445. [Google Scholar] [CrossRef]
- Liu, W.; Bie, Y.; Xu, T.; Cichon, A.; Królczyk, G.; Li, Z. Heat transfer enhancement of latent heat thermal energy storage in solar heating system: A state-of-the-art review. J. Energy Storage 2022, 46, 103727. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Aridi, R.; Yehya, A. Review on the sustainability of phase-change materials used in buildings. Energy Convers. Manag. X 2022, 15, 100237. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Aydın, A.A.; Okutan, H. High-chain fatty acid esters of myristyl alcohol with even carbon number: Novel organic phase change materials for thermal energy storage—1. Sol. Energy Mater. Sol. Cells 2011, 95, 2752–2762. [Google Scholar] [CrossRef]
- Vélez, C.; Ortiz de Zárate, J.M.; Khayet, M. Thermal properties of n-pentadecane, n-heptadecane and n-nonadecane in the solid/liquid phase change region. Int. J. Therm. Sci. 2015, 94, 139–146. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef]
- Cunha, S.; Aguiar, J.B.; Ferreira, V.M.; Tadeu, A. Influence of the Type of Phase Change Materials Microcapsules on the Properties of Lime-Gypsum Thermal Mortars. Adv. Eng. Mater. 2014, 16, 433–441. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Hawes, D.; Ghanbari, E. Obtaining an energy storing building material by direct incorporation of an organic phase change material in gypsum wallboard. Sol. Energy Mater. 1991, 22, 231–242. [Google Scholar] [CrossRef]
- Athienitis, A.K.; Liu, C.; Hawes, D.; Banu, D.; Feldman, D. Investigation of the thermal performance of a passive solar test-room with wall latent heat storage. Build. Environ. 1997, 32, 405–410. [Google Scholar] [CrossRef]
- Feijoo, J.; Alvarez-Feijoo, M.A.; Fort, R.; Arce, E.; Ergenç, D. Effects of paraffin additives, as phase change materials, on the behavior of a traditional lime mortar. Constr. Build. Mater. 2022, 361, 129734. [Google Scholar] [CrossRef]
- Lecompte, T.; Le Bideau, P.; Glouannec, P.; Nortershauser, D.; Le Masson, S. Mechanical and thermo-physical behaviour of concretes and mortars containing phase change material. Energy Build. 2015, 94, 52–60. [Google Scholar] [CrossRef]
- Beyhan, B.; Paksoy, H.; Daşgan, Y. Root zone temperature control with thermal energy storage in phase change materials for soilless greenhouse applications. Energy Convers. Manag. 2013, 74, 446–453. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Ren, W.; Liu, S. Thermal Environment Regulating Effects of Phase Change Material in Chinese Style Solar Greenhouse. Energy Procedia 2014, 61, 2071–2074. [Google Scholar] [CrossRef]
- Enibe, S.O. Performance of a natural circulation solar air heating system with phase change material energy storage. Renew. Energy 2002, 27, 69–86. [Google Scholar] [CrossRef]
- Azaizia, Z.; Kooli, S.; Hamdi, I.; Elkhal, W.; Guizani, A.A. Experimental study of a new mixed mode solar greenhouse drying system with and without thermal energy storage for pepper. Renew. Energy 2020, 145, 1972–1984. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Yinping, Z.; Yi, J. A simple method, the-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, Y.; Liu, X.; Luo, Y. Thermal performance of sodium acetate trihydrate based composite phase change material for thermal energy storage. Appl. Therm. Eng. 2018, 143, 172–181. [Google Scholar] [CrossRef]
- Wang, P.; Feng, X.; Zhu, Y.; Lian, J.; Zhang, H.; Fang, M. Preparation and thermal properties of colloidal mixtures of capric acid and Na2HPO4·12H2O as a phase change material for energy storage. Sol. Energy Mater. Sol. Cells 2020, 215, 110636. [Google Scholar] [CrossRef]
- Chen, W.; Liang, X.; Wang, S.; Ding, Y.; Gao, X.; Zhang, Z.; Fang, Y. SiO2 hydrophilic modification of expanded graphite to fabricate form-stable ternary nitrate composite room temperature phase change material for thermal energy storage. Chem. Eng. J. 2021, 413, 127549. [Google Scholar] [CrossRef]
- Xie, N.; Luo, J.; Li, Z.; Huang, Z.; Gao, X.; Fang, Y.; Zhang, Z. Salt hydrate/expanded vermiculite composite as a form-stable phase change material for building energy storage. Sol. Energy Mater. Sol. Cells 2019, 189, 33–42. [Google Scholar] [CrossRef]
- Zou, T.; Fu, W.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Hydrophilic modification of expanded graphite to develop form-stable composite phase change material based on modified CaCl2·6H2O. Energy 2020, 190, 116473. [Google Scholar] [CrossRef]
- Dong, O.; Li, D.; Zeng, D. A novel eutectic phase-change material: CaCl2·6H2O + NH4Cl + KCl. Calphad 2018, 63, 92–99. [Google Scholar] [CrossRef]
- Praveen, B.; Suresh, S.; Pethurajan, V. Heat transfer performance of graphene nano-platelets laden micro-encapsulated PCM with polymer shell for thermal energy storage based heat sink. Appl. Therm. Eng. 2019, 156, 237–249. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sarı, A.; Biçer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, W.; Li, S.; Zhang, J.; Yi, M. Preparation and properties of Na2SO4•10H2O-Na2HPO4•12H2O composite shaped phase change material for greenhouse. Chem. Ind. Eng. Prog. 2022, 41, 920–929. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Tie, S.; Wang, C. Preparation and Thermal Performance of Nano-Graphene Oxide/Mirabilite Composite Phase Change Materials. J. Chin. Ceram. Soc. 2022, 50, 1642–1651. [Google Scholar] [CrossRef]
- Llorach-Massana, P.; Peña, J.; Rieradevall, J.; Montero, J.I. LCA & LCCA of a PCM application to control root zone temperatures of hydroponic crops in comparison with conventional root zone heating systems. Renew. Energy 2016, 85, 1079–1089. [Google Scholar] [CrossRef]
- Llorach-Massana, P.; Peña, J.; Rieradevall, J.; Montero, J.I. Analysis of the technical, environmental and economic potential of phase change materials (PCM) for root zone heating in Mediterranean greenhouses. Renew. Energy 2017, 103, 570–581. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Chen, Y.; Liu, W. Usage strategy of phase change materials in plastic greenhouses, in hot summer and cold winter climate. Appl. Energy 2020, 277, 115416. [Google Scholar] [CrossRef]
- Arkar, C.; Vidrih, B.; Medved, S. Efficiency of free cooling using latent heat storage integrated into the ventilation system of a low energy building. Int. J. Refrig. 2007, 30, 134–143. [Google Scholar] [CrossRef]
- Kong, X.; Lu, S.; Huang, J.; Cai, Z.; Wei, S. Experimental research on the use of phase change materials in perforated brick rooms for cooling storage. Energy Build. 2013, 62, 597–604. [Google Scholar] [CrossRef]
- Benli, H.; Durmuş, A. Evaluation of ground-source heat pump combined latent heat storage system performance in greenhouse heating. Energy Build. 2009, 41, 220–228. [Google Scholar] [CrossRef]
- Chang, S.J.; Wi, S.; Jeong, S.-G.; Kim, S. Thermal performance evaluation of macro-packed phase change materials (PCMs) using heat transfer analysis device. Energy Build. 2016, 117, 120–127. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Zhu, Y.; Li, D.; Ma, L. Experimental investigation of optical and thermal performance of a PCM-glazed unit for building applications. Energy Build. 2018, 158, 794–800. [Google Scholar] [CrossRef]
- Silva, T.; Vicente, R.; Soares, N.; Ferreira, V. Experimental testing and numerical modelling of masonry wall solution with PCM incorporation: A passive construction solution. Energy Build. 2012, 49, 235–245. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Chung, O.; Jeong, S.-G.; Kim, S. Preparation of energy efficient paraffinic PCMs/expanded vermiculite and perlite composites for energy saving in buildings. Sol. Energy Mater. Sol. Cells 2015, 137, 107–112. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Lu, Z.; Li, Z. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites. Appl. Energy 2015, 160, 358–367. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Capric–myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage. Sol. Energy 2009, 83, 323–332. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Capric–myristic acid/expanded perlite composite as form-stable phase change material for latent heat thermal energy storage. Renew. Energy 2008, 33, 2599–2605. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Li, W.; Zheng, J.; Tian, W.; Li, X. Shape-stabilized phase change materials based on polyethylene glycol/porous carbon composite: The influence of the pore structure of the carbon materials. Sol. Energy Mater. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Wang, Z.; Situ, W.; Li, X.; Zhang, G.; Huang, Z.; Yuan, W.; Yang, C.; Yang, C. Novel shape stabilized phase change material based on epoxy matrix with ultrahigh cycle life for thermal energy storage. Appl. Therm. Eng. 2017, 123, 1006–1012. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Xuan, Y.; Huang, Y.; Li, Q. Experimental investigation on thermal conductivity and specific heat capacity of magnetic microencapsulated phase change material suspension. Chem. Phys. Lett. 2009, 479, 264–269. [Google Scholar] [CrossRef]
- Hawlader, M.N.A.; Uddin, M.S.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Fang, G. Encapsulation of inorganic phase change thermal storage materials and its effect on thermophysical properties: A review. Sol. Energy Mater. Sol. Cells 2022, 241, 111747. [Google Scholar] [CrossRef]
- Srinivasaraonaik, B.; Singh, L.P.; Sinha, S.; Tyagi, I.; Rawat, A. Studies on the mechanical properties and thermal behavior of microencapsulated eutectic mixture in gypsum composite board for thermal regulation in the buildings. J. Build. Eng. 2020, 31, 101400. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Yang, F.; Liu, X.; Wu, S. Preparation and characterization of nano-encapsulated n-tetradecane as phase change material for thermal energy storage. Chem. Eng. J. 2009, 153, 217–221. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Chang, T.; Wang, J.; Wang, X.; Zhou, G. Phase change material microcapsules with melamine resin shell via cellulose nanocrystal stabilized Pickering emulsion in-situ polymerization. Chem. Eng. J. 2022, 428, 131164. [Google Scholar] [CrossRef]
- Yan, B.; Lu, H.; Li, M.; Wang, X.; Wang, Z.; Pi, M.; Cui, W.; Ran, R. Preparation of phase change microcapsules with high thermal storage and temperature sensitive for thermal management. J. Energy Storage 2023, 64, 107003. [Google Scholar] [CrossRef]
- Song, S.; Dong, L.; Qu, Z.; Ren, J.; Xiong, C. Microencapsulated capric–stearic acid with silica shell as a novel phase change material for thermal energy storage. Appl. Therm. Eng. 2014, 70, 546–551. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, L.; Shan, F.; Fang, G. Preparation and characteristics of microencapsulated stearic acid as composite thermal energy storage material in buildings. Energy Build. 2013, 62, 469–474. [Google Scholar] [CrossRef]
- Thaler, S.M.; Zwatz, J.; Nicolay, P.; Hauser, R.; Lackner, R. An Innovative Heating Solution for Sustainable Agriculture: A Feasibility Study on the Integration of Phase Change Materials as Passive Heating Elements. Appl. Sci. 2024, 14, 7419. [Google Scholar] [CrossRef]
- Santamouris, M.; Balaras, C.A.; Dascalaki, E.; Vallindras, M. Passive solar agricultural greenhouses: A worldwide classification and evaluation of technologies and systems used for heating purposes. Sol. Energy 1994, 53, 411–426. [Google Scholar] [CrossRef]
- Sethi, V.P.; Sharma, S.K. Survey and evaluation of heating technologies for worldwide agricultural greenhouse applications. Sol. Energy 2008, 82, 832–859. [Google Scholar] [CrossRef]
- Sharma, V.; Rai, A.C. Performance assessment of residential building envelopes enhanced with phase change materials. Energy Build. 2020, 208, 109664. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Xu, H. Performance of phase change thermal storage wallboard of disodium hydrogen phosphate dodecahydrate in solar greenhouses. J. Shanghai Jiaotong Univ. 2014, 32, 88–94. [Google Scholar]
- Wang, X.; Sun, G.; Zhang, L.; Lei, W.; Zhang, W.; Li, H.; Zhang, C.; Guo, J. Application of green energy in smart rural passive heating: A case study of indoor temperature self-regulating greenhouse of winter in Jinan, China. Energy 2023, 278, 127770. [Google Scholar] [CrossRef]
- Ling, H.; Wang, L.; Chen, C.; Wang, Y.; Chen, H. Effect of thermophysical properties correlation of phase change material on numerical modelling of agricultural building. Appl. Therm. Eng. 2019, 157, 113579. [Google Scholar] [CrossRef]
- Kalbasi, R.; Tahmasebi, A.; Ghaderi, M.; Yari, M.; Izadi, F. Toward sustainable energy-based buildings with focusing on electricity demand reduction–Case studies in Middle East region climate. Sustain. Energy Technol. Assess. 2022, 52, 102294. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, W.; Jian, Y.; Arıcı, M.; Chen, Y.; Shen, Q. Thermal environment evaluation of plastic greenhouses in southern China and optimization by phase change materials. J. Build. Eng. 2022, 57, 104882. [Google Scholar] [CrossRef]
- Arias, F.J. On the use of encapsulated phase change materials pebbles and pellets as freeze protection method for low-stature plants/crops. Eng. Agric. Environ. Food 2016, 9, 274–279. [Google Scholar] [CrossRef]
- Jiang, Z.; Tie, S. Property and heat storage performances of Glauber's salt-based phase change materials for solar greenhouse in Qinghai-Tibet plateau. Trans. Chin. Soc. Agric. Eng. 2016, 32, 209–216. [Google Scholar]
- Bao, L.; Hou, Q.; Wang, K.; Jiang, Z. Study on preparation and application of inorganic composite phase change materials for solar greenhouse. Inorg. Chem. Ind. 2022, 54, 61–69. [Google Scholar] [CrossRef]
- Fan, L.; Khodadadi, J.M. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2011, 15, 24–46. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, X.; Ma, Z. A review of the composite phase change materials: Fabrication, characterization, mathematical modeling and application to performance enhancement. Appl. Energy 2016, 165, 472–510. [Google Scholar] [CrossRef]
- Reddy, V.J.; Ghazali, M.F.; Kumarasamy, S. Innovations in phase change materials for diverse industrial applications: A comprehensive review. Results Chem. 2024, 8, 101552. [Google Scholar] [CrossRef]
- ELSihy, E.S.; Xie, H.; Lin, H.; Du, X.; Wang, Z. Combined effects of upward eccentricity and volume fraction of graphene nanoparticles on the melting performance of a horizontal double-tube latent heat storage unit. Int. Commun. Heat Mass Transf. 2024, 158, 107906. [Google Scholar] [CrossRef]
- Ibrahem, A.M.; El-Amin, M.F.; Sun, S. Effects of nanoparticles on melting process with phase-change using the lattice Boltzmann method. Results Phys. 2017, 7, 1676–1682. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, X.; Zhang, C.; Huang, Y.; Chen, Y. Melting behaviors of PCM in porous metal foam characterized by fractal geometry. Int. J. Heat Mass Transf. 2017, 113, 1031–1042. [Google Scholar] [CrossRef]
- Younis, O.; Mozaffari, M.; Ahmed, A.; Ghalambaz, M. Improvement of Latent Heat Thermal Energy Storage Rate for Domestic Solar Water Heater Systems Using Anisotropic Layers of Metal Foam. Buildings 2024, 14, 2322. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, D.; Sun, D.; Liu, X. Experimental and numerical studies on the heat transfer improvement of a latent heat storage unit using gradient tree-shaped fins. Int. J. Heat Mass Transf. 2022, 182, 121920. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X. Charging and discharging enhancement of a vertical latent heat storage unit by fractal tree-shaped fins. Renew. Energy 2021, 174, 199–217. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Chen, Y. Improving the energy discharging performance of a latent heat storage (LHS) unit using fractal-tree-shaped fins. Appl. Energy 2020, 259, 114102. [Google Scholar] [CrossRef]
- Elsihy, E.S.; Cai, C.; Du, X.; Wang, Z. Influences of the number and length of longitudinal fins on the single and cyclic charging and discharging performance of vertical double-tube latent heat storage systems. J. Energy Storage 2024, 86, 111393. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Sheng, J.; Li, B.; Chen, Y. Dynamic thermal response behaviors of pumped two-phase loop with latent heat storage. Int. J. Heat Mass Transf. 2024, 225, 125382. [Google Scholar] [CrossRef]
- Öztürk, H.H. Experimental evaluation of energy and exergy efficiency of a seasonal latent heat storage system for greenhouse heating. Energy Convers. Manag. 2005, 46, 1523–1542. [Google Scholar] [CrossRef]
- Kooli, S.; Bouadila, S.; Lazaar, M.; Farhat, A. The effect of nocturnal shutter on insulated greenhouse using a solar air heater with latent storage energy. Sol. Energy 2015, 115, 217–228. [Google Scholar] [CrossRef]
- Baddadi, S.; Bouadila, S.; Ghorbel, W.; Guizani, A. Autonomous greenhouse microclimate through hydroponic design and refurbished thermal energy by phase change material. J. Clean. Prod. 2019, 211, 360–379. [Google Scholar] [CrossRef]
- Bouadila, S.; Kooli, S.; Skouri, S.; Lazaar, M.; Farhat, A. Improvement of the greenhouse climate using a solar air heater with latent storage energy. Energy 2014, 64, 663–672. [Google Scholar] [CrossRef]
- Nishad, S.; Krupa, I. Phase change materials for thermal energy storage applications in greenhouses: A review. Sustain. Energy Technol. Assess. 2022, 52, 102241. [Google Scholar] [CrossRef]
- Ziapour, B.M.; Hashtroudi, A. Performance study of an enhanced solar greenhouse combined with the phase change material using genetic algorithm optimization method. Appl. Therm. Eng. 2017, 110, 253–264. [Google Scholar] [CrossRef]
- Yang, X.; Sun, D.; Li, J.; Yu, C.; Deng, Y.; Yu, B. Demonstration study on ground source heat pump heating system with solar thermal energy storage for greenhouse heating. J. Energy Storage 2022, 54, 105298. [Google Scholar] [CrossRef]
- Luo, J.; Li, A.; Ma, X.; Pei, K. Stabilization of the temperature in a greenhouse using a Geothermal-Battery-Energy-Storage (GBES) system. Appl. Therm. Eng. 2023, 223, 120029. [Google Scholar] [CrossRef]
- Ananno, A.A.; Masud, M.H.; Dabnichki, P.; Ahmed, A. Design and numerical analysis of a hybrid geothermal PCM flat plate solar collector dryer for developing countries. Sol. Energy 2020, 196, 270–286. [Google Scholar] [CrossRef]
- El Khadraoui, A.; Bouadila, S.; Kooli, S.; Farhat, A.; Guizani, A. Thermal behavior of indirect solar dryer: Nocturnal usage of solar air collector with PCM. J. Clean. Prod. 2017, 148, 37–48. [Google Scholar] [CrossRef]
- Benli, H. Energetic performance analysis of a ground-source heat pump system with latent heat storage for a greenhouse heating. Energy Convers. Manag. 2011, 52, 581–589. [Google Scholar] [CrossRef]
- Andrews, R.; Pearce, J.M. Environmental and economic assessment of a greenhouse waste heat exchange. J. Clean. Prod. 2011, 19, 1446–1454. [Google Scholar] [CrossRef]
- Yan, S.; Fazilati, M.A.; Toghraie, D.; Khalili, M.; Karimipour, A. Energy cost and efficiency analysis of greenhouse heating system enhancement using phase change material: An experimental study. Renew. Energy 2021, 170, 133–140. [Google Scholar] [CrossRef]
- Dolado, P.; Lazaro, A.; Delgado, M.; Peñalosa, C.; Mazo, J.; Marin, J.M.; Zalba, B. An approach to the integrated design of PCM-air heat exchangers based on numerical simulation: A solar cooling case study. Resources 2015, 4, 796–818. [Google Scholar] [CrossRef]
- Marinković, M.; Nikolić, R.; Savović, J.; Gadžurić, S.; Zsigrai, I. Thermochromic complex compounds in phase change materials: Possible application in an agricultural greenhouse. Sol. Energy Mater. Sol. Cells 1998, 51, 401–411. [Google Scholar] [CrossRef]
- Najjar, A.; Hasan, A. Modeling of greenhouse with PCM energy storage. Energy Convers. Manag. 2008, 49, 3338–3342. [Google Scholar] [CrossRef]
- Vadiee, A.; Martin, V. Thermal energy storage strategies for effective closed greenhouse design. Appl. Energy 2013, 109, 337–343. [Google Scholar] [CrossRef]
- Benkaddour, A.; Faraji, M.; Faraji, H. Numerical study of the thermal energy storage behaviour of a novel composite PCM/Concrete wall integrated solar collector. Mater. Today Proc. 2020, 30, 905–908. [Google Scholar] [CrossRef]
- Laasri, I.A.; Es-sakali, N.; Charai, M.; Mghazli, M.O.; Outzourhit, A. Recent progress, limitations, and future directions of macro-encapsulated phase change materials for building applications. Renew. Sustain. Energy Rev. 2024, 199, 114481. [Google Scholar] [CrossRef]
- Figueiredo, A.; Silva, T.; Goncalves, M.; Samagaio, A. Application of Novel Phase Change Material Constructive Solution for Thermal Regulation of Passive Solar Buildings. Buildings 2024, 14, 493. [Google Scholar] [CrossRef]
- Jia, Z.; Cunha, S.; Aguiar, J.; Guo, P. The Effect of Phase Change Materials on the Physical and Mechanical Properties of Concrete Made with Recycled Aggregate. Buildings 2023, 13, 2601. [Google Scholar] [CrossRef]
- Mano, C.; Fazlizan, A.; Thongtha, A. Enhancing Thermal Efficiency through the Use of Graphite-Infused Phase Change Materials in Roof Structures to Reduce Building Cooling Demand. Buildings 2024, 14, 68. [Google Scholar] [CrossRef]
- Mahdaoui, M.; Hamdaoui, S.; Msaad, A.A.; Kousksou, T.; El Rhafiki, T.; Jamil, A.; Ahachad, M. Building bricks with phase change material (PCM): Thermal performances. Constr. Build. Mater. 2021, 269, 121315. [Google Scholar] [CrossRef]
- Iqbal, S.; Tang, J.; Raza, G.; Cheema, I.I.; Kazmi, M.A.; Li, Z.; Wang, B.; Liu, Y. Experimental and numerical analyses of thermal storage tile-bricks for efficient thermal management of buildings. Buildings 2021, 11, 357. [Google Scholar] [CrossRef]
- Mourid, A.; El Alami, M.; Kuznik, F. Experimental investigation on thermal behavior and reduction of energy consumption in a real scale building by using phase change materials on its envelope. Sustain. Cities Soc. 2018, 41, 35–43. [Google Scholar] [CrossRef]
- Bravo, J.P.; Venegas, T.; Correa, E.; Álamos, A.; Sepúlveda, F.; Vasco, D.A.; Barreneche, C. Experimental and computational study of the implementation of mPCM-modified gypsum boards in a test enclosure. Buildings 2020, 10, 15. [Google Scholar] [CrossRef]
- Dardouri, S.; Tunçbilek, E.; Khaldi, O.; Arıcı, M.; Sghaier, J. Optimizing PCM integrated wall and roof for energy saving in building under various climatic conditions of Mediterranean region. Buildings 2023, 13, 806. [Google Scholar] [CrossRef]
- Jaradat, M.; Al Majali, H.; Bendea, C.; Bungau, C.C.; Bungau, T. Enhancing Energy Efficiency in Buildings through PCM Integration: A Study across Different Climatic Regions. Buildings 2023, 14, 40. [Google Scholar] [CrossRef]
- Gao, X.; Sheng, Q.; Li, N. A Numerical Investigation of the Influence of Humid Environments on the Thermal Performance of a Phase Change Thermal Storage Cooling System in Buildings. Buildings 2024, 14, 1161. [Google Scholar] [CrossRef]
- Khadra, A.; Akander, J.; Myhren, J.A. Greenhouse Gas Payback Time of Different HVAC Systems in the Renovation of Nordic District-Heated Multifamily Buildings Considering Future Energy Production Scenarios. Buildings 2024, 14, 413. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y. HVAC Design Optimization for Pharmaceutical Facilities with BIM and CFD. Buildings 2024, 14, 1627. [Google Scholar] [CrossRef]
- Bekkouche, S.M.E.A.; Djeffal, R.; Cherier, M.K.; Hamdani, M.; Younsi, Z.; Al-Saadi, S.; Zaiani, M. Experimental Performance and Cost-Effectiveness of a Combined Heating System under Saharan Climate. Buildings 2023, 13, 635. [Google Scholar] [CrossRef]
- Kushwaha, P.K.; Sharma, N.K.; Kumar, A.; Meena, C.S. Recent advancements in augmentation of solar water heaters using nanocomposites with PCM: Past, present, and future. Buildings 2022, 13, 79. [Google Scholar] [CrossRef]
- Manirathnam, A.; Manikandan, M.D.; Prakash, R.H.; Kumar, B.K.; Amarnath, M.D. Experimental analysis on solar water heater integrated with Nano composite phase change material (SCi and CuO). Mater. Today Proc. 2021, 37, 232–240. [Google Scholar] [CrossRef]
- Pathak, S.K.; Tyagi, V.; Chopra, K.; Pandey, A.; Sari, A. Hot water generation for domestic use in residential buildings via PCM Integrated U-Tube based solar thermal collector: A 4-E analysis. Buildings 2023, 13, 1212. [Google Scholar] [CrossRef]
- Lee, T.; Sato, R.; Asawa, T.; Yoon, S. Indoor Air Temperature Distribution and Heat Transfer Coefficient for Evaluating Cold Storage of Phase-Change Materials during Night Ventilation. Buildings 2024, 14, 1872. [Google Scholar] [CrossRef]
- Jiao, F.; Li, G.; Zhang, C.; Liu, J. Study on the Coupling of Air-Source Heat Pumps (ASHPs) and Passive Heating in Cold Regions. Buildings 2024, 14, 2410. [Google Scholar] [CrossRef]
- Prakash, K.; Almeshaal, M.; Pasupathi, M.K.; Chinnasamy, S.; Saravanakumar, S.; Rajesh Ruban, S. Hybrid PV/T heat pump system with PCM for combined heating, cooling and power provision in buildings. Buildings 2023, 13, 1133. [Google Scholar] [CrossRef]
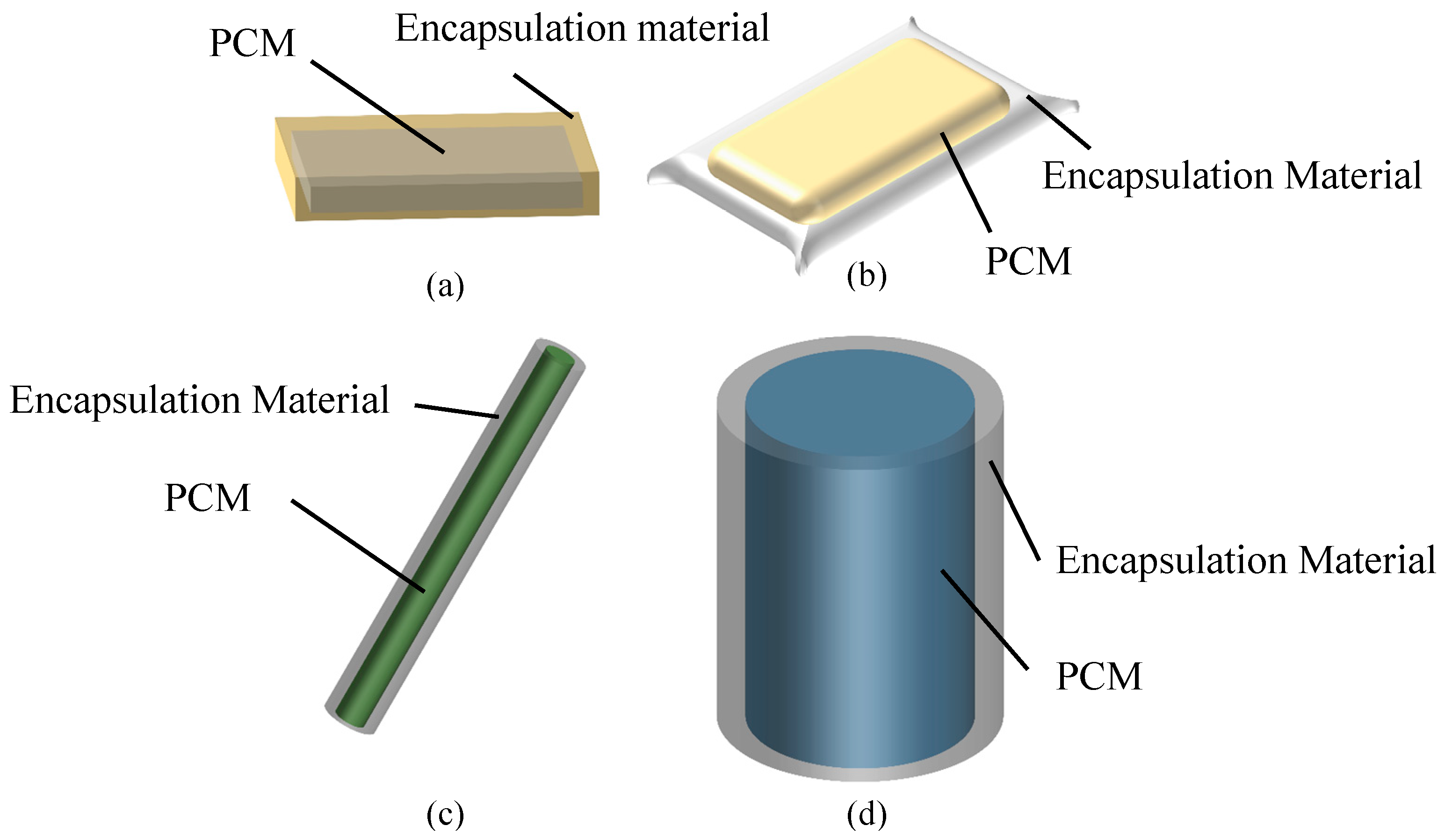



| PCM | Encapsulation Form | Size and Appearance | Reference |
|---|---|---|---|
| N-octadecane (C18) | Nylon packing tape encapsulation | The nylon bag is divided into three compartments, each containing 60 g of PCMs | [48] |
| Paraffin waxes (RT18HC) | Tubular low-density polyethylene (LDPE) bag | Tubular LDPE bags with a circumference of 200 mm and a thickness of 150 µm | [42] |
| Eutectic PCMs consisting of 70% paraffin waxes, 22% fatty acid, and 8% tetradecanol | Black aluminum foil bag | 88 cm × 60 cm × 10 cm | [44] |
| A mixture of CADE | PCM panel | Size 10 cm × 10 cm× 0.1 cm | [46] |
| A mixture of 40% oleic acid and 60% capric acid | Rectangular container | 0.16 m × 0.10 m × 0.03 m | [26] |
| CaCl2·6H2O | Cylindrical plastic cans | Diameter of 800 mm, length of 1500 mm | [47] |
| PCM 1, PCM 2 and PCM 3 | PCM-filled glass units | 500 mm × 450 mm × 4 mm | [49] |
| Paraffin waxes (RT18) | Hong capsule construction steel | 30 cm × 17 cm × 2.8 cm Thickness of 0.75 mm | [50] |
| Method | Organic | Inorganic | |
|---|---|---|---|
| Chemical | Suspension polymerization | Suitable | Unsuitable |
| Physical–chemical | Coacervation | Suitable | Unsuitable |
| Physical | Spray drying | Suitable | Unsuitable |
| PCM | Application | Type | Recommendation |
|---|---|---|---|
| CaCl2·6H2O | PCWs | Passive PCHS (Buildings) | Effective for greenhouse walls and temperature regulation [11] |
| Paraffin wax | PCWs | Passive PCHS (Buildings) | Widely used for moderate thermal performance and compatibility with building materials [78,79] |
| Paraffin wax + fatty acids | PCHS units | Passive PCHS (Agriculture) | Suitable for greenhouses and improving indoor temperature control [44] |
| Na2SO4·10H2O | PCHS units | Passive PCHS (Agriculture) | High latent heat for consistent temperature regulation in greenhouses [83,84] |
| Paraffin wax + graphite | Solar heat source active PCHS | Active PCHS (Buildings, Agriculture) | Enhanced thermal conductivity for heat storage in solar systems [79] |
| S19 | Geothermal heat source PCHS | Active PCHS (Agriculture) | Effective for geothermal systems with high heat storage capacity [114] |
| Stearic acid | Solar collection systems | Active PCHS (Buildings) | Reliable for solar-based heating systems, especially in agricultural facilities [132] |
| HS36 | Hybrid photovoltaic evaporator | Active PCHS (Buildings) | Ideal for zero-energy buildings with advanced integration [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Gulfam, R.; Ishrat, Y.; Iqbal, S.; Yao, F. Recent Progress of Phase Change Materials and Their Applications in Facility Agriculture and Related-Buildings—A Review. Buildings 2024, 14, 2999. https://doi.org/10.3390/buildings14092999
Cui Y, Gulfam R, Ishrat Y, Iqbal S, Yao F. Recent Progress of Phase Change Materials and Their Applications in Facility Agriculture and Related-Buildings—A Review. Buildings. 2024; 14(9):2999. https://doi.org/10.3390/buildings14092999
Chicago/Turabian StyleCui, Yijing, Raza Gulfam, Yousaf Ishrat, Saqib Iqbal, and Feng Yao. 2024. "Recent Progress of Phase Change Materials and Their Applications in Facility Agriculture and Related-Buildings—A Review" Buildings 14, no. 9: 2999. https://doi.org/10.3390/buildings14092999







