Entrapment Syndrome in a Kidney Transplant Recipient with Cryptococcal Meningitis
Abstract
:1. Introduction
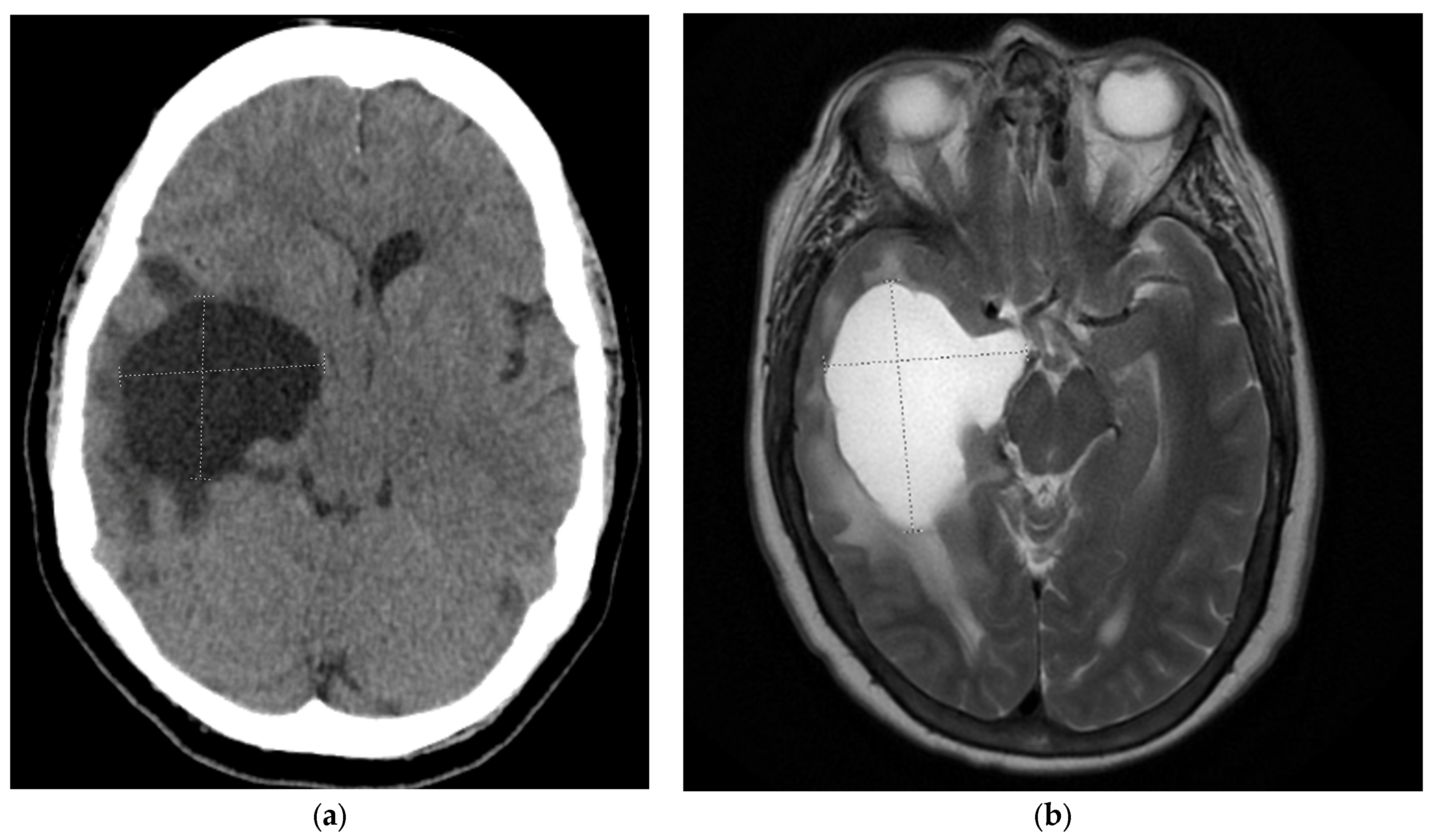
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Shi, Z.W.; Strickland, A.B.; Shi, M. Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen. J. Fungi 2022, 8, 1069. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.S.; Sathiyamoorthy, J.; Lalitha, C.; Ramakrishnan, J. A holistic review on Cryptococcus neoformans. Microb. Pathog. 2022, 166, 105521. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.M.; Ding, M.; Nielsen, K. Importance of Clinical Isolates in Cryptococcus neoformans Research. J. Fungi 2023, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Tardieu, L.; Divard, G.; Lortholary, O.; Scemla, A.; Rondeau, É.; Accoceberry, I.; Agbonon, R.; Alanio, A.; Angoulvant, A.; Albano, L.; et al. Cryptococcal Meningitis in Kidney Transplant Recipients: A Two-Decade Cohort Study in France. Pathogens 2022, 11, 699. [Google Scholar] [CrossRef]
- Van der Torre, M.H.; Andrews, R.A.; Hooker, E.L.; Rankin, A.; Dodd, S. Systematic review on Cryptococcus neoformans/Cryptococcus gattii species complex infections with recommendations for practice in health and care settings. Clin. Infect. Pract. 2022, 15, 100154. [Google Scholar] [CrossRef]
- Sharma, C.; Acharya, M.; Kumawat, B.L.; Kochar, A. ‘Trapped temporal horn’ of lateral ventricle in tuberculous meningitis. BMJ Case Rep. 2014, 2014, bcr2014203837. [Google Scholar] [CrossRef] [PubMed]
- Maurice-Williams, R.S.; Choksey, M. Entrapment of the temporal horn: A form of focal obstructive hydrocephalus. J. Neurol. Neurosurg. Psychiatry 1986, 49, 238–242. [Google Scholar] [CrossRef]
- Shaariah, W.; Morad, Z.; Suleiman, A.B. Cryptococcosis in renal transplant recipients. Transplant. Proc. 1992, 24, 1898–1899. [Google Scholar] [PubMed]
- Zhuang, Y.; Richard, S.; Zhou, J.; Liu, J.; Fang, Z.; Chen, Z. Entrapped temporal horn syndrome: A retrospective analysis of 5 case series. Int. J. Surg. Glob. Health 2022, 5, e73. [Google Scholar] [CrossRef]
- Rhoton, A.L., Jr.; Gomez, M.R. Conversion of multilocular hydrocephalus to unilocular. Case report. J. Neurosurg. 1972, 36, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl, A.K.; Baldauf, J.; Gaab, M.R.; Schroeder, H.W. Endoscopic temporal ventriculocisternostomy: An option for the treatment of trapped temporal horns. J. Neurosurg. Pediatr. 2013, 11, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Kovoor, J.M.; Mahadevan, A.; Narayan, J.P.; Govindappa, S.S.; Satishchandra, P.; Taly, A.V.; Shankar, S.K. Cryptococcal choroid plexitis as a mass lesion: MR imaging and histopathologic correlation. AJNR Am. J. Neuroradiol. 2002, 23, 273–276. [Google Scholar] [PubMed]
- Kumari, R.; Raval, M.; Dhun, A. Cryptococcal choroid plexitis: Rare imaging findings of central nervous system cryptococcal infection in an immunocompetent individual. Br. J. Radiol. 2010, 83, e14–e17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, L.; Thompson, G.R., III; Koff, A.; Cohen, S.H. Entrapment Syndrome in a Kidney Transplant Recipient with Cryptococcal Meningitis. Pathogens 2023, 12, 711. https://doi.org/10.3390/pathogens12050711
Reddy L, Thompson GR III, Koff A, Cohen SH. Entrapment Syndrome in a Kidney Transplant Recipient with Cryptococcal Meningitis. Pathogens. 2023; 12(5):711. https://doi.org/10.3390/pathogens12050711
Chicago/Turabian StyleReddy, Laya, George R. Thompson, III, Alan Koff, and Stuart H. Cohen. 2023. "Entrapment Syndrome in a Kidney Transplant Recipient with Cryptococcal Meningitis" Pathogens 12, no. 5: 711. https://doi.org/10.3390/pathogens12050711
APA StyleReddy, L., Thompson, G. R., III, Koff, A., & Cohen, S. H. (2023). Entrapment Syndrome in a Kidney Transplant Recipient with Cryptococcal Meningitis. Pathogens, 12(5), 711. https://doi.org/10.3390/pathogens12050711






