Toxicity of Common Acaricides, Disinfectants, and Natural Compounds against Eggs of Rhipicephalus annulatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acaricides, Chemicals, and Natural Products
2.2. Preparation of Different Common Acaricides
2.3. Ivermectin Preparation
2.4. Disinfectants Preparations
2.5. Natural Compounds Preparation
2.6. Eggs Collection of R. annulatus
2.7. Egg Hatch Assay (EHA)
2.8. Extended In Vitro Inhibition of Egg Hatching Bioassay
2.9. Statistical Analysis
3. Results
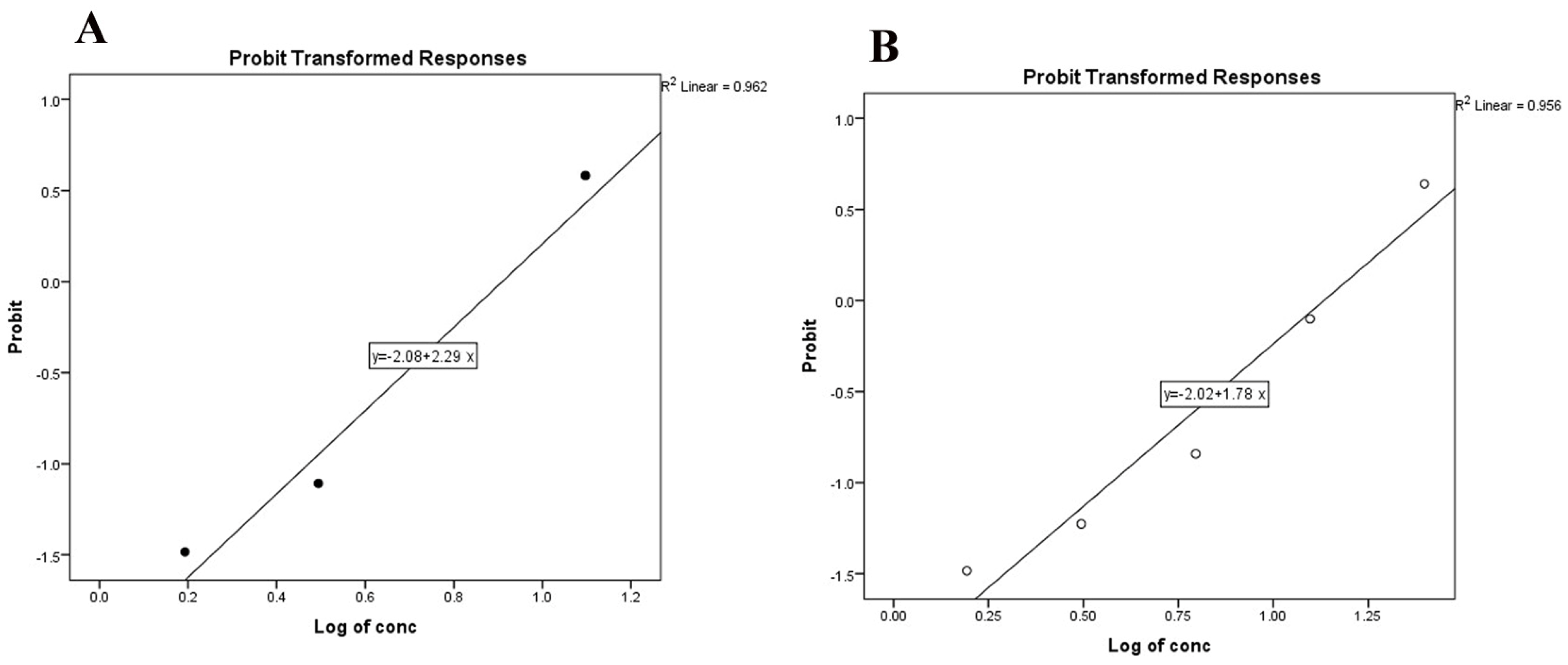
3.1. Ovicidal Activity of Acaricides
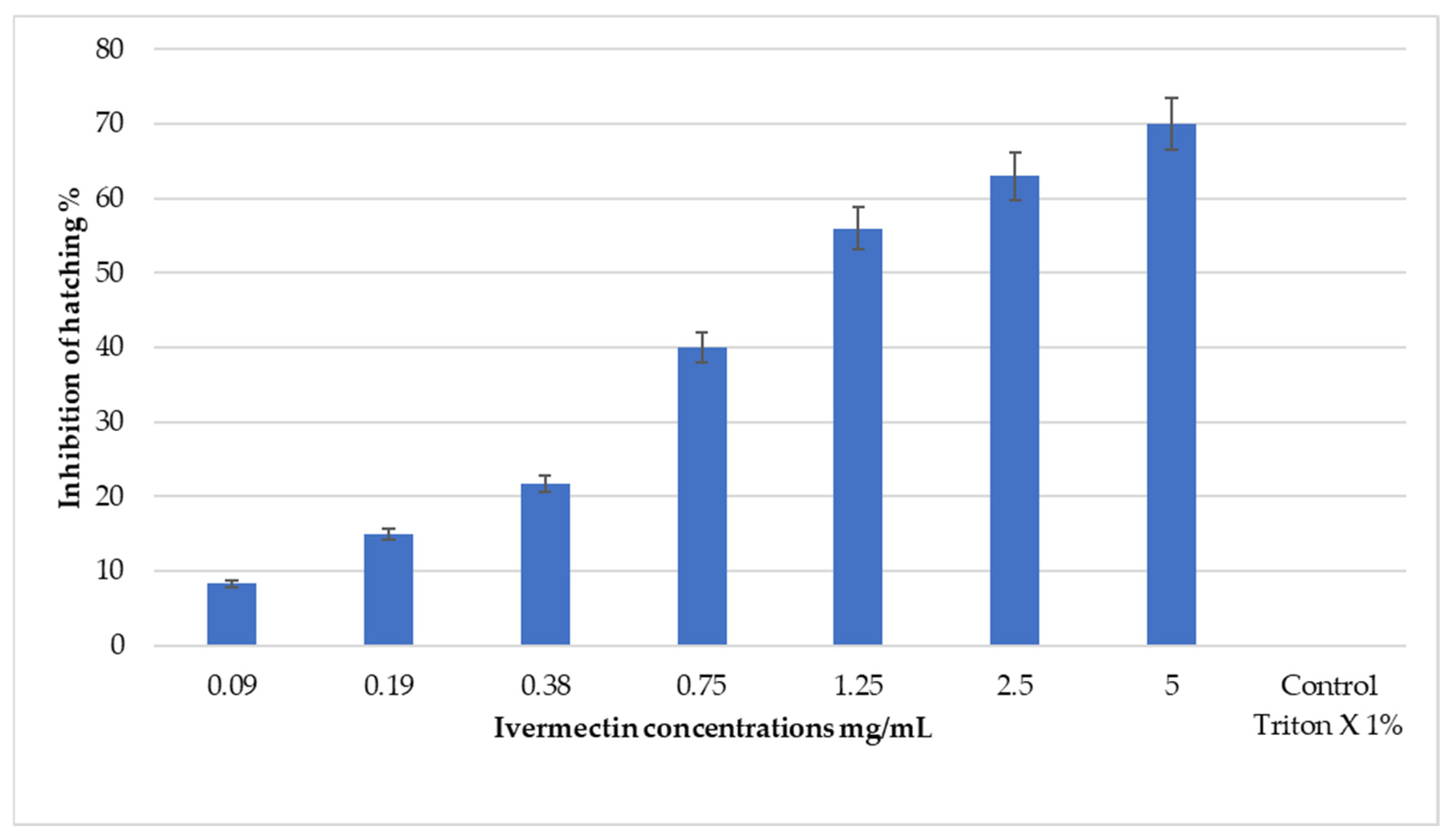
3.2. Ovicidal Activity of Ivermectin
3.3. Ovicidal Effect of Disinfectants
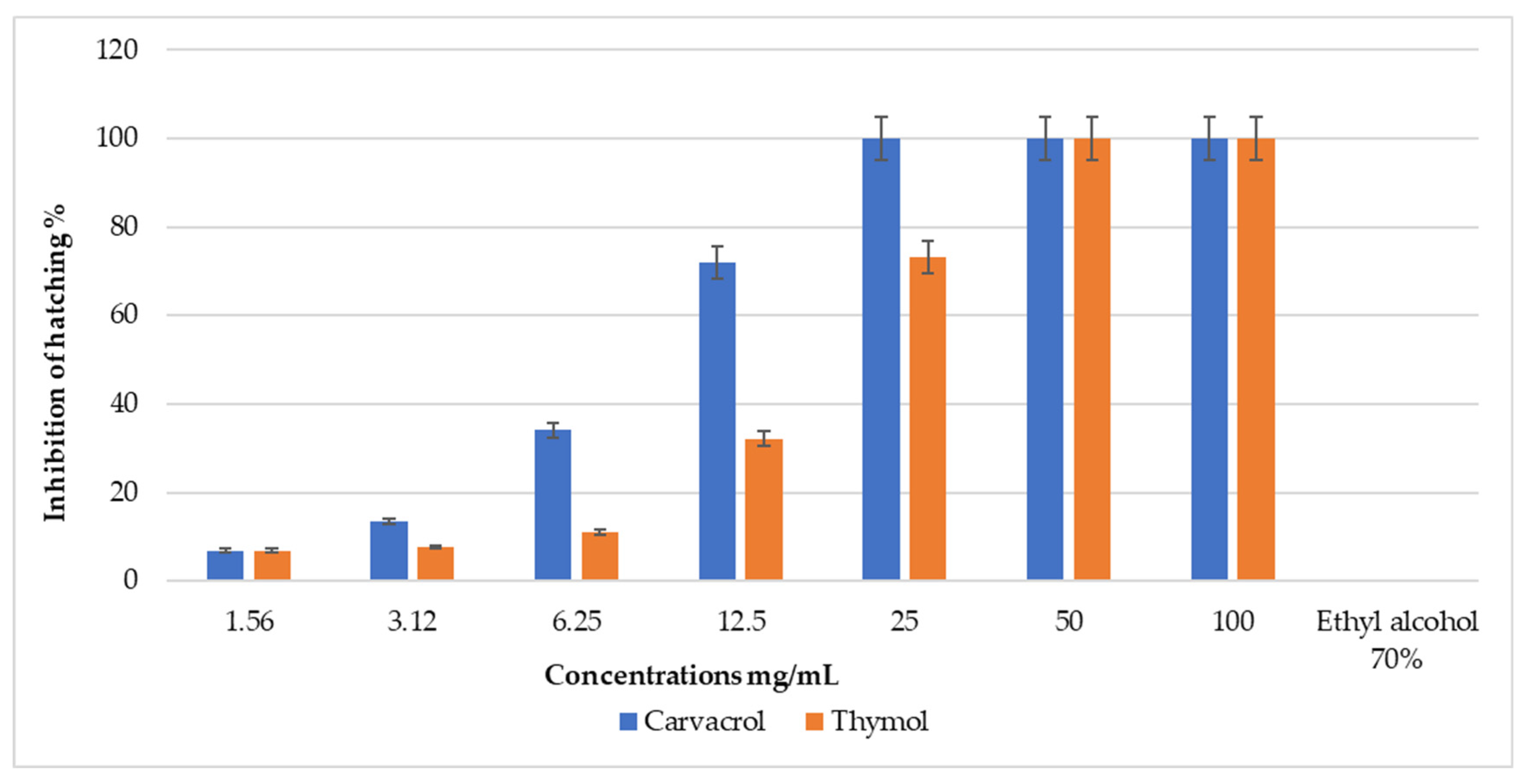
3.4. Ovicidal Effect of Selected Natural Products
3.5. Extended In Vitro Inhibition of Egg Hatching Bioassay Results Using Soil
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouattour, A.; Darghouth, M.A.; Daoud, A. Distribution and ecology of ticks (Acari: Ixodidae) infesting livestock in Tunisia: An overview of eighth years field collections. Parassitologia 1999, 41 (Suppl. 1), 5–10. [Google Scholar] [PubMed]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of domestic animals in the Mediterranean region. In A Guide to Identification of Species, 1st ed.; Bioscience Reports: Edinburgh, UK, 2004; p. 13. [Google Scholar]
- Adham, F.K.; Abd-El-Samie, E.M.; Gabre, R.M.; Hussein, H.E. Detection of tick blood parasites in Egypt using PCR assay I-Babesia bovis and Babesia bigemina. Parasitol. Res. 2009, 105, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Ueti, M.W.; Noh, S.M.; Knowles, D.P.; Palmer, G.H. Conservation of transmission phenotype of Anaplasma marginale (Rickettsiales: Anaplasmataceae) strains among Dermacentor and Rhipicephalus ticks (Acari: Ixodidae). J. Med. Entomol. 2007, 44, 484–491. [Google Scholar] [CrossRef]
- Rouby, S.R.; Hussein, K.H.; Aboelhadid, S.M.; El-Sherif, A.M. Role of Rhipicephalus annulatus tick in transmission of lumpy skin disease virus in naturally infected cattle in Egypt. Adv. Anim. Vet. Sci. 2017, 5, 185–191. [Google Scholar]
- de Castro, J.J. Sustainable tick and tick borne disease control in livestock improvement in developing countries. Vet. Parasitol. 1997, 71, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Perez de Leon, A.A.; Mitchell, R.D., 3rd; Watson, D.W. Ectoparasites of cattle. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 173–185. [Google Scholar] [CrossRef]
- Abdel-Shafy, S. Is the cattle tick Rhipicephalus annulatus Say, 1821 reared on the rabbit? J. Parasit. Dis. 2018, 42, 297–302. [Google Scholar] [CrossRef]
- Haytham, S.; Kanta, H.L.; Aiswarya, B.; Kumar, B.D.; Badal, B.; Sahidur, R. Life cycle of the southern cattle tick, Rhipicephalus (Boophilus) microplus Canestrini 1888 (Acari: Ixodidae) under laboratory conditions. Syst. Appl. Acarol. 2018, 23, 1169. [Google Scholar]
- Jagannath, M.S.; Muraleedharan, K.; Hiregoudar, L.S. Life-cycle of Boophilus annulatus (Say 1821) (Acari: Ixodidae). Indian J. Anim. Sci. 1982, 52, 502–505. [Google Scholar]
- Davey, R.B. Effect of temperature on the ovipositional biology and egg viability of the cattle tick Boophilus annulatus (Acari: Ixodidae). Exp. Appl. Acarol. 1988, 5, 1–14. [Google Scholar] [CrossRef]
- Booth, T.F. Observation on the composition and biosynthesis of egg wax lipids in the cattle tick, Boophilus microplus. Exp. Appl. Acarol. 1992, 14, 137–149. [Google Scholar] [CrossRef]
- Esteves, E.; Fogaça, A.C.; Maldonado, R.; Silva, F.D.; Manso, P.P.A.; Pelajo-Machado, M.; Valle, D. Daffre Antimicrobial activity in the tick Rhipicephalus (Boophilus) microplus eggs: Cellular localization and temporal expression of microplusin during oogenesis and embryogenesis. Dev. Comp. Immunol. 2009, 33, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Leskiw, B.K.; Kaufman, W.R. Antimicrobial activity in the egg wax of the African cattle tick Amblyomma hebraeum (Acari: Ixodidae). Exp. Appl. Acarol. 2006, 39, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Thomson, E.L.; Liu, J.; Dennis, J.J.; Jacobs, R.L.; Kaufman, W.R. Antimicrobial activity in the egg of the Amblyomma hebraeum (Acari: Ixodidae) is associated with free fatty acids C16:1 and C18:2. Exp. Appl. Acarol. 2012, 58, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S. Tick ecology: Processes and patterns behind the epidemiological risk posed by Ixodid ticks as vectors. Parasitology 2004, 129, S37–S65. [Google Scholar] [CrossRef]
- Davey, R.B.; Cooksey, L.M.; Despins, J.L. Survival of larvae of Boophilus annulatus, Boophilus microplus, and Boophilus hybrids (Acari: Ixodidae) in different temperature and humidity regimes in the laboratory. Vet. Parasitol. 1991, 40, 305–313. [Google Scholar] [CrossRef]
- Davey, R.B. Daily dynamics of egg development and fecundity and effect of age of larvae on attachment rate to cattle in Boophilus annulatus. Southwest. Entomol. 1986, 11, 17–22. [Google Scholar]
- Esteves, E.; Pohl, P.C.; Klafke, G.M.; Reck, J.; Fogaça, A.C.; Martins, J.R.; Daffre, S. Low temperature affects cattle tick reproduction but does not lead to transovarial transmission of Anaplasma marginale. Vet. Parasitol. 2015, 214, 322–326. [Google Scholar] [CrossRef]
- Canevari, J.T.; Mangold, A.J.; Guglielmone, A.A.; Nava, S. Population dynamics of the cattle tick Rhipicephalus (Boophilus) microplus in a subtropical subhumid region of Argentina for use in the design of control strategies. Med. Vet. Entomol. 2017, 31, 6–14. [Google Scholar] [CrossRef]
- de Barros, M.N.D.; Riet-Correa, F.; Azevedo, S.S.; Labruna, M.B. Off-host development and survival of Rhipicephalus (Boophilus) microplus in the Brazilian semiarid. Vet. Parasitol. Reg. Stud. Rep. 2017, 9, 17–24. [Google Scholar] [CrossRef]
- Haque, M.; Jyoti; Singh, N.K.; Rath, S.S. Effect of various acaricides on hatchability of eggs of Rhipicephalus (Boophilus) microplus. BioMed Res. Int. 2014, 2014, 425423. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singh, V.; Shyma, K.P.; Solanki, V.; Gupta, J.P. Detection of deltamethrin resistance in eggs and larva of Rhipicephalus (Boophilus) microplus. Indian J. Anim. Sci. 2017, 87, 143–146. [Google Scholar] [CrossRef]
- Prado-Ochoa, M.; Muñoz-Guzmán, M.; Abrego-Reyes, V.; Velázquez-Sánchez, A.; Lara-Rocha, M.; Cuenca-Verde, C.; Angeles, E.; Alba-Hurtado, F. Effect of new ethyl and methyl carbamates on biological parameters and reproduction of the cattle tick Rhipicephalus microplus. Vet. Parasitol. 2013, 194, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Aboelhadid, S.M.; Ibrahium, S.M.; Abdel-Baki, A.S.; Hassan, K.M.; Arafa, W.M.; Aboud, H.M.; Mohy, S.; Al-Quraishy, S.; Hassan, A.O.; Abdelgelil, N.H.; et al. An investigation of the acaricidal activity of benzyl alcohol on Rhipicephalus annulatus and Rhipicephalus sanguineus and its synergistic or antagonistic interaction with commonly used acaricides. Med. Vet. Entomol. 2024, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, A.A.; Abdelfattah, A.M.; Khalil, W.F.; Mahmoud, A.E.; Abouelhassan, E. In vitro and in vivo Evaluation of the Efficacy of Phoxim and Deltamethrin against Life Stages of Rhipicephalus sanguineus (Brown Dog Tick). J. Adv. Vet. Res. 2023, 13, 827–832. [Google Scholar]
- Batiha, G.E.; El-Far, A.H.; El-Mleeh, A.A.; Alsenosy, A.A.; Abdelsamei, E.K.; Abdel-Daim, M.M.; El-Sayed, Y.S.; Shaheen, H.M. In vitro study of ivermectin efficiency against the cattle tick, Rhipicephalus (Boophilus) annulatus, among cattle herds in El-Beheira, Egypt. Vet. World 2019, 12, 1319–1326. [Google Scholar] [CrossRef]
- Gamal, A.; Aboelhadid, S.M.; Abo El-Ela, F.I.; Abdel-Baki, A.S.; Ibrahium, S.M.; El-Mallah, A.M.; Al-Quraishy, S.; Hassan, A.O.; Gadelhaq, S.M. Synthesis of Carvacrol-Loaded Invasomes Nanoparticles Improved Acaricide Efficacy, Cuticle Invasion and Inhibition of Acetylcholinestrase against Hard Ticks. Microorganisms 2023, 11, 733. [Google Scholar] [CrossRef]
- Arafa, W.M.; Klafke, G.M.; Tidwell, J.P.; de Leon, A.A.P.; Esteve- Gassent, M. Detection of single nucleotide polymor-phism in the para-sodium channel gene of Rhipicephalus annulatus populations from Egypt resistant to deltamethrin. Ticks Tick-Borne Dis. 2020, 11, 101488. [Google Scholar] [CrossRef]
- Gadelhaq, S.M.; Ibrahium, S.M.; Abdel-Baki, A.S.; Arafa, W.M.; Al-Quraishy, S.; Hassan, A.O.; Abdelgelil, N.H.; Ahmed, M.; Aboelhadid, S.M. Efficacy and safety of geranium-oregano-thymol formulations to control of dog tick Rhipicephalus sanguineus sensu lato under laboratory and field conditions. Vet. Parasitol. 2024, 327, 110112. [Google Scholar] [CrossRef]
- Geering, W.A.; Penrith, M.L.; Nayakahuma, D. Manual on Procedures for Disease Eradication by Stamping Out; Food and Agriculture Organization of The United Nations: Rome, Italy, 2001. [Google Scholar]
- Gadelhaq, S.M.; Arafa, W.M.; Abolhadid, S.M. In vitro activity of natural and chemical products on sporulation of Eimeria species oocysts of chickens. Vet. Parasitol. 2018, 251, 12–16. [Google Scholar] [CrossRef]
- de Oliveira Monteiro, C.M.; Daemon, E.; Clemente, M.A.; dos Santos Rosa, L.; Maturano, R. Acaricidal efficacy of thymol on engorged nymphs and females of Rhipicephalus sanguineus (Latreille, 1808) (Acari: Ixodidae). Parasitol. Res. 2009, 105, 1093. [Google Scholar] [CrossRef] [PubMed]
- Camargo-Mathias, M.I. Inside Ticks: Morphophysiology, Toxicology and Therapeutic Perspectives; Editora UNESP: São Paulo, Brazil, 2018; 199p, ISBN 978-85954-62-86-1. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.; Miller, R.; Ojeda-Chi, M.; Rosado-Aguilar, J.; Trinidad-Martínez, I.; de León, A.P. Acaricide and ivermectin resistance in a field population of Rhipicephalus microplus (Acari: Ixodidae) collected from red deer (Cervus elaphus) in the Mexican tropics. Vet. Parasitol. 2014, 200, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Arafa, W.M.; Aboelhadid, S.M.; Moawad, A.; Shokeir, K.M.; Ahmed, O. Toxicity, repellency and anti-cholinesterase activities of thymol-eucalyptus combinations against phenotypically resistant Rhipicephalus annulatus ticks. Exp. Appl. Acarol. 2020, 81, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.B.; Ahrens, E.H.; George, J.E. Ovicidal Activity of Topically Applied Acaricides Against Eggs of the Southern Cattle Tick (Acari: Ixodidae). J. Econ. Entomol. 1989, 82, 539–542. [Google Scholar] [CrossRef]
- Ribeiro, V.L.S.; Avancini, C.; Gonçalves, K.; Toigo, E.; von Poser, G. Acaricidal activity of Calea serrata (Asteraceae) on Boophilus microplus and Rhipicephalus sanguineus. Vet. Parasitol. 2008, 151, 351–354. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve, 2nd ed.; Cambridge University Press: Cambridge, UK, 1952. [Google Scholar]
- Rodriguez-Vivas, R.I.; Jonsson, N.N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2017, 117, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Mastropaolo, M.; Mangold, A.J.; Guglielmone, A.A.; Nava, S. Non-parasitic life cycle of the cattle tick Rhipicephalus (Boophilus) microplus in Panicum maximum pastures in northern Argentina. Res. Vet. Sci. 2017, 115, 138–145. [Google Scholar] [CrossRef]
- Ibrahium, S.M.; Aboelhadid, S.M.; Wahba, A.A.; Farghali, A.A.; Miller, R.J.; Abdel-Baki, A.-A.S.; Al-Quraishy, S. Preparation of geranium oil formulations effective for control of phenotypic resistant cattle tick Rhipicephalus annulatus. Sci. Rep. 2022, 12, 11693. [Google Scholar] [CrossRef]
- Aboelhadid, S.; Arafa, W.M.; Mahrous, L.N.; Fahmy, M.M.; Kamel, A.A. Molecular detection of Rhipicephalus (Boophilus) annulatus resistance against deltamethrin in middle Egypt. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 198–204. [Google Scholar] [CrossRef]
- El-Bahy, N.M.; Bazh, E.K.; Shaheen, H.M. Efficacy of deltamethrin, diazinon, and ivermectin on Boophilus annulatus ticks (in vitro and in vivo study). Parasitol. Res. 2014, 114, 29–36. [Google Scholar] [CrossRef]
- Ravindran, R.; Kumar, K.G.A.; Suresh, N.N.; Amithamol, K.K.; Sunil, A.R.; Adarshkrishna, T.P.; Chithra, N.D.; Jyothimol, G.; Ghosh, S.; Juliet, S. Acaricidal effects of fenvalerate and cypermethrin against Rhipicephalus (Boophilus) annulatus. Trop. Biomed. 2014, 31, 449–455. [Google Scholar] [PubMed]
- Khalaf-Allah, S.S. Acaricidal efficacy of cypermethrine (a new synthetic pyrethroid) against Boophilus annulatus ticks in cattle. Dtsch. Tierarztl. Wochenschr. 1996, 103, 463–464. [Google Scholar]
- Rodríguez-Hidalgo, R.; Pérez-Otáñez, X.; Garcés-Carrera, S.; Vanwambeke, S.O.; Madder, M.; Benítez-Ortiz, W. The current status of resistance to alpha-cypermethrin, ivermectin, and amitraz of the cattle tick (Rhipicephalus microplus) in Ecuador. PLoS ONE 2017, 12, e0174652. [Google Scholar] [CrossRef]
- de Sousa, A.B.B.; Bianchi, D.; Santos, E.M.; Dias, S.R.; Peleja, P.L.; Santos, R.R.; Caruso, N.M.; Minervino, A.H.H. First Description of Acaricide Resistance in Populations of Rhipicephalus microplus Tick from the Lower Amazon, Brazil. Animals 2022, 12, 2931. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jyothimol, G.; Amithamol, K.K.; Sunil, A.R.; Chandrasekhar, L.; Lenka, D.R.; Amritha, A.; Sreelekha, K.; Sathish, N.; Udayan, D.; et al. In vitro efficacy of amitraz, coumaphos, deltamethrin and lindane against engorged female Rhipicephalus (Boophilus) annulatus and Haemaphysalis bispinosa ticks. Exp. Appl. Acarol. 2018, 75, 241–253. [Google Scholar] [CrossRef]
- Iken, I.; Abdessadek, M.; El Attari, A.; Achour, S. Poisoning by Amitraz, uncommon pesticide revealed by high per-formance liquid chromatography: About two cases. Toxicol. Anal. Et Clin. 2020, 32, 200–204. [Google Scholar]
- Kanapadinchareveetil, S.; Chandrasekhar, L.; Pious, A.; Kartha, H.S.; Ravindran, R.; Juliet, S.; Nair, S.N.; Ajithkumar, K.G.; Ghosh, S. Molecular, histological and ultrastructural characterization of cytotoxic effects of amitraz on the ovaries of engorged females of Rhipicephalus (Boophilus) annulatus. Exp. Parasitol. 2019, 204, 107732. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Barrero, R.A.; Black, M.; Bellgard, M.I.; van Dalen, E.M.; Fourie, J.; MaritzOlivier, C. Differentially expressed genes in response to amitraz treatment suggests a proposed model of resistance to amitraz in R decoloratus ticks. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 361–371. [Google Scholar] [CrossRef]
- Eddleston, M. The pathophysiology of organophosphorus pesticide self-poisoning is not so simple. Neth. J. Med. 2008, 66, 146–148. [Google Scholar]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current research on the safety of pyrethroids used as insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef]
- Lima, H.G.; Santos, F.O.; Santos, A.C.V.; Silva, G.D.D.; Santos, R.J.D.; Carneiro, K.O.; Reis, I.M.A.; Estrela, I.O.; Freitas, H.F.; Bahiense, T.C.; et al. Anti-tick effect and cholines-terase inhibition caused by Prosopis juliflora alkaloids: In vitro and in silico studies. Rev. Bras. Parasitol. Vet. 2020, 29, e019819. [Google Scholar] [CrossRef] [PubMed]
- El-Ashram, S.; Aboelhadid, S.M.; Kamel, A.A.; Mahrous, L.N.; Fahmy, M.M. First Report of Cattle Tick Rhipicephalus (Boophilus) annulatus in Egypt Resistant to Ivermectin. Insects 2019, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Cully, D.F.; Vassilatis, D.K.; Liu, K.K.; Paress, P.S.; Van der Ploeg, L.H.; Schaeffer, J.M.; Arena, J.P. Cloning of an avermec-tin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 1994, 371, 707–711. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, P.; Beato, M.S.; Capua, I. Inactivation of Avian Influenza Viruses by Chemical Agents and Physical Conditions: A Review. Zoonoses Public. Health 2007, 54, 51–68. [Google Scholar] [CrossRef]
- Gasparini, R.; Pozzi, T.; Magnelli, R.; Fatighenti, D.; Giotti, E.; Poliseno, G.; Pratelli, M.; Severini, R.; Bonanni, P.; De Feo, L. Evaluation of in vitro efficacy of the disinfectant Virkon. Eur. J. Epidemiol. 1995, 11, 193–197. [Google Scholar] [CrossRef]
- Bloomfield, S.F. Chlorine and iodine formulations. In Handbook of Disinfectants and Antiseptics; Ascenzi, J.M., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 133–158. [Google Scholar]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Scott, E.M.; Gorman, S.P. Glutaraldehyde. In Disinfection, Sterilization and Preservation, 4th ed.; Block, S.S., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1991; pp. 596–614. [Google Scholar]
- Power, E.G.M. Aldehydes as biocides. Prog. Med. Chem. 1995, 34, 149–201. [Google Scholar]
- Senra, T.d.O.S.; Zeringóta, V.; Monteiro, C.M.d.O.; Calmon, F.; Maturano, R.; Gomes, G.A.; Faza, A.; de Carvalho, M.G.; Daemon, E. Assessment of the acaricidal activity of carvacrol, (E)-cinnamaldehyde, trans-anethole, and linalool on larvae of Rhipicephalus microplus and Dermacentor nitens (Acari: Ixodidae). Parasitol. Res. 2013, 112, 1461–1466. [Google Scholar] [CrossRef]
- Araújo, L.X.; Novato, T.P.L.; Zeringota, V.; Maturano, R.; Melo, D.; DA Silva, B.C.; Daemon, E.; DE Carvalho, M.G.; Monteiro, C.M.O. Synergism of thymol, carvacrol and eugenol in larvae of the cattle tick, Rhipicephalus microplus, and brown dog tick, Rhipicephalus sanguineus. Med. Vet. Entomol. 2016, 30, 377–382. [Google Scholar] [CrossRef]
- Novato, T.; Gomes, G.A.; Zeringóta, V.; Franco, C.T.; de Oliveira, D.R.; Melo, D.; de Carvalho, M.G.; Daemon, E.; Monteiro, C.M.d.O. In vitro assessment of the acaricidal activity of carvacrol, thymol, eugenol and their acetylated derivatives on Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol. 2018, 260, 1–4. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Monteiro, C.M.; Daemon, E.; Silva, A.M.R.; Maturano, R.; Amaral, C. Acaricide and ovicide activities of thymol on engorged females and eggs of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol. Res. 2010, 106, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.A.; Tirloni, L.; Pinto, A.F.; Diedrich, J.K.; Yates, J.R.; Gonzales, S.; Farber, M.; Vaz, I.d.S.; Termignoni, C. Tick Gené’s organ engagement in lipid metabolism revealed by a combined transcriptomic and proteomic approach. Ticks Tick-Borne Dis. 2019, 10, 787–797. [Google Scholar] [CrossRef]
- Aktar, W.; Paramasivam, M.; Sengupta, D.; Purkait, S.; Ganguly, M.; Banerjee, S. Impact assessment of pesticide residues in fish of Ganga river around Kolkata in West Bengal. Environ. Monit. Assess. 2009, 157, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Critical Peak Pricing-San Diego Gas & Electric. Available online: https://www.sdge.com/businesses/savings-center/energy-management-programs/demand-response/critical-peak-pricing (accessed on 4 March 2022).
- Favari, L.; López, E.; Martínez-Tabche, L.; Díaz-Pardo, E. Effect of Insecticides on Plankton and Fish of Ignacio Ramirez Reservoir (Mexico): A Biochemical and Biomagnification Study. Ecotoxicol. Environ. Saf. 2002, 51, 177–186. [Google Scholar] [CrossRef]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef]
- Bradberry, S.M.; A Cage, S.; Proudfoot, A.T.; Vale, J.A. Poisoning due to pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef]
- Costa, L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar]
- Heaney, K.; Lindahl, R. Safety of a topically applied spot-on formulation of metaflumizone plus amitraz for flea and tick control in dogs. Vet. Parasitol. 2007, 150, 225–232. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef]
- Vudriko, P.; Okwee-Acai, J.; Tayebwa, D.S.; Byaruhanga, J.; Kakooza, S.; Wampande, E.; Omara, R.; Muhindo, J.B.; Tweyongyere, R.; Owiny, D.O.; et al. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasites Vectors 2016, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.S.; Sciuto, M.; Dahl, J. The use of phoxim and bendiocarb for control of fleas in farmed mink (Mustela vison). Acta Vet. Scand. 2018, 60, 58. [Google Scholar] [CrossRef]
- Maghraby, M.; Mahmoud, A.; Abdelkhalek, D.; Sallam, N.; Aly, A.; Elaziz, A.; Soliman, E. Insecticidal efficacy and safety of phoxim and influence on hematological, biochemical, and antioxidant profiles in german shepherd dogs. Open Vet. J. 2022, 12, 888–902. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Omura, S. Ivermectin,‘wonder drug’from Japan: The human use perspective. Proc. Jpn. Acad. Ser. B 2011, 87, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Mbassi, D.E.; Mombo-Ngoma, G.; Held, J.; Okwu, D.G.; Ndzebe-Ndoumba, W.; Kalkman, L.C.; Zoleko-Manego, R. Efficacy and safety of ivermectin for the treatment of Plasmodium falciparum infections in asymptomatic male and female Gabonese adults–A pilot randomized, double-blind, placebo-controlled single-centre phase Ib/IIa clinical trial. EBioMedicine 2023, 97, 104814. [Google Scholar]
- Navarro, M.; Camprubí, D.; Requena-Méndez, A.; Buonfrate, D.; Giorli, G.; Kamgno, J.; Gardon, J.; Boussinesq, M.; Muñoz, J.; Krolewiecki, A. Safety of high-dose ivermectin: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2020, 75, 827–834. [Google Scholar] [CrossRef]
- Rúa, J.; del Valle, P.; de Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of carvacrol and thymol: Antimicrobial activity against Staphylococcus aureus and antioxidant activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial activity of thyme oil, oregano oil, thymol and carvacrol against sensitive and resistant microbial isolates from dogs with otitis externa. Vet. Dermatol. 2019, 30, 524-e159. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil—New Insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A natural phenolic compound with antimicrobial properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
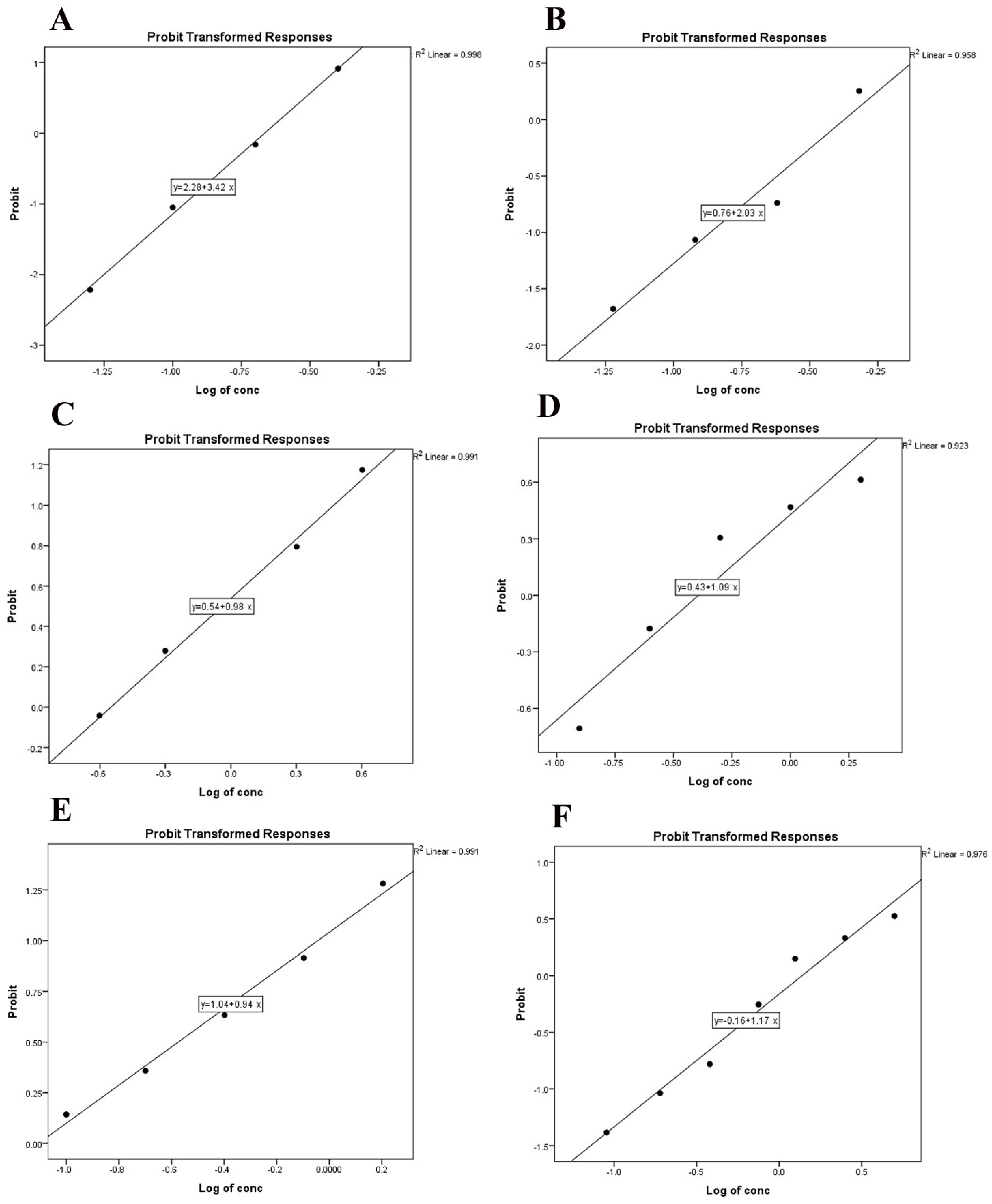
| Acaricide | LC50 (95% CL) mg/mL | LC90 (95% CL) mg/mL | Χ2 | df | Slope ± SE | p * | R2 |
|---|---|---|---|---|---|---|---|
| Deltamethrin | 0.216 (0.195–0.242) | 0.520 (0.436–0.658) | 0.526 | 3 | 3.36 ± 0.29 | 0.913 | 0.998 |
| Alpha-cypermethrin | 0.408 (0.342–0.514) | 1.544 (1.079–2.617) | 5.008 | 3 | 2.22 ± 0.23 | 0.171 | 0.958 |
| Cholopyrifos | 0.398 (0.303–0.506) | 5.96 (3.44–14.59) | 5.177 | 3 | 1.09 ± 0.14 | 0.159 | 0.923 |
| Phoxim | 0.279 (0.156–0.401) | 5.88 (3.54–14.27) | 0.392 | 3 | 0.97 ± 0.15 | 0.942 | 0.991 |
| Amitraz | 0.077 (0.035–0.120) | 1.86 (1.14–4.53) | 0.336 | 3 | 0.93 ± 0.15 | 0.953 | 0.991 |
| Ivermectin | 1.25 (1.02–1.57) | 15.8 (10.02–29.40) | 9.655 | 5 | 1.18 ± 0.09 | 0.086 | 0.976 |
| Carvacrol | 9.10 (8.21–9.97) | 15.4 (14.37–17.99) | 1.32 | 5 | 2.02 ± 0.09 | 0.933 | 0.990 |
| Thymol | 18.0 (16.49–19.97) | 30.7 (28.70–35.62) | 0.997 | 5 | 1.01 ± 0.04 | 0.963 | 0.993 |
| Disinfectants/Conc | Percentage Inhibition of Hatching (IH%) ± SE | ||
|---|---|---|---|
| 5 mg/mL | 10 mg/mL | 20 mg/mL | |
| Virkon® S | 7.66 ± 2.89 | 7.28 ± 2.39 | 9.95 ± 1.52 * |
| TH4 | 6.89 ± 2.29 | 8.81 ± 3.51 | 11.1 ± 2.39 * |
| 1 mg/mL | 2 mg/mL | 4 mg/mL | |
| Chlorox (5%) | 6.89 ± 3.04 | 7.28 ± 1.75 | 8.81 ± 1.75 |
| 50 mg/mL | 100 mg/mL | 200 mg/mL | |
| Formalin | 6.51 ± 1.75 | 6.89 ± 2.29 | 34.1 ± 2.89 * |
| Control group Distilled water | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Treatments | Phoxim (0.5 mg/mL) Treated Eggs ± SE | Deltamethrin (0.05 mg/mL) Treated Eggs ± SE | Untreated Eggs DW Control |
|---|---|---|---|
| Percentage inhibition of hatching (IH%) | 40.0 ± 5.08 * | 8.79 ± 4.06 | 0.00 ± 0.00 |
| Viability of hatched larvae | Slow motion or dead | Viable | Viable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahium, S.M.; Abdel-Baki, A.-A.S.; Gadelhaq, S.M.; Aboelhadid, S.M.; Mahran, H.A.; Al-Quraishy, S.; Reyad, A.; Kamel, A.A. Toxicity of Common Acaricides, Disinfectants, and Natural Compounds against Eggs of Rhipicephalus annulatus. Pathogens 2024, 13, 824. https://doi.org/10.3390/pathogens13100824
Ibrahium SM, Abdel-Baki A-AS, Gadelhaq SM, Aboelhadid SM, Mahran HA, Al-Quraishy S, Reyad A, Kamel AA. Toxicity of Common Acaricides, Disinfectants, and Natural Compounds against Eggs of Rhipicephalus annulatus. Pathogens. 2024; 13(10):824. https://doi.org/10.3390/pathogens13100824
Chicago/Turabian StyleIbrahium, Samar M., Abdel-Azeem S. Abdel-Baki, Sahar M. Gadelhaq, Shawky M. Aboelhadid, Hesham A. Mahran, Saleh Al-Quraishy, Abdulrahman Reyad, and Asmaa A. Kamel. 2024. "Toxicity of Common Acaricides, Disinfectants, and Natural Compounds against Eggs of Rhipicephalus annulatus" Pathogens 13, no. 10: 824. https://doi.org/10.3390/pathogens13100824







