Galacto-Oligosaccharides as an Anti-Infective and Anti-Microbial Agent for Macrolide-Resistant and -Sensitive Mycoplasma pneumoniae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. NDOs and Antibiotics
2.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.4. Human Respiratory Epithelial Cell Culture
2.5. Lactate Dehydrogenase (LDH) Assay
2.6. MP and MRMP Adhesion to A549 Cells
2.7. ELISA
2.8. Statistical Analysis
3. Results
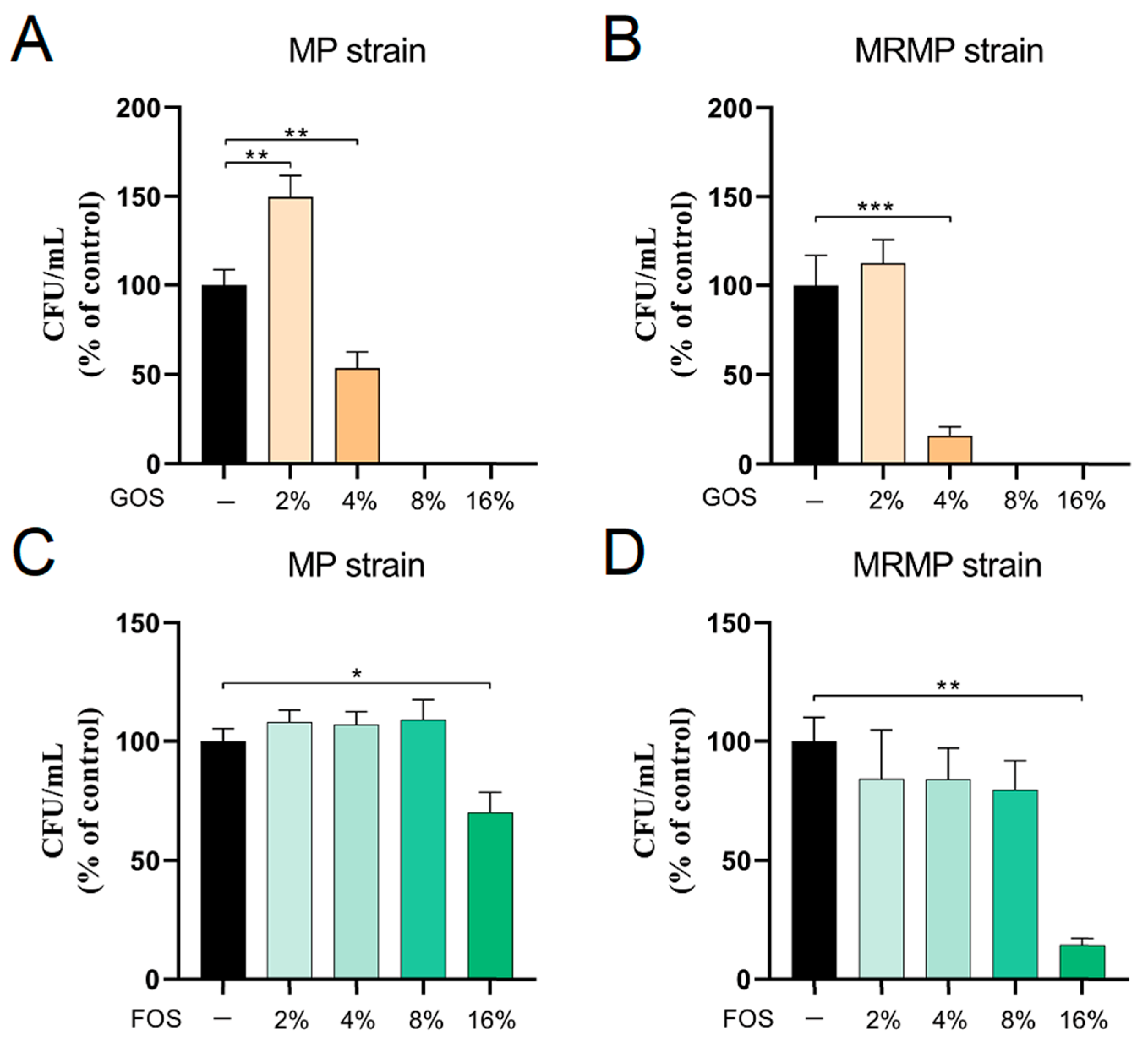
3.1. Inhibition of MP and MRMP Growth by GOS and FOS
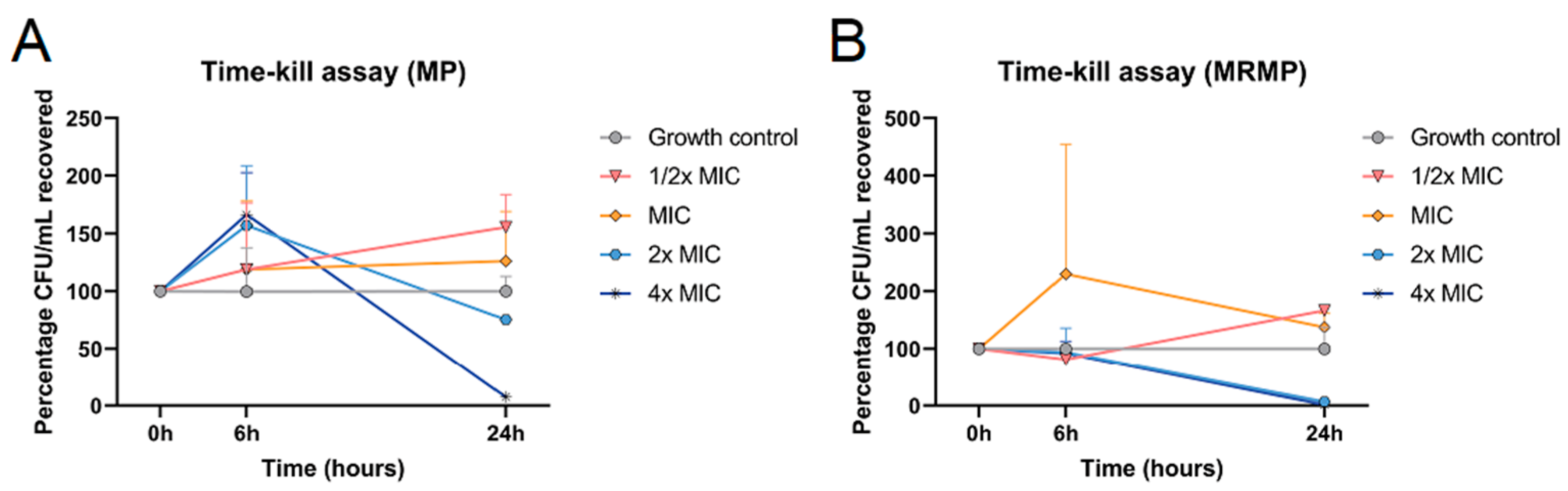
3.2. Bactericidal Effect of GOS and FOS on MP and MRMP
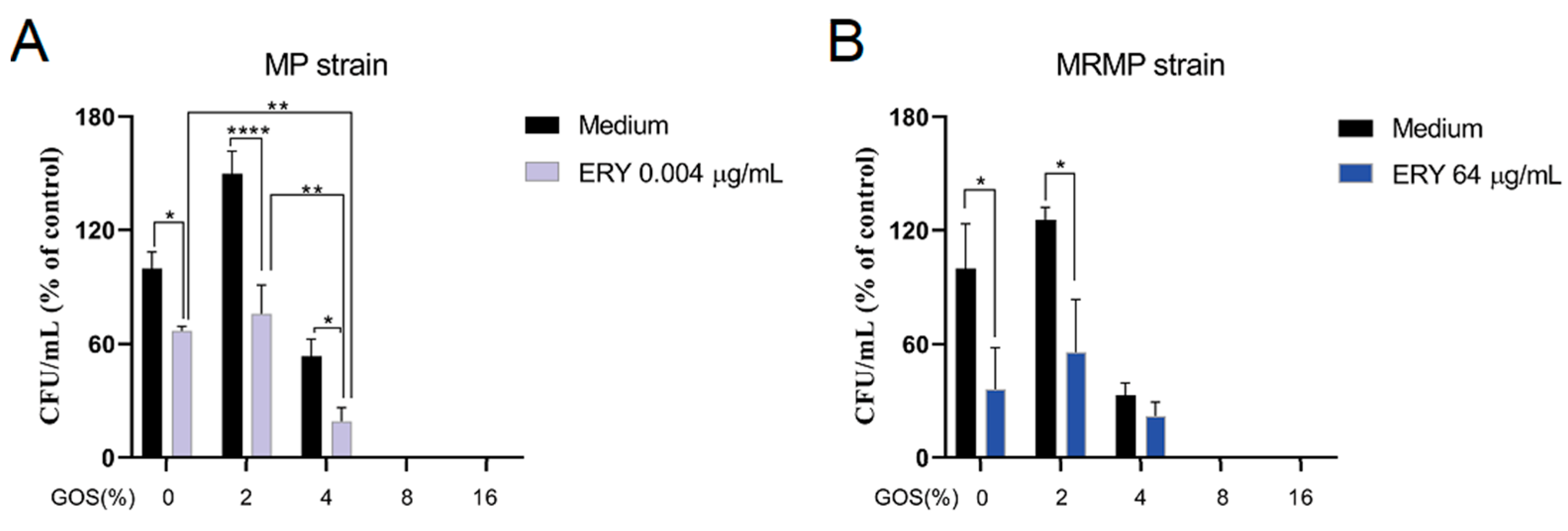
3.3. GOS Act Additive with ERY on the Viability of MP but Not MRMP
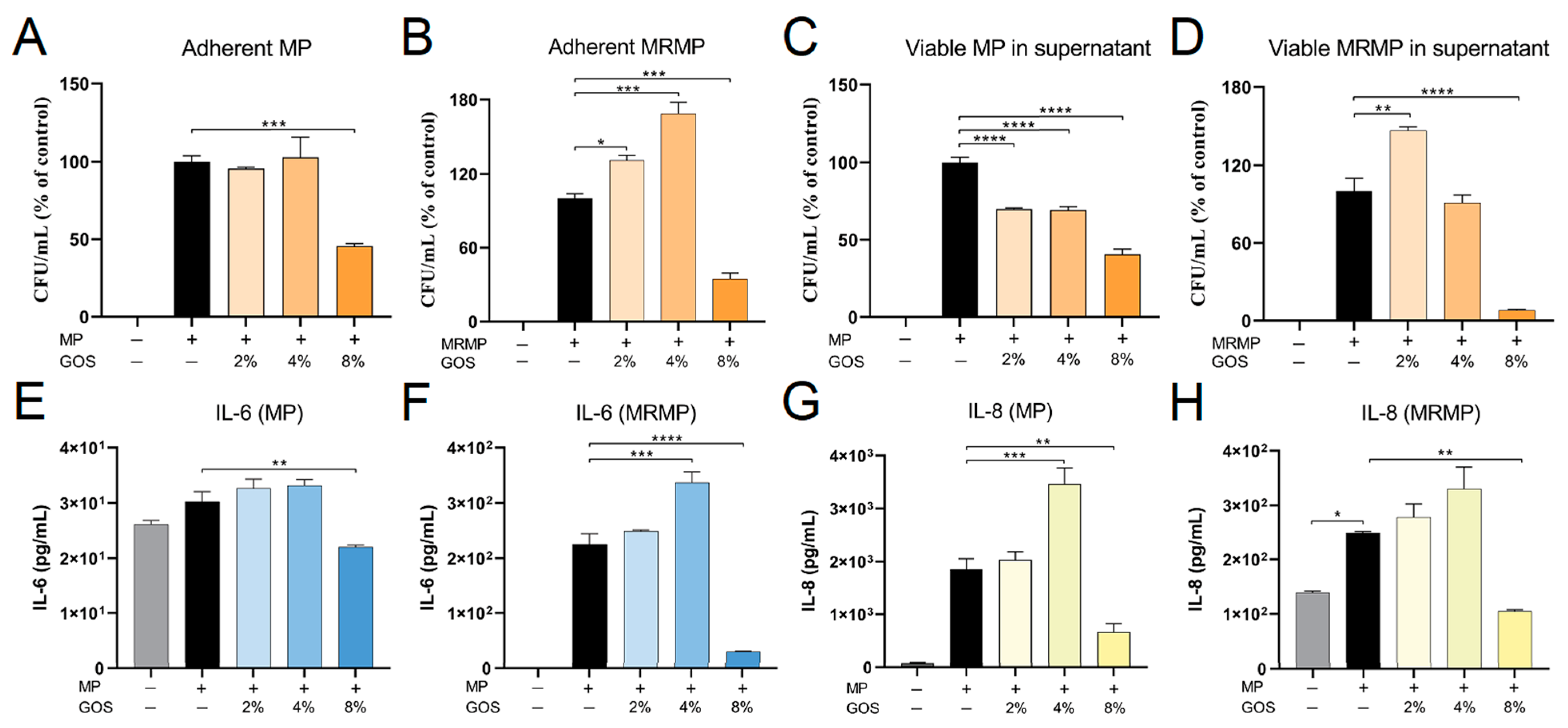
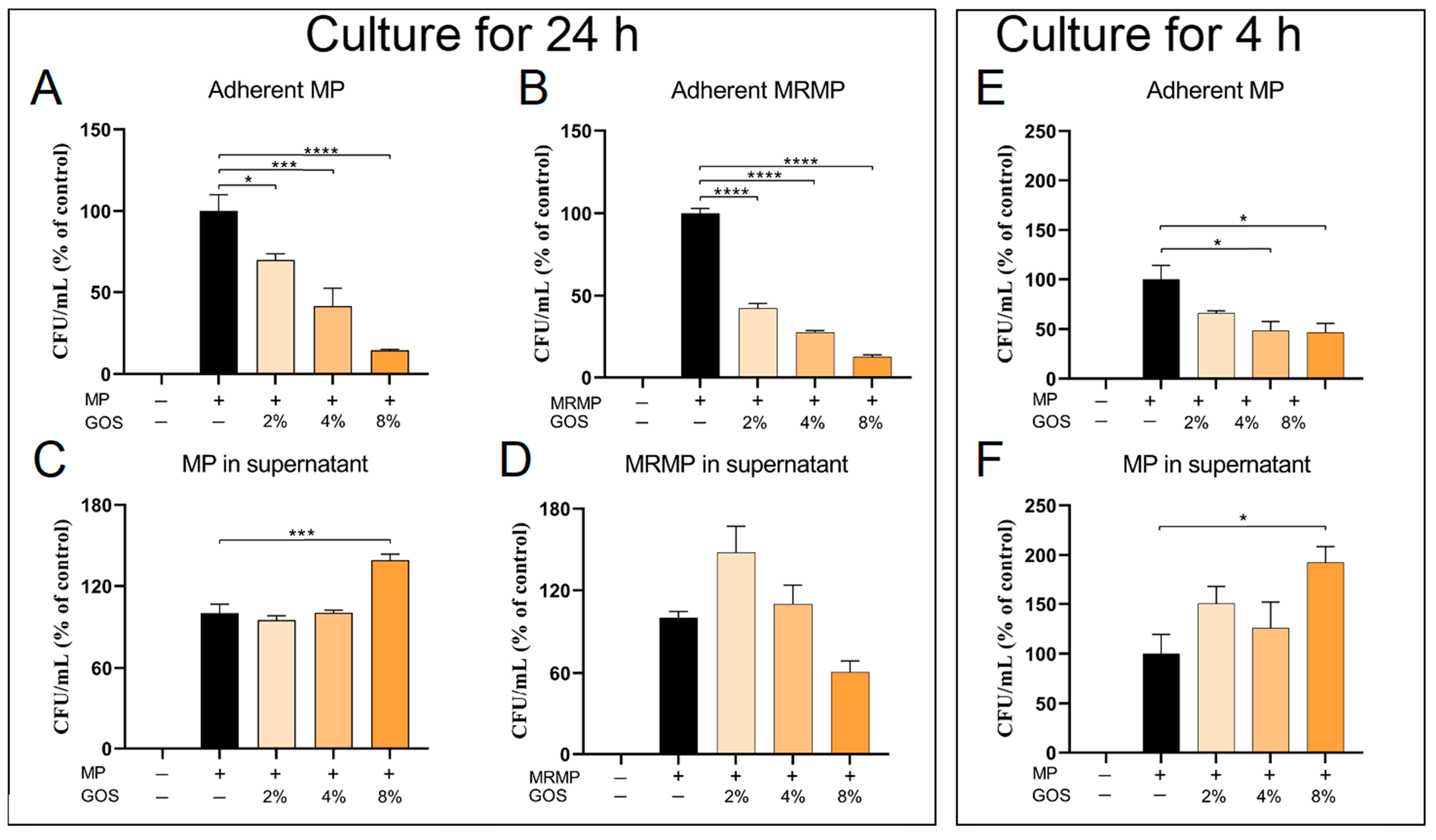
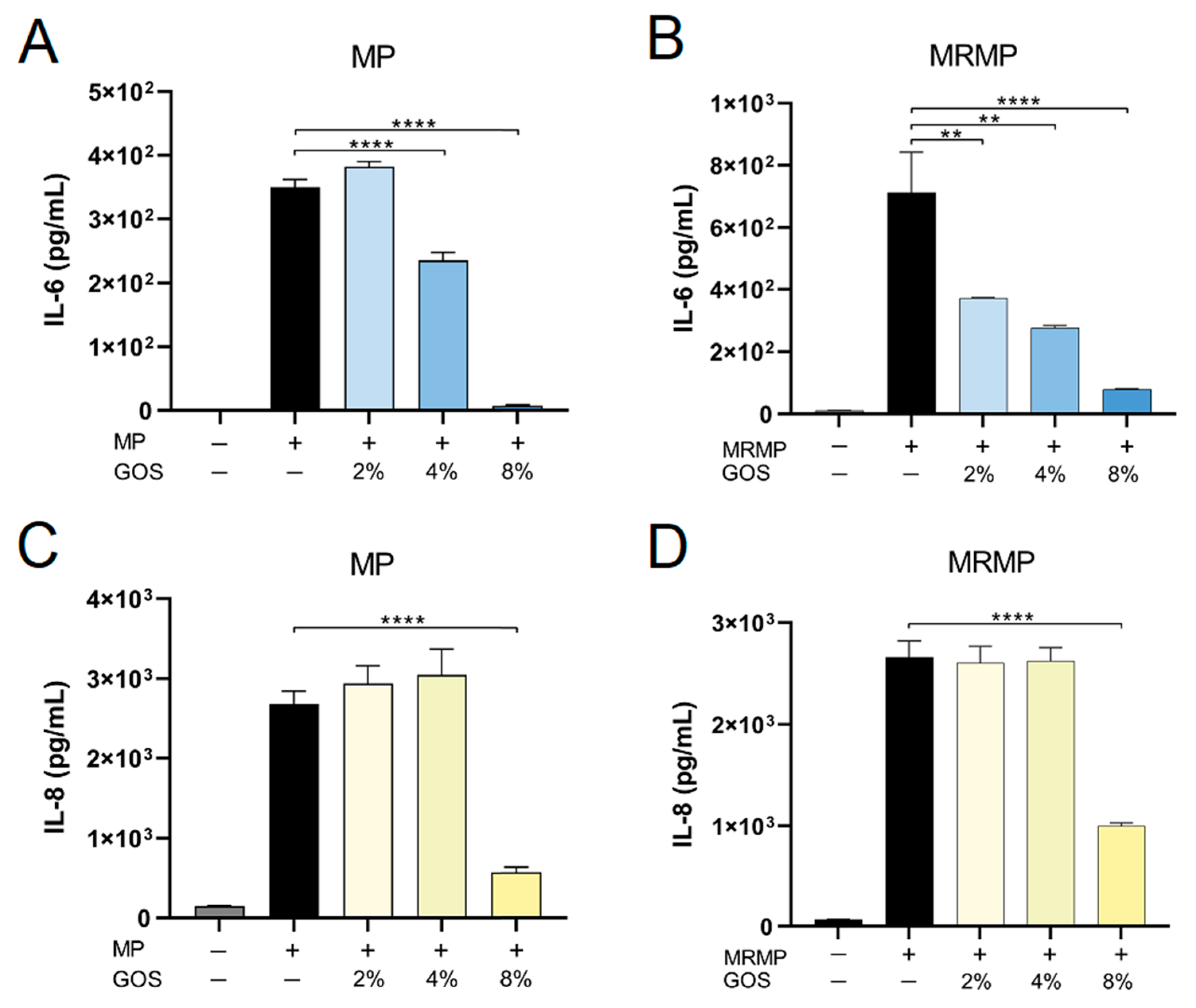
3.4. GOS Kills MP and MRMP That are Adherent to Respiratory Epithelial Cells
3.5. GOS Prevents the Adhesion of MP and MRMP to A549 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.C.; Teoh, O.H.; Chong, C.Y.; Goh, A.; Tang, J.P.; Chay, O.M. Epidemiology, clinical characteristics and antimicrobial resistance patterns of community-acquired pneumonia in 1702 hospitalized children in Singapore. Respirology 2007, 12, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Cheon, B.R.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Increased risk of refractory Mycoplasma pneumoniae pneumonia in children with atopic sensitization and asthma. Korean J. Pediatr. 2014, 57, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Argentiero, A.; Gramegna, A.; Principi, N. Mycoplasma pneumoniae: A pathogen with unsolved therapeutic problems. Expert. Opin. Pharmacother. 2021, 22, 1193–1202. [Google Scholar] [CrossRef]
- Okada, T.; Morozumi, M.; Sakata, H.; Takayanagi, R.; Ishiwada, N.; Sato, Y.; Oishi, T.; Tajima, T.; Haruta, T.; Kawamura, N. A practical approach estimating etiologic agents using real-time PCR in pediatric inpatients with community-acquired pneumonia. J. Infect. Chemother. 2012, 18, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Saikku, P. Atypical respiratory pathogens. Clin. Microbiol. Infect. 1997, 3, 599–604. [Google Scholar] [CrossRef]
- Matsubara, K.; Morozumi, M.; Okada, T.; Matsushima, T.; Komiyama, O.; Shoji, M.; Ebihara, T.; Ubukata, K.; Sato, Y.; Akita, H. A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J. Infect. Chemother. 2009, 15, 380–383. [Google Scholar] [CrossRef]
- Lin, K.; Zahlanie, Y.; Ortwine, J.K.; Mang, N.S.; Wei, W.; Brown, L.S.; Prokesch, B.C. Decreased Outpatient Fluoroquinolone Prescribing Using a Multimodal Antimicrobial Stewardship Initiative. Open Forum Infect. Dis. 2020, 7, ofaa182. [Google Scholar] [CrossRef]
- Arnold, F.W.; Summersgill, J.T.; Ramirez, J.A. Role of Atypical Pathogens in the Etiology of Community-Acquired Pneumonia. Semin. Respir. Crit. Care Med. 2016, 37, 819–828. [Google Scholar] [CrossRef]
- Kutty, P.K.; Jain, S.; Taylor, T.H.; Bramley, A.M.; Diaz, M.H.; Ampofo, K.; Arnold, S.R.; Williams, D.J.; Edwards, K.M.; McCullers, J.A. Mycoplasma pneumoniae Among Children Hospitalized with Community-acquired Pneumonia. Clin. Infect. Dis. 2019, 68, 5–12. [Google Scholar] [CrossRef]
- Kurkela, S.; Puolakkainen, M.; Hokynar, K.; Nieminen, T.; Saxen, H.; Mannonen, L.; Pietikäinen, R. Mycoplasma pneumoniae outbreak, Southeastern Finland, 2017–2018: Molecular epidemiology and laboratory diagnostic lessons. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ratliff, A.E.; Crabb, D.M.; Mixon, E.; Qin, X.; Selvarangan, R.; Tang, Y.W.; Zheng, X.; Dien Bard, J.; Hong, T. Molecular Characterization of Mycoplasma pneumoniae Isolates in the United States from 2012 to 2018. J. Clin. Microbiol. 2020, 58, e00710-20. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Buller, R.; Bledsoe, S.; Storch, G.A. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr. Infect. Dis. J. 2012, 31, 409–410. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, X.; Zhang, H.; Xu, X.; Li, W.; Zhu, D.; Wang, M. Characterization of macrolide resistance in Mycoplasma pneumoniae isolated from children in Shanghai, China. Diagn. Microbiol. Infect. Dis. 2010, 67, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, D.; Demirkaya, E.; Sazonov, V.; Romano, M. Mycoplasma pneumoniae Infections and Primary Immune Deficiencies. Int. J. Clin. Pract. 2022, 2022, 6343818. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Morozumi, M.; Tajima, T.; Hasegawa, M.; Sakata, H.; Ohnari, S.; Chiba, N.; Iwata, S.; Ubukata, K. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin. Infect. Dis. 2012, 55, 1642–1649. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Sheng, Y.; Zhang, L.; Shen, Z.; Chen, Z. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1034–1038. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, W.Y.; Chang, T.H. Macrolide-Resistant Mycoplasma pneumoniae Infections in Pediatric Community-Acquired Pneumonia. Emerg. Infect. Dis. 2020, 26, 1382–1391. [Google Scholar] [CrossRef]
- Asadpoor, M.; Peeters, C.; Henricks, P.A.J.; Varasteh, S.; Pieters, R.J.; Folkerts, G.; Braber, S. Anti-Pathogenic Functions of Non-Digestible Oligosaccharides In Vitro. Nutrients 2020, 12, 1789. [Google Scholar] [CrossRef]
- Laucirica, D.R.; Triantis, V.; Schoemaker, R.; Estes, M.K.; Ramani, S. Milk Oligosaccharides Inhibit Human Rotavirus Infectivity in MA104 Cells. J. Nutr. 2017, 147, 1709–1714. [Google Scholar] [CrossRef]
- Newburg, D.S.; Grave, G. Recent advances in human milk glycobiology. Pediatr. Res. 2014, 75, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef]
- Zuurveld, M.; van Witzenburg, N.P.; Garssen, J.; Folkerts, G.; Stahl, B.; Van’t Land, B.; Willemsen, L.E.M. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.; Difilippo, E.; Schols, H.A.; Schoterman, M.H.C.; Garssen, J.; Braber, S. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size: Structure-activity relationships of non-digestible oligosaccharides. Eur. J. Nutr. 2017, 56, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef]
- Cai, Y.; van Putten, J.P.M.; Gilbert, M.S.; Gerrits, W.J.J.; Folkerts, G.; Braber, S. Galacto-oligosaccharides as an anti-bacterial and anti-invasive agent in lung infections. Biomaterials 2022, 283, 121461. [Google Scholar] [CrossRef]
- Ortega-González, M.; Sánchez de Medina, F.; Molina-Santiago, C.; López-Posadas, R.; Pacheco, D.; Krell, T.; Martínez-Augustin, O.; Abdelali, D. Fructooligosacharides reduce Pseudomonas aeruginosa PAO1 pathogenicity through distinct mechanisms. PLoS ONE 2014, 9, e85772. [Google Scholar] [CrossRef]
- Spuesens, E.B.; Fraaij, P.L.; Visser, E.G.; Hoogenboezem, T.; Hop, W.C.; van Adrichem, L.N.; Weber, F.; Moll, H.A.; Broekman, B.; Berger, M.Y. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: An observational study. PLoS Med. 2013, 10, e1001444. [Google Scholar] [CrossRef]
- Spuesens, E.B.; Hoogenboezem, T.; Sluijter, M.; Hartwig, N.G.; van Rossum, A.M.; Vink, C. Macrolide resistance determination and molecular typing of Mycoplasma pneumoniae by pyrosequencing. J. Microbiol. Methods 2010, 82, 214–222. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, J.; Shi, W.; Huang, F.; Liu, L.; Zhao, S.; Zhang, J. Antimicrobial susceptibility and genotyping of Mycoplasma pneumoniae isolates in Beijing, China, from 2014 to 2016. Antimicrob. Resist. Infect. Control 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Meyer Sauteur, P.M.; Unger, W.W.; Nadal, D.; Berger, C.; Vink, C.; van Rossum, A.M. Infection with and Carriage of Mycoplasma pneumoniae in Children. Front. Microbiol. 2016, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.C. Mycoplasma pneumoniae cytadherence: Organization and assembly of the attachment organelle. Trends Microbiol. 1998, 6, 15–18. [Google Scholar] [CrossRef]
- Seto, S.; Layh-Schmitt, G.; Kenri, T.; Miyata, M. Visualization of the attachment organelle and cytadherence proteins of Mycoplasma pneumoniae by immunofluorescence microscopy. J. Bacteriol. 2001, 183, 1621–1630. [Google Scholar] [CrossRef]
- Bredt, W. Motility and multiplication of Mycoplasma pneumoniae. A phase contrast study. Pathol. Microbiol. 1968, 32, 321–326. [Google Scholar]
- Meng, Y.L.; Wang, W.M.; Lv, D.D.; An, Q.X.; Lu, W.H.; Wang, X.; Tang, G. The effect of Platycodin D on the expression of cytoadherence proteins P1 and P30 in Mycoplasma pneumoniae models. Env. Toxicol. Pharm. 2017, 49, 188–193. [Google Scholar] [CrossRef]
- Shim, S. Effects of Prebiotics, Probiotics and Synbiotics in the Diet of Young Pigs; Wageningen University and Research: Wageningen, The Netherlands, 2005. [Google Scholar]
- Csernus, B.; Czeglédi, L. Physiological, antimicrobial, intestine morphological, and immunological effects of fructooligosaccharides in pigs. Arch. Anim. Breed 2020, 63, 325–335. [Google Scholar] [CrossRef]
- Shim, J.H.; Xiao, C.; Paschal, A.E.; Bailey, S.T.; Rao, P.; Hayden, M.S.; Lee, K.Y.; Bussey, C.; Steckel, M.; Tanaka, N. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005, 19, 2668–2681. [Google Scholar] [CrossRef]
- Shim, S.B.; Williams, I.H.; Verstegen, M.W.A. Effects of dietary fructo-oligosaccharide on villous height and disaccharidase activity of the small intestine, pH, VFA and ammonia concentrations in the large intestine of weaned pigs. Acta Agric. Scand. Sect. A-Anim. Sci. 2005, 55, 91–97. [Google Scholar] [CrossRef]
- Okazaki, N.; Narita, M.; Yamada, S.; Izumikawa, K.; Umetsu, M.; Kenri, T.; Sasaki, Y.; Arakawa, Y.; Sasaki, T. Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol. Immunol. 2001, 45, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, M.; Takahashi, T.; Ubukata, K. Macrolide-resistant Mycoplasma pneumoniae: Characteristics of isolates and clinical aspects of community-acquired pneumonia. J. Infect. Chemother. 2010, 16, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Pereyre, S.; Guyot, C.; Renaudin, H.; Charron, A.; Bébéar, C.; Bébéar, C.M. In vitro selection and characterization of resistance to macrolides and related antibiotics in Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 460–465. [Google Scholar] [CrossRef]
- Tabassum, I.; Chaudhry, R.; Chourasia, B.K.; Malhotra, P. Identification of an N-terminal 27 kDa fragment of Mycoplasma pneumoniae P116 protein as specific immunogen in M. pneumoniae infections. BMC Infect. Dis. 2010, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.C.; Leith, D.K.; Wilson, R.M.; Baseman, J.B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect. Immun. 1982, 35, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Yiwen, C.; Yueyue, W.; Lianmei, Q.; Cuiming, Z.; Xiaoxing, Y. Infection strategies of mycoplasmas: Unraveling the panoply of virulence factors. Virulence 2021, 12, 788–817. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, D.; Gumpel, J.M.; Hill, A.; Swannell, A.J. Isolation of Mycoplasma pneumoniae from the synovial fluid of a hypogrammaglobulinaemic patient in a survey of patients with inflammatory polyarthritis. Ann. Rheum. Dis. 1978, 37, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, D.; Webster, A.D.; Furr, P.M.; Asherson, G.L. Prolonged persistence of Mycoplasma pneumoniae in a patient with hypogammaglobulinaemia. J. Infect. 1980, 2, 171–175. [Google Scholar] [CrossRef]
- Roifman, C.M.; Rao, C.P.; Lederman, H.M.; Lavi, S.; Quinn, P.; Gelfand, E.W. Increased susceptibility to Mycoplasma infection in patients with hypogammaglobulinemia. Am. J. Med. 1986, 80, 590–594. [Google Scholar] [CrossRef]
- Furr, P.M.; Taylor-Robinson, D.; Webster, A.D. Mycoplasmas and ureaplasmas in patients with hypogammaglobulinaemia and their role in arthritis: Microbiological observations over twenty years. Ann. Rheum. Dis. 1994, 53, 183–187. [Google Scholar] [CrossRef]
- Franz, A.; Webster, A.D.; Furr, P.M.; Taylor-Robinson, D. Mycoplasmal arthritis in patients with primary immunoglobulin deficiency: Clinical features and outcome in 18 patients. Br. J. Rheumatol. 1997, 36, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Ploton, M.C.; Sommet, J.; Koehl, B.; Gaschignard, J.; Holvoet, L.; Mariani-Kurkdjian, P.; Benkerrou, M.; Le Roux, E.; Bonacorsi, S.; Faye, A. Respiratory pathogens and acute chest syndrome in children with sickle cell disease. Arch. Dis. Child 2020, 105, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Lu, X.; Gao, B.; Liu, Y.; Aslam, M.Z.; Wang, X.; Li, Z. Lactiplantibacillus plantarum subs plantarum and Fructooligosaccharides Combination Inhibits the Growth, Adhesion, Invasion, and Virulence of Listeria monocytogenes. Foods 2022, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shin, E.C.; Park, H.G. Fructooligosaccharides decreased the ability of probiotic Escherichia coli Nissle 1917 to adhere to co-cultures of human intestinal cell lines. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 45–52. [Google Scholar] [CrossRef]
- Piotrowski, M.; Wultańska, D.; Obuch-Woszczatyński, P.; Pituch, H. Fructooligosaccharides and mannose affect Clostridium difficile adhesion and biofilm formation in a concentration-dependent manner. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1975–1984. [Google Scholar] [CrossRef]
- Piotrowski, M.; Wultańska, D.; Pituch, H. Effect of prebiotics on Bacteroides sp. adhesion and biofilm formation and synbiotic effect on Clostridioides difficile. Future Microbiol. 2022, 17, 363–375. [Google Scholar] [CrossRef]
- Yang, J.; Hooper, W.C.; Phillips, D.J.; Talkington, D.F. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infect. Immun. 2002, 70, 3649–3655. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.; Lu, J.; Chen, S.; Zhang, X.; Mao, W. Hyperoside inhibits proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Mol. Cell Biochem. 2019, 453, 179–186. [Google Scholar] [CrossRef]
- Waites, K.B.; Xiao, L.; Liu, Y.; Balish, M.F.; Atkinson, T.P. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin. Microbiol. Rev. 2017, 30, 747–809. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kwon, H.J.; Lee, B.J. Effect of clarithromycin on rhinovirus-16 infection in A549 cells. Eur. Respir. J. 2006, 27, 12–19. [Google Scholar] [CrossRef]
- Kudoh, S. Erythromycin treatment in diffuse panbronchiolitis. Curr. Opin. Pulm. Med. 1998, 4, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Khair, O.A.; Devalia, J.L.; Abdelaziz, M.M.; Sapsford, R.J.; Davies, R.J. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur. Respir. J. 1995, 8, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Boehm, G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J. Nutr. 2007, 137, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
| MIC Values | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strains | MP | MRMP | ||||||
| M129 | FH | A103 | H010 | A58 | M688/98 | T79 | P05/132 | |
| GOS (%) | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| FOS (%) | 16 | 16 | 8 | 16 | 16 | 16 | 16 | 16 |
| ERY (µg/mL) | 0.032 | 0.032 | 0.032 | 0.032 | 256 | 256 | 256 | 256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Cai, Y.; Slimmen, L.J.M.; de Bruijn, A.C.J.M.; van Rossum, A.M.C.; Folkerts, G.; Braber, S.; Unger, W.W.J. Galacto-Oligosaccharides as an Anti-Infective and Anti-Microbial Agent for Macrolide-Resistant and -Sensitive Mycoplasma pneumoniae. Pathogens 2023, 12, 659. https://doi.org/10.3390/pathogens12050659
Zhu H, Cai Y, Slimmen LJM, de Bruijn ACJM, van Rossum AMC, Folkerts G, Braber S, Unger WWJ. Galacto-Oligosaccharides as an Anti-Infective and Anti-Microbial Agent for Macrolide-Resistant and -Sensitive Mycoplasma pneumoniae. Pathogens. 2023; 12(5):659. https://doi.org/10.3390/pathogens12050659
Chicago/Turabian StyleZhu, Hongzhen, Yang Cai, Lisa J. M. Slimmen, Adrianus C. J. M. de Bruijn, Annemarie M. C. van Rossum, Gert Folkerts, Saskia Braber, and Wendy W. J. Unger. 2023. "Galacto-Oligosaccharides as an Anti-Infective and Anti-Microbial Agent for Macrolide-Resistant and -Sensitive Mycoplasma pneumoniae" Pathogens 12, no. 5: 659. https://doi.org/10.3390/pathogens12050659
APA StyleZhu, H., Cai, Y., Slimmen, L. J. M., de Bruijn, A. C. J. M., van Rossum, A. M. C., Folkerts, G., Braber, S., & Unger, W. W. J. (2023). Galacto-Oligosaccharides as an Anti-Infective and Anti-Microbial Agent for Macrolide-Resistant and -Sensitive Mycoplasma pneumoniae. Pathogens, 12(5), 659. https://doi.org/10.3390/pathogens12050659







