Review and Perspectives on the Structure–Function Relationships of the Gag Subunits of Feline Immunodeficiency Virus
Abstract
:1. Introduction
2. The Importance of the Gag Polyprotein for the Replication of Lentiviruses
3. FIV MA
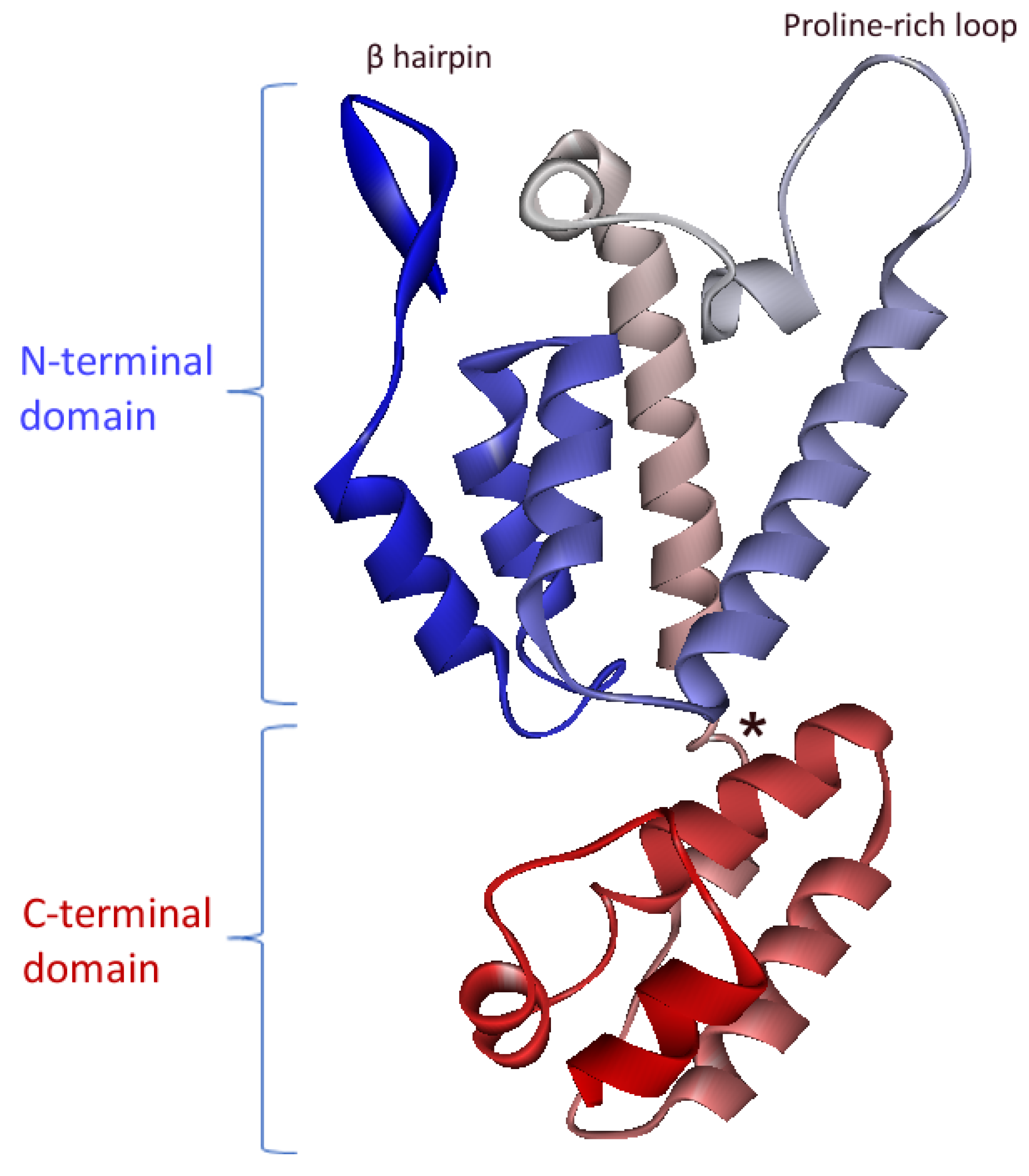
4. FIV CA
5. FIV SP
6. FIV p13
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bendinelli, M.; Pistello, M.; Lombardi, S.; Poli, A.; Garzelli, C.; Matteucci, D.; Ceccherini-Nelli, L.; Malvaldi, G.; Tozzini, F. Feline immunodeficiency virus: An interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 1995, 8, 87–112. [Google Scholar] [CrossRef]
- Krishnan, A.; Pillai, V.N.; Chameettachal, A.; Mohamed Ali, L.; Nuzra Nagoor Pitchai, F.; Tariq, S.; Mustafa, F.; Marquet, R.; Rizvi, T.A. Purification and Functional Characterization of a Biologically Active Full-Length Feline Immunodeficiency Virus (FIV) Pr50Gag. Viruses 2019, 11, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.; Abdo, Z.; Ericsson, A.; Elder, J.; VandeWoude, S. Applications of the FIV Model to Study HIV Pathogenesis. Viruses 2018, 10, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, V.L.; Burgess, S.C.; Shack, L.A.; Lockett, N.N.; Coats, K.S. Expression of CD134 and CXCR4 mRNA in term placentas from FIV-infected and control cats. Vet. Immunol. Immunopathol. 2008, 123, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troyer, J.L.; Vandewoude, S.; Pecon-Slattery, J.; McIntosh, C.; Franklin, S.; Antunes, A.; Johnson, W.; O’Brien, S.J. FIV cross-species transmission: An evolutionary prospective. Vet. Immunol. Immunopathol. 2008, 123, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.; Yang, D.; Green, J.; Norris, J.; Malik, R.; Parr, Y.A.; McDonald, M.; Hosie, M.J.; VandeWoude, S.; Miller, C. Antibody Responses in Cats Following Primary and Annual Vaccination against Feline Immunodeficiency Virus (FIV) with an Inactivated Whole-Virus Vaccine (Fel-O-Vax((R)) FIV). Viruses 2021, 13, 470. [Google Scholar] [CrossRef]
- Ott, D.E.; Coren, L.V.; Shatzer, T. The nucleocapsid region of human immunodeficiency virus type 1 Gag assists in the coordination of assembly and Gag processing: Role for RNA-Gag binding in the early stages of assembly. J. Virol. 2009, 83, 7718–7727. [Google Scholar] [CrossRef] [Green Version]
- Dubois, N.; Khoo, K.K.; Ghossein, S.; Seissler, T.; Wolff, P.; McKinstry, W.J.; Mak, J.; Paillart, J.-C.; Marquet, R.; Bernacchi, S. The C-terminal p6 domain of the HIV-1 Pr55Gag precursor is required for specific binding to the genomic RNA. RNA Biol. 2018, 15, 923–936. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.R.; Schooler, J.B.; Ding, H.J.; Kieffer, C.; Fillmore, C.; Sundquist, W.I.; Jensen, G.J. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 2007, 26, 2218–2226. [Google Scholar] [CrossRef] [Green Version]
- Briggs, J.A.; Riches, J.D.; Glass, B.; Bartonova, V.; Zanetti, G.; Krausslich, H.G. Structure and assembly of immature HIV. Proc. Natl. Acad. Sci. USA 2009, 106, 11090–11095. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Loeliger, E.; Luncsford, P.; Kinde, I.; Beckett, D.; Summers, M.F. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. USA 2004, 101, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Rose, K.M.; Hirsch, V.M.; Bouamr, F. Budding of a Retrovirus: Some Assemblies Required. Viruses 2020, 10, 1188. [Google Scholar] [CrossRef] [PubMed]
- Sarca, A.D.; Sardo, L.; Fukuda, H.; Matsui, H.; Shirakawa, K.; Horikawa, K.; Takaori-Kondo, A.; Izumi, T. FRET-Based Detection and Quantification of HIV-1 Virion Maturation. Front. Microbiol. 2021, 12, 647452. [Google Scholar] [CrossRef] [PubMed]
- Bester, S.M.; Wei, G.; Zhao, H.; Adu-Ampratwum, D.; Iqbal, N.; Courouble, V.V.; Francis, A.C.; Annamalai, A.S.; Singh, P.K.; Shkriabai, N.; et al. Structural and mechanistic bases for a potent HIV-1 capsid inhibitor. Science 2020, 370, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.N.; Kyere, S.; Kinde, I.; Tang, C.; Howard, B.R.; Robinson, H.; Sundquist, W.I.; Summers, M.F.; Hill, C.P. Structure of the antiviral assembly inhibitor CAP-1 complex with the HIV-1 CA protein. J. Mol. Biol. 2007, 373, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnes, S.K.; Sheehan, J.H.; Aiken, C. Inhibitors of the HIV-1 capsid, a target of opportunity. Curr. Opin. HIV AIDS 2018, 13, 359–365. [Google Scholar] [CrossRef]
- McArthur, C.; Gallazzi, F.; Quinn, T.P.; Singh, K. HIV Capsid Inhibitors Beyond PF74. Diseases 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef]
- Manrique, M.L.; Rauddi, M.L.; Gonzalez, S.A.; Affranchino, J.L. Functional domains in the Feline Immunodeficiency Virus nucleocapsid protein. Virology 2004, 327, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Serrière, J.; Robert, X.; Perez, M.; Gouet, P.; Guillon, C. Biophysical characterization and crystal structure of the Feline Immunodeficiency Virus p15 matrix protein. Retrovirology 2013, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Khwaja, A.; Galilee, M.; Marx, A.; Alian, A. Structure of FIV capsid C-terminal domain demonstrates lentiviral evasion of genetic fragility by coevolved substitutions. Sci. Rep. 2016, 6, 24957. [Google Scholar] [CrossRef] [Green Version]
- Folio, C.; Sierra, N.; Dujardin, M.; Alvarez, G.; Guillon, C. Crystal structure of the full-length Feline Immunodeficiency Virus capsid protein shows an N-terminal beta-hairpin in the absence of N-terminal proline. Viruses 2017, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Burkala, E.; Poss, M. Evolution of feline immunodeficiency virus Gag proteins. Virus Genes 2007, 35, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Cox, C.; Baptiste, J.; Summers, H.; Button, R.; Bahlow, K.; Spurrier, V.; Kyser, J.; Luttge, B.G.; Kuo, L.; et al. NMR structure of the myristylated feline immunodeficiency virus matrix protein. Viruses 2015, 7, 2210–2229. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.B.; Summers, H.R.; Brown, L.A.; Marchant, J.; Canova, P.N.; O’Hern, C.T.; Abbott, S.T.; Nyaunu, C.; Maxwell, S.; Johnson, T.; et al. Structural and Mechanistic Studies of the Rare Myristoylation Signal of the Feline Immunodeficiency Virus. J. Mol. Biol. 2020, 432, 4076–4091. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Ke, Z.; Zila, V.; Anders-Osswein, M.; Glass, B.; Mucksch, F.; Muller, R.; Schultz, C.; Muller, B.; Krausslich, H.G.; et al. Maturation of the matrix and viral membrane of HIV-1. Science 2021, 373, 700–704. [Google Scholar] [CrossRef]
- Abdusetir Cerfoglio, J.C.; Gonzalez, S.A.; Affranchino, J.L. Structural elements in the Gag polyprotein of feline immunodeficiency virus involved in Gag self-association and assembly. J. Gen. Virol. 2014, 95, 2050–2059. [Google Scholar] [CrossRef]
- Bukrinskaya, A. HIV-1 matrix protein: A mysterious regulator of the viral life cycle. Virus Res. 2007, 124, 1–11. [Google Scholar] [CrossRef]
- Ono, A.; Orenstein, J.M.; Freed, E.O. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J. Virol. 2000, 74, 2855–2866. [Google Scholar] [CrossRef] [Green Version]
- Chukkapalli, V.; Hogue, I.B.; Boyko, V.; Hu, W.S.; Ono, A. Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient gag membrane binding. J. Virol. 2008, 82, 2405–2417. [Google Scholar] [CrossRef] [Green Version]
- Ono, A.; Ablan, S.D.; Lockett, S.J.; Nagashima, K.; Freed, E.O. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. USA 2004, 101, 14889–14894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Feigenson, G.W.; Vogt, V.M.; Dick, R.A. Mechanisms of PI(4,5)P2 Enrichment in HIV-1 Viral Membranes. J. Mol. Biol. 2020, 432, 5343–5364. [Google Scholar] [CrossRef]
- Freed, E.O.; Martin, M.A. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 1996, 70, 341–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luttge, B.G.; Freed, E.O. FIV Gag: Virus assembly and host-cell interactions. Vet. Immunol. Immunopathol. 2010, 134, 3. [Google Scholar] [CrossRef] [Green Version]
- Affranchino, J.L.; Gonzalez, S.A. Understanding the process of envelope glycoprotein incorporation into virions in simian and feline immunodeficiency viruses. Viruses 2014, 6, 264–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Perilla, J.R.; Yufenyuy, E.L.; Meng, X.; Chen, B.; Ning, J.; Ahn, J.; Gronenborn, A.M.; Schulten, K.; Aiken, C.; et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 2013, 497, 643–646. [Google Scholar] [CrossRef]
- Briggs, J.A.; Simon, M.N.; Gross, I.; Krausslich, H.G.; Fuller, S.D.; Vogt, V.M.; Johnson, M.C. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 2004, 11, 672–675. [Google Scholar] [CrossRef]
- Arhel, N. Revisiting HIV-1 uncoating. Retrovirology 2010, 7, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Russell, R.W.; Bryer, A.J.; Quinn, C.M.; Hou, G.; Zhang, H.; Schwieters, C.D.; Perilla, J.R.; Gronenborn, A.M.; Polenova, T. Atomic-resolution structure of HIV-1 capsid tubes by magic-angle spinning NMR. Nat. Struct. Mol. Biol. 2020, 27, 863–869. [Google Scholar] [CrossRef]
- Pornillos, O.; Ganser-Pornillos, B.K.; Kelly, B.N.; Hua, Y.; Whitby, F.G.; Stout, C.D.; Sundquist, W.I.; Hill, C.P.; Yeager, M. X-ray structures of the hexameric building block of the HIV capsid. Cell 2009, 137, 1282–1292. [Google Scholar] [CrossRef] [Green Version]
- Barr, M.C.; Zou, L.; Holzschu, D.L.; Phillips, L.; Scott, F.W.; Casey, J.W.; Avery, R.J. Isolation of a highly cytopathic lentivirus from a nondomestic cat. J. Virol. 1995, 69, 7371–7374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.; Cantrelle, F.X.; Robert, X.; Boll, E.; Sierra, N.; Gouet, P.; Hanoulle, X.; Alvarez, G.I.; Guillon, C. Identification of a Potential Inhibitor of the FIV p24 Capsid Protein and Characterization of Its Binding Site. Biochemistry 2021, 60, 1896–1908. [Google Scholar] [CrossRef]
- Xu, C.; Fischer, D.K.; Rankovic, S.; Li, W.; Dick, R.A.; Runge, B.; Zadorozhnyi, R.; Ahn, J.; Aiken, C.; Polenova, T.; et al. Permeability of the HIV-1 capsid to metabolites modulates viral DNA synthesis. PLoS Biol. 2020, 18, e3001015. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Uncovering the release mechanism of nucleotide import by HIV-1 capsid. Phys. Biol. 2020, 18, 016004. [Google Scholar] [CrossRef]
- Renner, N.; Mallery, D.L.; Faysal, K.M.R.; Peng, W.; Jacques, D.A.; Bocking, T.; James, L.C. A lysine ring in HIV capsid pores coordinates IP6 to drive mature capsid assembly. PLoS Pathog. 2021, 17, e1009164. [Google Scholar] [CrossRef] [PubMed]
- Novikova, M.; Zhang, Y.; Freed, E.O.; Peng, K. Multiple Roles of HIV-1 Capsid during the Virus Replication Cycle. Virol. Sin. 2019, 34, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Santos da Silva, E.; Shanmugapriya, S.; Malikov, V.; Gu, F.; Delaney, M.K.; Naghavi, M.H. HIV-1 capsids mimic a microtubule regulator to coordinate early stages of infection. EMBO J. 2020, 39, e104870. [Google Scholar] [CrossRef]
- Malikov, V.; da Silva, E.S.; Jovasevic, V.; Bennett, G.; de Souza Aranha Vieira, D.A.; Schulte, B.; Diaz-Griffero, F.; Walsh, D.; Naghavi, M.H. HIV-1 capsids bind and exploit the kinesin-1 adaptor FEZ1 for inward movement to the nucleus. Nat. Commun. 2015, 6, 6660. [Google Scholar] [CrossRef]
- Dharan, A.; Campbell, E.M. Role of Microtubules and Microtubule-Associated Proteins in HIV-1 Infection. J. Virol. 2018, 92, e00085-18. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Craigie, R. The road to chromatin—Nuclear entry of retroviruses. Nat. Rev. Microbiol. 2007, 5, 187–196. [Google Scholar] [CrossRef]
- Vernekar, S.K.V.; Sahani, R.L.; Casey, M.C.; Kankanala, J.; Wang, L.; Kirby, K.A.; Du, H.; Zhang, H.; Tedbury, P.R.; Xie, J.; et al. Toward Structurally Novel and Metabolically Stable HIV-1 Capsid-Targeting Small Molecules. Viruses 2020, 12, 452. [Google Scholar] [CrossRef]
- Blair, W.S.; Pickford, C.; Irving, S.L.; Brown, D.G.; Anderson, M.; Bazin, R.; Cao, J.; Ciaramella, G.; Isaacson, J.; Jackson, L.; et al. HIV capsid is a tractable target for small molecule therapeutic intervention. PLoS Pathog. 2010, 6, e1001220. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Gallazzi, F.; Hill, K.J.; Burke, D.H.; Lange, M.J.; Quinn, T.P.; Neogi, U.; Sonnerborg, A. GS-CA Compounds: First-In-Class HIV-1 Capsid Inhibitors Covering Multiple Grounds. Front. Microbiol. 2019, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Sierra, N.; Folio, C.; Robert, X.; Long, M.; Guillon, C.; Alvarez, G. Looking for novel capsid protein multimerization inhibitors of Feline Immunodeficiency Virus. Pharmaceuticals 2018, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Towers, G.J. The control of viral infection by tripartite motif proteins and cyclophilin A. Retrovirology 2007, 4, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwan, W.A.; Schaller, T.; Ylinen, L.M.; Hosie, M.J.; Towers, G.J.; Willett, B.J. Truncation of TRIM5 in the Feliformia explains the absence of retroviral restriction in cells of the domestic cat. J. Virol. 2009, 83, 8270–8275. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Griffero, F.; Kar, A.; Lee, M.; Stremlau, M.; Poeschla, E.; Sodroski, J. Comparative requirements for the restriction of retrovirus infection by TRIM5α and TRIMCyp. Virology 2007, 369, 400–410. [Google Scholar] [CrossRef] [Green Version]
- Selyutina, A.; Persaud, M.; Simons, L.M.; Bulnes-Ramos, A.; Buffone, C.; Martinez-Lopez, A.; Scoca, V.; Di Nunzio, F.; Hiatt, J.; Marson, A.; et al. Cyclophilin A Prevents HIV-1 Restriction in Lymphocytes by Blocking Human TRIM5α Binding to the Viral Core. Cell Rep. 2020, 30, 3766–3777.e6. [Google Scholar] [CrossRef]
- Gonzalez, S.A.; Affranchino, J.L. Properties and Functions of Feline Immunodeficiency Virus Gag Domains in Virion Assembly and Budding. Viruses 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accola, M.A.; Hoglund, S.; Gottlinger, H.G. A putative alpha-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly. J. Virol. 1998, 72, 2072–2078. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.E.; Salzwedel, K.; Allaway, G.P. Bevirimat: A novel maturation inhibitor for the treatment of HIV-1 infection. Antivir. Chem. Chemother. 2008, 19, 107–113. [Google Scholar] [CrossRef]
- Gupta, S.; Louis, J.M.; Tycko, R. Effects of an HIV-1 maturation inhibitor on the structure and dynamics of CA-SP1 junction helices in virus-like particles. Proc. Natl. Acad. Sci. USA 2020, 117, 10286–10293. [Google Scholar] [CrossRef]
- Adamson, C.S.; Sakalian, M.; Salzwedel, K.; Freed, E.O. Polymorphisms in Gag spacer peptide 1 confer varying levels of resistance to the HIV-1 maturation inhibitor bevirimat. Retrovirology 2010, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Chen, C.H.; Aiken, C. Human immunodeficiency virus type 1 resistance to the small molecule maturation inhibitor 3-O-(3′,3′-dimethylsuccinyl)-betulinic acid is conferred by a variety of single amino acid substitutions at the CA-SP1 cleavage site in Gag. J. Virol. 2006, 80, 12095–12101. [Google Scholar] [CrossRef] [Green Version]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Del Vecchio, C.; Celestino, M.; Celegato, M.; Palu, G.; Parolin, C.; Bouamr, F.; Calistri, A. Alix-Mediated Rescue of Feline Immunodeficiency Virus Budding Differs from That Observed with Human Immunodeficiency Virus. J. Virol. 2020, 94, e02019-19. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, W.; Naiyer, N.; Fichtenbaum, E.; Qualley, D.F.; McCauley, M.J.; Gorelick, R.J.; Rouzina, I.; Musier-Forsyth, K.; Williams, M.C. Single aromatic residue location alters nucleic acid binding and chaperone function of FIV nucleocapsid protein. Virus Res. 2014, 193, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Amarasinghe, G.K.; De Guzman, R.N.; Turner, R.B.; Chancellor, K.J.; Wu, Z.R.; Summers, M.F. NMR structure of the HIV-1 nucleocapsid protein bound to stem-loop SL2 of the psi-RNA packaging signal. Implications for genome recognition. J. Mol. Biol. 2000, 301, 491–511. [Google Scholar] [CrossRef] [Green Version]
- James, L.; Sargueil, B. RNA secondary structure of the feline immunodeficiency virus 5′UTR and Gag coding region. Nucleic Acids Res. 2008, 36, 4653–4666. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.C.; Ghazawi, A.; Cheung, W.K.; Phillip, P.S.; Rizvi, T.A.; Lever, A.M. The secondary structure of the 5′ end of the FIV genome reveals a long-range interaction between R/U5 and gag sequences, and a large, stable stem-loop. RNA 2008, 14, 2597–2608. [Google Scholar] [CrossRef] [Green Version]
- Asquith, C.R.M.; Laitinen, T.; Konstantinova, L.S.; Tizzard, G.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R.; Hilton, S.T. Investigation of the Pentathiepin Functionality as an Inhibitor of Feline Immunodeficiency Virus (FIV) via a Potential Zinc Ejection Mechanism, as a Model for HIV Infection. ChemMedChem 2019, 14, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Lever, A.M. Wrapping up the bad news: HIV assembly and release. Retrovirology 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fossen, T.; Wray, V.; Bruns, K.; Rachmat, J.; Henklein, P.; Tessmer, U.; Maczurek, A.; Klinger, P.; Schubert, U. Solution structure of the human immunodeficiency virus type 1 p6 protein. J. Biol. Chem. 2005, 280, 42515–42527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gifford, R.J. Viral evolution in deep time: Lentiviruses and mammals. Trends Genet. 2012, 28, 89–100. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, M.; Toesca, J.; Guillon, C. Review and Perspectives on the Structure–Function Relationships of the Gag Subunits of Feline Immunodeficiency Virus. Pathogens 2021, 10, 1502. https://doi.org/10.3390/pathogens10111502
Long M, Toesca J, Guillon C. Review and Perspectives on the Structure–Function Relationships of the Gag Subunits of Feline Immunodeficiency Virus. Pathogens. 2021; 10(11):1502. https://doi.org/10.3390/pathogens10111502
Chicago/Turabian StyleLong, Mathieu, Johan Toesca, and Christophe Guillon. 2021. "Review and Perspectives on the Structure–Function Relationships of the Gag Subunits of Feline Immunodeficiency Virus" Pathogens 10, no. 11: 1502. https://doi.org/10.3390/pathogens10111502
APA StyleLong, M., Toesca, J., & Guillon, C. (2021). Review and Perspectives on the Structure–Function Relationships of the Gag Subunits of Feline Immunodeficiency Virus. Pathogens, 10(11), 1502. https://doi.org/10.3390/pathogens10111502






