Aerotolerancy of Campylobacter spp.: A Comprehensive Review
Abstract
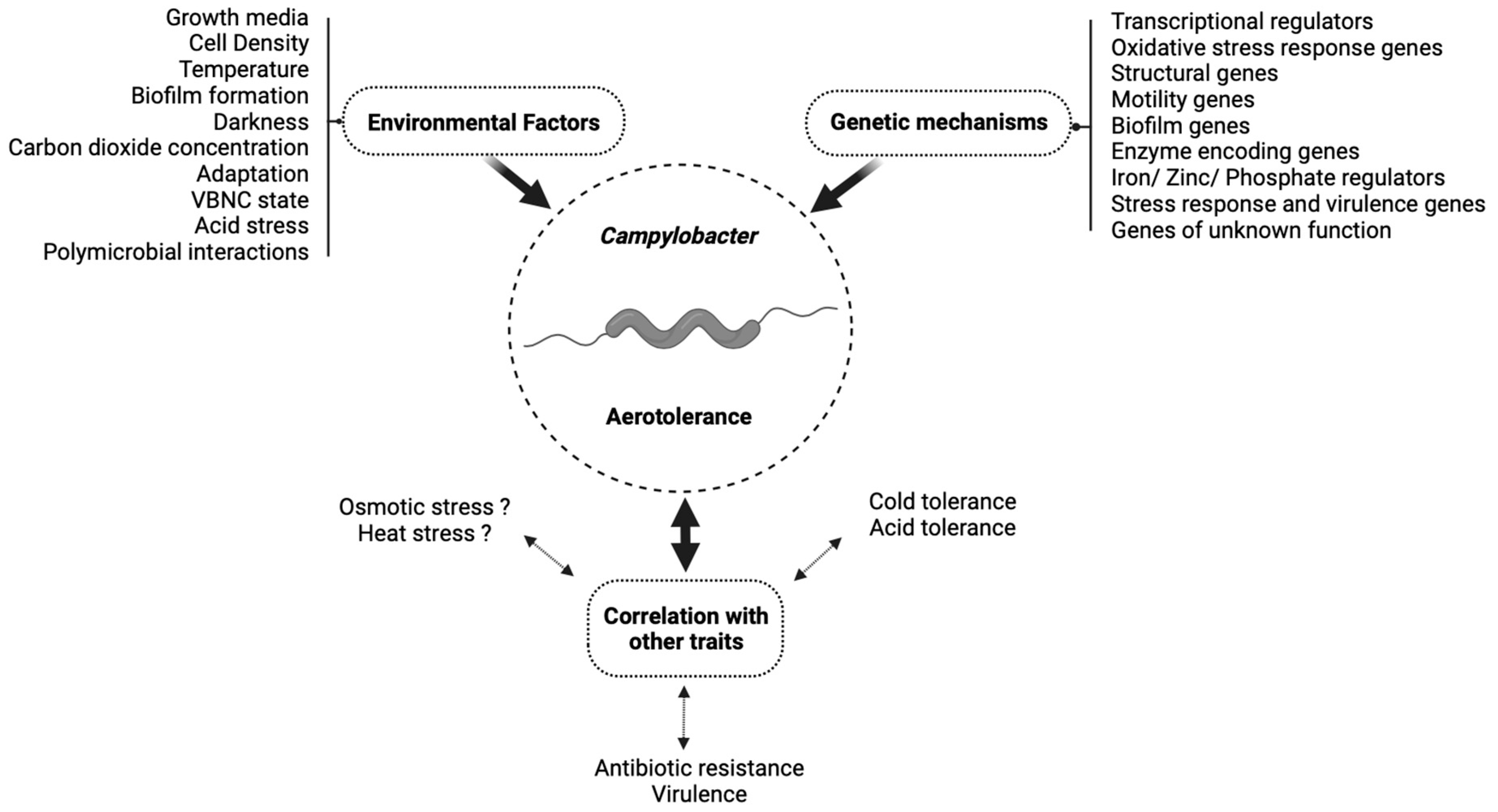
:1. Introduction
2. Prevalence of Aerotolerant Campylobacter Strains in Meat Production
3. Effects of Environmental Conditions on Aerotolerance
3.1. Growth Media and Supplementation
3.2. Effects of Polymicrobial Interactions and Biofilms
3.3. Influence of Other Environmental Factors on Aerotolerance
3.4. Influence of Intrinsic Factors on Aerotolerance
4. Correlations between Aerotolerance and Virulence or Survival Ability
5. Mechanisms of Aerotolerance
5.1. Role of Oxidative Stress Genes katA, sodB, and ahpC on Aerotolerance
5.2. Role of Temperature on Aerotolerance
5.3. Role of perR, mutY, and cosR on Aerotolerance
5.4. Role of Biofilms and Polysaccharides on Aerotolerance
5.5. Role of Genes Involved in Iron Metabolism and Other Metals
5.6. Role of Other Genes on Campylobacter Aerotolerance
5.7. Proteins Known to Be Involved in Campylobacter Aerotolerance
5.8. Genes and Proteins Involved in Aerotolerance That Require Further Characterization
6. Concluding Remarks
7. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Skirrow, M.B. Campylobacter Enteritis: A “New” Disease. Br. Med. J. 1977, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- WHO Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 8 August 2023).
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-Specific Burdens of Community Diarrhoea in Developing Countries: A Multisite Birth Cohort Study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef] [PubMed]
- Allos, B.M. Campylobacter jejuni Infections: Update on Emerging Issues and Trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Blaser, M.J. Fatalities Associated with Campylobacter jejuni Infections. JAMA 1985, 253, 2873–2875. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, A.; Muñoz, P.; Mohedano, R.; Valerio, M.; Marín, M.; Alcalá, L.; Rodriguez-Créixems, M.; Cercenado, E.; Bouza, E. Campylobacter Bacteremia: Clinical Characteristics, Incidence, and Outcome over 23 Years. Medicine 2010, 89, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Keithlin, J.; Sargeant, J.; Thomas, M.K.; Fazil, A. Systematic Review and Meta-Analysis of the Proportion of Campylobacter Cases That Develop Chronic Sequelae. BMC Public Health 2014, 14, 1203. [Google Scholar] [CrossRef]
- CDC Information for Health Professionals|Campylobacter|CDC. Available online: https://www.cdc.gov/campylobacter/technical.html (accessed on 8 August 2023).
- Skirrow, M.B. Epidemiology of Campylobacter Enteritis. Int. J. Food Microbiol. 1991, 12, 9–16. [Google Scholar] [CrossRef]
- Moore, J.E.; Corcoran, D.; Dooley, J.S.G.; Fanning, S.; Lucey, B.; Matsuda, M.; McDowell, D.A.; Mégraud, F.; Millar, B.C.; O’Mahony, R.; et al. Campylobacter. Vet. Res. 2005, 36, 351–382. [Google Scholar] [CrossRef]
- Solow, B.T.; Cloak, O.M.; Fratamico, P.M. Effect of Temperature on Viability of Campylobacter jejuni and Campylobacter coli on Raw Chicken or Pork Skin. J. Food Prot. 2003, 66, 2023–2031. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Miller, W.G.; De Reuse, H.; Mendz, G.L. Oxygen Requirement and Tolerance of Campylobacter jejuni. Res. Microbiol. 2007, 158, 644–650. [Google Scholar] [CrossRef]
- Keener, K.M.; Bashor, M.P.; Curtis, P.A.; Sheldon, B.W.; Kathariou, S. Comprehensive Review of Campylobacter and Poultry Processing. Compr. Rev. Food Sci. Food Saf. 2004, 3, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Kiatsomphob, S.; Taniguchi, T.; Tarigan, E.; Latt, K.M.; Jeon, B.; Misawa, N. Aerotolerance and Multilocus Sequence Typing among Campylobacter jejuni Strains Isolated from Humans, Broiler Chickens, and Cattle in Miyazaki Prefecture, Japan. J. Vet. Med. Sci. 2019, 81, 19–0228. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Kim, J.; Kim, J.H.; Jung, J.I.; Cho, S.; Ryu, S.; Jeon, B. Comparative Analysis of Aerotolerance, Antibiotic Resistance, and Virulence Gene Prevalence in Campylobacter jejuni Isolates from Retail Raw Chicken and Duck Meat in South Korea. Microorganisms 2019, 7, 433. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Chui, L.; Bae, J.; Li, V.; Ma, A.; Mutschall, S.K.; Taboada, E.N.; McMullen, L.M.; Jeon, B. Frequent Implication of Multistress-Tolerant Campylobacter jejuni in Human Infections. Emerg. Infect. Dis. 2018, 24, 1037–1044. [Google Scholar] [CrossRef]
- Oh, E.; McMullen, L.; Jeon, B. High Prevalence of Hyper-Aerotolerant Campylobacter jejuni in Retail Poultry with Potential Implication in Human Infection. Front. Microbiol. 2015, 6, 1263. [Google Scholar] [CrossRef]
- Pokhrel, D.; Thames, H.T.; Zhang, L.; Dinh, T.; Schilling, M.W.; White, S.; Ramachandran, R.; Sukumaran, A.T. Aerotolerance and Multi-Locus Sequence Typing of Campylobacter jejuni Isolated from Commercial Broiler Processing Plants. Foods 2023, 12, 3305. [Google Scholar] [CrossRef]
- Mouftah, S.F.; Cobo-Díaz, J.F.; Álvarez-Ordóñez, A.; Mousa, A.; Calland, J.K.; Pascoe, B.; Sheppard, S.K.; Elhadidy, M. Stress Resistance Associated with Multi-Host Transmission and Enhanced Biofilm Formation at 42 °C among Hyper-Aerotolerant Generalist Campylobacter jejuni. Food Microbiol. 2021, 95, 103706. [Google Scholar] [CrossRef]
- Guk, J.H.; Kim, J.; Song, H.; Kim, J.; An, J.U.; Kim, J.; Ryu, S.; Jeon, B.; Cho, S. Hyper-Aerotolerant Campylobacter coli from Duck Sources and Its Potential Threat to Public Health: Virulence, Antimicrobial Resistance, and Genetic Relatedness. Microorganisms 2019, 7, 579. [Google Scholar] [CrossRef]
- Karki, A.B.; Marasini, D.; Oakey, C.K.; Mar, K.; Fakhr, M.K. Campylobacter coli from Retail Liver and Meat Products Is More Aerotolerant than Campylobacter jejuni. Front. Microbiol. 2018, 9, 2951. [Google Scholar] [CrossRef]
- Jarusirivait, N.; Luangtongkum, T. Study of Aerotolerant Campylobacter jejuni in Broiler Production Process. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 2021. [Google Scholar]
- Guk, J.H.; Song, H.; Yi, S.; An, J.U.; Lee, S.; Kim, W.H.; Cho, S. Hyper-Aerotolerant Campylobacter coli from Swine May Pose a Potential Threat to Public Health Based on Its Quinolone Resistance, Virulence Potential, and Genetic Relatedness. Front. Microbiol. 2021, 12, 703993. [Google Scholar] [CrossRef]
- Song, H.; Kim, J.; Guk, J.H.; An, J.U.; Lee, S.; Cho, S. Complete Genome Sequence and Comparative Genomic Analysis of Hyper-Aerotolerant Campylobacter Lari Strain SCHS02 Isolated from Duck for Its Potential Pathogenicity. Microb. Pathog. 2020, 142, 104110. [Google Scholar] [CrossRef] [PubMed]
- Bowdre, J.H.; Krieg, N.R.; Hoffman, P.S.; Smibert, F.M. Stimulatory Effect of Dihydroxyphenyl Compounds on the Aerotolerance of Spirillum Volutans and Campylobacter fetus Subspecies Jejuni. Appl. Environ. Microbiol. 1976, 31, 127–133. [Google Scholar] [CrossRef] [PubMed]
- George, H.A.; Hoffman, P.S.; Smibert, T.M.; Krieg, N.R. Improved Media for Growth and Aerotolerance of Campylobacter fetus. J. Clin. Microbiol. 1978, 8, 36–41. [Google Scholar] [CrossRef]
- Hoffman, P.S.; George, H.A.; Krieg, N.R.; Smibert, R.M. Studies of the Microaerophilic Nature of Campylobacter fetus Subsp. jejuni. II. Role of Exogenous Superoxide Anions and Hydrogen Peroxide. Can. J. Microbiol. 1979, 25, 8–16. [Google Scholar] [CrossRef]
- Koidis, P.; Doyle, M.P. Survival Of Campylobacter jejuni in the Presence of Bisulfite and Different Atmospheres. Eur. J. Clin. Microbiol. 1983, 2, 384–388. [Google Scholar] [CrossRef]
- Juven, B.J.; Rosenthal, I. Effect of Free-Radical and Oxygen Scavengers on Photochemically Generated Oxygen Toxicity and on the Aerotolerance of Campylobacter jejuni. J. Appl. Bacteriol. 1985, 59, 413–419. [Google Scholar] [CrossRef]
- Moran, A.P.; Upton, M.E. Factors Affecting Production of Coccoid Forms by Campylobacter jejuni on Solid Media during Incubation. J. Appl. Bacteriol. 1987, 62, 527–537. [Google Scholar] [CrossRef]
- Lee, M.H.T.; Smibert, R.M.; Krieg, N.R. Effect of Incubation Temperature, Ageing, and Bisulfite Content of Unsupplemented Brucella Agar on Aerotolerance of Campylobacter jejuni. Can. J. Microbiol. 1988, 34, 1069–1074. [Google Scholar] [CrossRef]
- Hodge, J.P.; Krieg, N.R. Oxygen Tolerance Estimates in Campylobacter Species Depend on the Testing Medium. J. Appl. Bacteriol. 1994, 77, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.P.E.; Lyte, M.; Neal, C.P.; Maggs, A.F.; Haigh, R.D.; Williams, P.H. The Mammalian Neuroendocrine Hormone Norepinephrine Supplies Iron for Bacterial Growth in the Presence of Transferrin or Lactoferrin. J. Bacteriol. 2000, 182, 6091–6098. [Google Scholar] [CrossRef]
- Tangwatcharin, P.; Chanthachum, S.; Khopaibool, P.; Chambers, J.R.; Griffiths, M.W. Media for the Aerobic Resuscitation of Campylobacter jejuni. J. Food Prot. 2007, 70, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Peterz, M. Comparison of Preston Agar and a Blood-Free Selective Medium for Detection of Campylobacter jejuni in Food. J. Assoc. Off. Anal. Chem. 1991, 74, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Bolton, F.J.; Coates, D. Development of a Blood-Free Campylobacter Medium: Screening Tests on Basal Media and Supplements, and the Ability of Selected Supplements to Facilitate Aerotolerance. J. Appl. Bacteriol. 1983, 54, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Oyarzabal, O.A.; Macklin, K.S.; Barbaree, J.M.; Miller, R.S. Evaluation of Agar Plates for Direct Enumeration of Campylobacter spp. from Poultry Carcass Rinses. Appl. Environ. Microbiol. 2005, 71, 3351–3354. [Google Scholar] [CrossRef]
- Chou, S.P.; Dular, R.; Kasatiya, S. Effect of Ferrous Sulfate, Sodium Metabisulfite, and Sodium Pyruvate on Survival of Campylobacter jejuni. J. Clin. Microbiol. 1983, 18, 986–987. [Google Scholar] [CrossRef]
- Karki, A.B.; Wells, H.; Fakhr, M.K. Retail Liver Juices Enhance the Survivability of Campylobacter jejuni and Campylobacter coli at Low Temperatures. Sci. Rep. 2019, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Hinton, A. Growth of Campylobacter Incubated Aerobically in Fumarate-Pyruvate Media or Media Supplemented with Dairy, Meat, or Soy Extracts and Peptones. Food Microbiol. 2016, 58, 23–28. [Google Scholar] [CrossRef]
- Oh, E.; Andrews, K.J.; Jeon, B. Enhanced Biofilm Formation by Ferrous and Ferric Iron Through Oxidative Stress in Campylobacter jejuni. Front. Microbiol. 2018, 9, 1204. [Google Scholar] [CrossRef]
- Turonova, H.; Briandet, R.; Rodrigues, R.; Hernould, M.; Hayek, N.; Stintzi, A.; Pazlarova, J.; Tresse, O. Biofilm Spatial Organization by the Emerging Pathogen Campylobacter jejuni: Comparison between NCTC 11168 and 81-176 Strains under Microaerobic and Oxygen-Enriched Conditions. Front Microbiol 2015, 6, 146600. [Google Scholar] [CrossRef]
- Asakura, H.; Yamasaki, M.; Yamamoto, S.; Igimi, S. Deletion of Peb4 Gene Impairs Cell Adhesion and Biofilm Formation in Campylobacter jejuni. FEMS Microbiol. Lett. 2007, 275, 278–285. [Google Scholar] [CrossRef]
- Joshua, G.W.P.; Guthrie-Irons, C.; Karlyshev, A.V.; Wren, B.W. Biofilm Formation in Campylobacter jejuni. Microbiology (N Y) 2006, 152, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Mallett, A.; Pearson, B.M.; Van Vliet, A.H.M. Biofilm Formation by Campylobacter jejuni Is Increased under Aerobic Conditions. Appl. Environ. Microbiol. 2010, 76, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.; Mulholland, F.; Pearson, B.M.; Pin, C.; McNicholl-Kennedy, J.; Ketley, J.M.; Wells, J.M. Campylobacter jejuni Gene Expression in Response to Iron Limitation and the Role of Fur. Microbiology 2005, 151, 243–257. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.; Ryu, S.; Jeon, B. Regulation of PerR Expression by Iron and PerR in Campylobacter jejuni. J. Bacteriol. 2011, 193, 6171–6178. [Google Scholar] [CrossRef]
- Palyada, K.; Threadgill, D.; Stintzi, A. Iron Acquisition and Regulation in Campylobacter jejuni. J. Bacteriol. 2004, 186, 4714. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase. An Enzymic Function for Erythrocuprein (Hemocuprein). Available online: https://www.researchgate.net/publication/17780338_McCOrd_J_M_and_Fridovich_I_Superoxide_dismutase_An_enzymic_function_for_erythrocuprein_hemocuprein_J_Biol_Chem (accessed on 9 July 2023).
- Wonglumsom, W.; Vishnubhatla, A.; Kim, J.M.; Fung, D.Y.C. Enrichment Media for Isolation of Campylobacter jejuni from Inoculated Ground Beef and Chicken Skin under Normal Atmosphere. J. Food Prot. 2001, 64, 630–634. [Google Scholar] [CrossRef]
- Adler, H.I.; Crow, W.D. Novel Approach to the Growth of Anaerobic Microorganisms. Biotechnol. Bioeng. Symp. (U. S.) Oak Ridge Natl. Lab. TN 1981, 11. Available online: https://www.osti.gov/biblio/5297983 (accessed on 12 May 2024).
- Verhoeff-Bakkenes, L.; Arends, A.P.; Snoep, J.L.; Zwietering, M.H.; De Jonge, R. Pyruvate Relieves the Necessity of High Induction Levels of Catalase and Enables Campylobacter jejuni to Grow under Fully Aerobic Conditions. Lett. Appl. Microbiol. 2008, 46, 377–382. [Google Scholar] [CrossRef]
- Hinton, A. Aerobic Growth of Campylobacter in Media Supplemented with C3-Monocarboxylates and C4-Dicarboxylates. J. Food Prot. 2013, 76, 685–690. [Google Scholar] [CrossRef]
- Lee, Y.-D.; Choi, J.-P.; Noh, B.-S.; Moon, B.-Y.; Chang, H.-G.; Park, J.-H. Isolation, Identification, and Characterization of Aero-Adaptive Campylobacter jejuni. J. Microbiol. Biotechnol. 2005, 15, 992–1000. Available online: https://koreascience.kr/article/JAKO200507521913540.page (accessed on 12 May 2024).
- Zhong, X.; Wu, Q.; Zhang, J.; Ma, Z.; Wang, J.; Nie, X.; Ding, Y.; Xue, L.; Chen, M.; Wu, S.; et al. Campylobacter jejuni Biofilm Formation Under Aerobic Conditions and Inhibition by ZnO Nanoparticles. Front. Microbiol. 2020, 11, 499084. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.P.; Upton, M.E. Effect of Medium Supplements, Illumination and Superoxide Dismutase on the Production of Coccoid Forms of Campylobacter jejuni ATCC 29428. J. Appl. Bacteriol. 1987, 62, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.; Leach, S. Analysis of Coccal Cell Formation by Campylobacter jejuni Using Continuous Culture Techniques, and the Importance of Oxidative Stress. J. Appl. Microbiol. 1998, 85, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, F.; Scherwitzel, M.; Paulsen, P.; Szostak, M.P. Survival of Campylobacter jejuni under Conditions of Atmospheric Oxygen Tension with the Support of Pseudomonas spp. Appl. Environ. Microbiol. 2010, 76, 5911–5917. [Google Scholar] [CrossRef] [PubMed]
- Anis, N.; Bonifait, L.; Quesne, S.; Baugé, L.; Yassine, W.; Guyard-Nicodème, M.; Chemaly, M. Survival of Campylobacter jejuni Co-Cultured with Salmonella spp. in Aerobic Conditions. Pathogens 2022, 11, 812. [Google Scholar] [CrossRef]
- Karki, A.B.; Ballard, K.; Harper, C.; Sheaff, R.J.; Fakhr, M.K. Staphylococcus aureus Enhances Biofilm Formation, Aerotolerance, and Survival of Campylobacter Strains Isolated from Retail Meats. Sci. Rep. 2021, 11, 13837. [Google Scholar] [CrossRef]
- Šimunović, K.; Stefanic, P.; Klančnik, A.; Erega, A.; Mandic Mulec, I.; Smole Možina, S. Bacillus subtilis PS-216 Antagonistic Activities against Campylobacter jejuni NCTC 11168 Are Modulated by Temperature, Oxygen, and Growth Medium. Microorganisms 2022, 10, 289. [Google Scholar] [CrossRef]
- Wongjaroen, P.; Kittiniyom, K.; Srimanote, P.; Tiyasuttipan, W.; Wonglumsom, W. Evaluation of Membrane Fragments Extracted from Escherichia Coli and Pseudomonas Aeruginosa on Campylobacter jejuni Growth under Normal Atmosphere. Agric. Nat. Resour. 2008, 42, 213–218. [Google Scholar]
- Axelsson-Olsson, D.; Ellström, P.; Waldenström, J.; Haemig, P.D.; Brudin, L.; Olsen, B. Acanthamoeba-Campylobacter Coculture as a Novel Method for Enrichment of Campylobacter Species. Appl. Environ. Microbiol. 2007, 73, 6864–6869. [Google Scholar] [CrossRef] [PubMed]
- Bui, X.T.; Winding, A.; Qvortrup, K.; Wolff, A.; Bang, D.D.; Creuzenet, C. Survival of Campylobacter jejuni in Co-Culture with Acanthamoeba Castellanii: Role of Amoeba-Mediated Depletion of Dissolved Oxygen. Environ. Microbiol. 2012, 14, 2034–2047. [Google Scholar] [CrossRef]
- Siringan, P.; Connerton, P.L.; Cummings, N.J.; Connerton, I.F. Alternative Bacteriophage Life Cycles: The Carrier State of Campylobacter jejuni. Open Biol. 2013, 4, 130200. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; Butucel, E.; Stef, L.; Pet, I.; Gradisteanu-Pircalabioru, G.; Chifiriuc, C.; Gundogdu, O.; Mccleery, D.; Corcionivoschi, N. Anti- Campylobacter Probiotics: Latest Mechanistic Insights. Foodborne Pathog. Dis. 2022, 19, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Kim, J.C.; Jeon, B. Stimulation of Biofilm Formation by Oxidative Stress in Campylobacter jejuni under Aerobic Conditions. Virulence 2016, 7, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, M.H.G. Effect of Biofilm Formation in a Hostile Oxidative Stress Environment on the Survival of Campylobacter jejuni Recovered from Poultry in Iraqi Markets. Vet. World 2024, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Efimochkina, N.R.; Stetsenko, V.V.; Sheveleva, S.A. Peculiarities of Biofilms Formation by Campylobacter Bacteria in Mixed Populations with Other Microbial Contaminants of Food Products. Bull. Exp. Biol. Med. 2019, 168, 62–65. [Google Scholar] [CrossRef]
- Sulaeman, S.; Hernould, M.; Schaumann, A.; Coquet, L.; Bolla, J.M.; Dé, E.; Tresse, O. Enhanced Adhesion of Campylobacter jejuni to Abiotic Surfaces Is Mediated by Membrane Proteins in Oxygen-Enriched Conditions. PLoS ONE 2012, 7, e46402. [Google Scholar] [CrossRef]
- Kassem, I.I.; Candelero-Rueda, R.A.; Esseili, K.A.; Rajashekara, G. Formate Simultaneously Reduces Oxidase Activity and Enhances Respiration in Campylobacter jejuni. Sci. Rep. 2017, 7, 40117. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lamour, G.; Xue, R.; Mirvakliki, M.N.; Hatzikiriakos, S.G.; Xu, J.; Li, H.; Wang, S.; Lu, X. Chemical, Physical and Morphological Properties of Bacterial Biofilms Affect Survival of Encased Campylobacter jejuni F38011 under Aerobic Stress. Int. J. Food Microbiol. 2016, 238, 172–182. [Google Scholar] [CrossRef]
- Sanders, S.Q.; Boothe, D.H.; Frank, J.F.; Arnold, J.W. Culture and Detection of Campylobacter jejuni within Mixed Microbial Populations of Biofilms on Stainless Steel. J. Food Prot. 2007, 70, 1379–1385. [Google Scholar] [CrossRef]
- Teh, K.H.; Flint, S.; French, N. Biofilm Formation by Campylobacter jejuni in Controlled Mixed-Microbial Populations. Int. J. Food Microbiol. 2010, 143, 118–124. [Google Scholar] [CrossRef]
- Ica, T.; Caner, V.; Istanbullu, O.; Nguyen, H.D.; Ahmed, B.; Call, D.R.; Beyenal, H. Characterization of Mono- and Mixed-Culture Campylobacter jejuni Biofilms. Appl. Environ. Microbiol. 2012, 78, 1033. [Google Scholar] [CrossRef] [PubMed]
- Scheik, L.K.; Volcan Maia, D.S.; Wurfel, S.D.F.R.; Ramires, T.; Kleinubing, N.R.; Haubert, L.; Lopes, G.V.; Da Silva, W.P. Biofilm-Forming Ability of Poultry Campylobacter jejuni Strains in the Presence and Absence of Pseudomonas Aeruginosa. Can. J. Microbiol. 2021, 67, 301–309. [Google Scholar] [CrossRef]
- Garénaux, A.; Jugiau, F.; Rama, F.; De Jonge, R.; Denis, M.; Federighi, M.; Ritz, M. Survival of Campylobacter jejuni Strains from Different Origins under Oxidative Stress Conditions: Effect of Temperature. Curr. Microbiol. 2008, 56, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; McMullen, L.M.; Chui, L.; Jeon, B. Differential Survival of Hyper-Aerotolerant Campylobacter jejuni under Different Gas Conditions. Front. Microbiol. 2017, 8, 954. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Zorman, T.; Smole Možina, S. Effects of Low Temperature, Starvation and Oxidative Stress on the Physiology of Campylobacter jejuni Cells. Croat. Chem. Acta 2008, 81, 41–46. [Google Scholar]
- Askoura, M.; Sarvan, S.; Couture, J.F.; Stintzi, A. The Campylobacter jejuni Ferric Uptake Regulator Promotes Acid Survival and Cross-Protection against Oxidative Stress. Infect. Immun. 2016, 84, 1287. [Google Scholar] [CrossRef]
- Jones, D.M.; Sutcliffe, E.M.; Rios, R.; Fox, A.J.; Curry, A. Campylobacter jejuni Adapts to Aerobic Metabolism in the Environment. J. Med. Microbiol. 1993, 38, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Chynoweth, R.W.; Hudson, J.A.; Thom, K. Aerobic Growth and Survival of Campylobacter jejuni in Food and Stream Water. Lett. Appl. Microbiol. 1998, 27, 341–344. [Google Scholar] [CrossRef]
- Shagieva, E.; Demnerova, K.; Michova, H. Waterborne Isolates of Campylobacter jejuni Are Able to Develop Aerotolerance, Survive Exposure to Low Temperature, and Interact with Acanthamoeba Polyphaga. Front. Microbiol. 2021, 12, 3162. [Google Scholar] [CrossRef] [PubMed]
- Nennig, M.; Clément, A.; Longueval, E.; Bernardi, T.; Ragimbeau, C.; Tresse, O. Metaphenotypes Associated with Recurrent Genomic Lineages of Campylobacter jejuni Responsible for Human Infections in Luxembourg. Front. Microbiol. 2022, 13, 901192. [Google Scholar] [CrossRef]
- O’Kane, P.M.; Connerton, I.F. Characterisation of Aerotolerant Forms of a Robust Chicken Colonizing Campylobacter coli. Front. Microbiol. 2017, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.; Kim, S.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Kim, Y.; Lee, Y.; Yoon, Y. The Risk of Aerotolerant Campylobacter jejuni Strains in Poultry Meat Distribution and Storage. Microb. Pathog. 2019, 134, 103537. [Google Scholar] [CrossRef] [PubMed]
- Carney, G.; Weimer, B.C.; Clyne, M.; Cróinín, T. Different Stages of the Infection Cycle Are Enriched for Campylobacter Strains with Distinct Phenotypes and Levels of Fluoroquinolone Resistance. Microbiology 2023, 169, 001349. [Google Scholar] [CrossRef]
- Jeon, B.; Wang, Y.; Hao, H.; Barton, Y.W.; Zhang, Q. Contribution of CmeG to Antibiotic and Oxidative Stress Resistance in Campylobacter jejuni. J. Antimicrob. Chemother. 2011, 66, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.I.; Kim, J.; Kang, M.S.; Kim, H.J.; Ryu, S.; Jeon, B. Cold Tolerance in Campylobacter jejuni and Its Impact on Food Safety. Food Res. Int. 2024, 175, 113683. [Google Scholar] [CrossRef]
- Oh, E.; Andrews, K.J.; McMullen, L.M.; Jeon, B. Tolerance to Stress Conditions Associated with Food Safety in Campylobacter jejuni Strains Isolated from Retail Raw Chicken. Sci. Rep. 2019, 9, 11915. [Google Scholar] [CrossRef] [PubMed]
- Jaakkonen, A.; Kivistö, R.; Aarnio, M.; Kalekivi, J.; Hakkinen, M. Persistent Contamination of Raw Milk by Campylobacter jejuni ST-883. PLoS ONE 2020, 15, e0231810. [Google Scholar] [CrossRef]
- Stead, D.; Park, S.F. Roles of Fe Superoxide Dismutase and Catalase in Resistance of Campylobacter coli to Freeze-Thaw Stress. Appl. Environ. Microbiol. 2000, 66, 3110. [Google Scholar] [CrossRef]
- Stintzi, A.; Whitworth, L. Investigation of the Campylobacter jejuni Cold-Shock Response by Global Transcript Profiling. Genome Lett. 2003, 2, 18–27. [Google Scholar]
- Garénaux, A.; Ritz, M.; Jugiau, F.; Rama, F.; Federighi, M.; De Jonge, R. Role of Oxidative Stress in C. jejuni Inactivation during Freeze-Thaw Treatment. Curr. Microbiol. 2009, 58, 134–138. [Google Scholar] [CrossRef]
- Grant, K.A.; Park, S.F. Molecular Characterization of KatA from Campylobacter jejuni and Generation of a Catalase-Deficient Mutant of Campylobacter coli by Interspecific Allelic Exchange. Microbiology 1995, 141 Pt 6, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; McMullen, L.; Jeon, B. Impact of Oxidative Stress Defense on Bacterial Survival and Morphological Change in Campylobacter jejuni under Aerobic Conditions. Front. Microbiol. 2015, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Vercellone, P.A.; Smibert, R.M.; Krieg, N.R. Catalase Activity in Campylobacter jejuni: Comparison of a Wild-Type Strain with an Aerotolerant Variant. Can. J. Microbiol. 1990, 36, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Haddad, N.; Chevret, D.; Cappelier, J.M.; Tresse, O. Comparison of Proteomics Profiles of Campylobacter jejuni Strain Bf under Microaerobic and Aerobic Conditions. Front. Microbiol. 2016, 7, 1596. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide Radical: An Endogenous Toxicant. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 239–257. [Google Scholar] [CrossRef]
- Purdy, D.; Park, S.F. Cloning, Nucleotide Sequence and Characterization of a Gene Encoding Superoxide Dismutase from Campylobacter jejuni and Campylobacter coli. Microbiology 1994, 140 Pt 5, 1203–1208. [Google Scholar] [CrossRef]
- Kikuchi, H.E.; Suzuki, T. An Electrophoretic Analysis of Superoxide Dismutase in Campylobacter spp. J. Gen. Microbiol. 1984, 130, 2791–2796. [Google Scholar] [CrossRef]
- Purdy, D.; Cawthraw, S.; Dickinson, J.H.; Newell, D.G.; Park, S.F. Generation of a Superoxide Dismutase (SOD)-Deficient Mutant of Campylobacter coli: Evidence for the Significance of SOD in Campylobacter Survival and Colonization. Appl. Environ. Microbiol. 1999, 65, 2540. [Google Scholar] [CrossRef]
- Garénaux, A.; Guillou, S.; Ermel, G.; Wren, B.; Federighi, M.; Ritz, M. Role of the Cj1371 Periplasmic Protein and the Cj0355c Two-Component Regulator in the Campylobacter jejuni NCTC 11168 Response to Oxidative Stress Caused by Paraquat. Res. Microbiol. 2008, 159, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, F.S.; Morgan, R.W.; Christman, M.F.; Ames, B.N. An Alkyl Hydroperoxide Reductase from Salmonella Typhimurium Involved in the Defense of DNA against Oxidative Damage. J. Biol. Chem. 1989, 264, 1488–1496. [Google Scholar] [CrossRef]
- Baillon, M.L.A.; Van Vliet, A.H.M.; Ketley, J.M.; Constantinidou, C.; Penn, C.W. An Iron-Regulated Alkyl Hydroperoxide Reductase (AhpC) Confers Aerotolerance and Oxidative Stress Resistance to the Microaerophilic Pathogen Campylobacter jejuni. J. Bacteriol. 1999, 181, 4798–4804. [Google Scholar] [CrossRef] [PubMed]
- Stoakes, E.; Chen, X.; Kalmar, L.; Baker, D.; Evans, R.; Rudder, S.; Grant, A.J. Identification of Campylobacter jejuni and Campylobacter coli Genes Contributing to Oxidative Stress Response Using TraDIS Analysis. BMC Microbiol. 2024, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Saisom, T.; Sasipreeyajan, J.; Luangtongkum, T. Live-Attenuated Oral Vaccines to Reduce Campylobacter Colonization in Poultry. Vaccines 2022, 10, 685. [Google Scholar] [CrossRef]
- Van Vliet, A.H.M.; Baillon, M.L.A.; Penn, C.W.; Ketley, J.M. Campylobacter jejuni Contains Two Fur Homologs: Characterization of Iron-Responsive Regulation of Peroxide Stress Defense Genes by the PerR Repressor. J. Bacteriol. 1999, 181, 6371–6376. [Google Scholar] [CrossRef]
- Handley, R.A.; Mulholland, F.; Reuter, M.; Ramachandran, V.K.; Musk, H.; Clissold, L.; Le Brun, N.E.; Van Vliet, A.H.M. PerR Controls Oxidative Stress Defence and Aerotolerance but Not Motility-Associated Phenotypes of Campylobacter jejuni. Microbiology 2015, 161, 1524–1536. [Google Scholar] [CrossRef]
- Atack, J.M.; Harvey, P.; Jones, M.A.; Kelly, D.J. The Campylobacter jejuni Thiol Peroxidases Tpx and Bcp Both Contribute to Aerotolerance and Peroxide-Mediated Stress Resistance but Have Distinct Substrate Specificities. J. Bacteriol. 2008, 190, 5279–5290. [Google Scholar] [CrossRef]
- Flint, A.; Sun, Y.Q.; Butcher, J.; Stahl, M.; Huang, H.; Stintzi, A. Phenotypic Screening of a Targeted Mutant Library Reveals Campylobacter jejuni Defenses against Oxidative Stress. Infect. Immun. 2014, 82, 2266. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Igimi, S.; Katayama, Y.; Yamamoto, S.; Amano, F. Identification of an Oxidative Stress-Sensitive Protein from Campylobacter jejuni, Homologous to Rubredoxin Oxidoreductase/Rubrerythrin. FEMS Microbiol. Lett. 2004, 235, 57–63. [Google Scholar] [CrossRef]
- Kendall, J.J.; Barrero-Tobon, A.M.; Hendrixson, D.R.; Kelly, D.J. Hemerythrins in the Microaerophilic Bacterium Campylobacter jejuni Help Protect Key Iron-Sulphur Cluster Enzymes from Oxidative Damage. Environ. Microbiol. 2014, 16, 1105–1121. [Google Scholar] [CrossRef]
- van Vliet, A.H.M.; Ketley, J.M.; Park, S.F.; Penn, C.W. The Role of Iron in Campylobacter Gene Regulation, Metabolism and Oxidative Stress Defense. FEMS Microbiol. Rev. 2002, 26, 173–186. [Google Scholar] [CrossRef]
- van Vliet, A.H.M.; Baillon, M.-L.A.; Penn, C.W.; Ketley, J.M. The Iron-Induced Ferredoxin FdxA of Campylobacter jejuni Is Involved in Aerotolerance. FEMS Microbiol. Lett. 2001, 196, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Atack, J.M.; Kelly, D.J. Contribution of the Stereospecific Methionine Sulphoxide Reductases MsrA and MsrB to Oxidative and Nitrosative Stress Resistance in the Food-Borne Pathogen Campylobacter jejuni. Microbiology 2008, 154, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Mizunoe, Y.; Kawabata, S.I.; Takade, A.; Harada, M.; Wai, S.N.; Yoshida, S.I. The Iron-Binding Protein Dps Confers Hydrogen Peroxide Stress Resistance to Campylobacter jejuni. J. Bacteriol. 2003, 185, 1010–1017. [Google Scholar] [CrossRef]
- Wai, S.N.; Nakayama, K.; Umene, K.; Moriya, T.; Amako, K. Construction of a Ferritin-Deficient Mutant of Campylobacter jejuni: Contribution of Ferritin to Iron Storage and Protection against Oxidative Stress. Mol. Microbiol. 1996, 20, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Brøndsted, L.; Andersen, M.T.; Parker, M.; Jørgensen, K.; Ingmer, H. The HtrA Protease of Campylobacter jejuni Is Required for Heat and Oxygen Tolerance and for Optimal Interaction with Human Epithelial Cells. Appl. Environ. Microbiol. 2005, 71, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Lind, J.; Backert, S.; Tegtmeyer, N. Campylobacter jejuni Serine Protease HtrA Plays an Important Role in Heat Tolerance, Oxygen Resistance, Host Cell Adhesion, Invasion, and Transmigration. Eur. J. Microbiol. Immunol. 2015, 5, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.T.; Brøndsted, L.; Pearson, B.M.; Mulholland, F.; Parker, M.; Pin, C.; Wells, J.M.; Ingmer, H. Diverse Roles of HspR in Campylobacter jejuni Revealed by the Proteome, Transcriptome and Phenotypic Characterization of an HspR Mutant. Microbiology 2005, 151, 905–915. [Google Scholar] [CrossRef]
- Takata, T.; Ono, J.; Amako, K.; Nvunt Wai, S.; Takade, A.; Sawae, Y. The Purification of a GroEL-like Stress Protein from Aerobically Adapted Campylobacter jejuni. Microbiol. Immunol. 1995, 39, 639–645. [Google Scholar] [CrossRef]
- Fields, J.A.; Thompson, S.A. Campylobacter jejuni CsrA Mediates Oxidative Stress Responses, Biofilm Formation, and Host Cell Invasion. J. Bacteriol. 2008, 190, 3411. [Google Scholar] [CrossRef]
- Palyada, K.; Sun, Y.Q.; Flint, A.; Butcher, J.; Naikare, H.; Stintzi, A. Characterization of the Oxidative Stress Stimulon and PerR Regulon of Campylobacter jejuni. BMC Genom. 2009, 10, 481. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, M.; Ryu, S.; Jeon, B. Regulation of Oxidative Stress Response by CosR, an Essential Response Regulator in Campylobacter jejuni. PLoS ONE 2011, 6, e22300. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, O.; da Silva, D.T.; Mohammad, B.; Elmi, A.; Mills, D.C.; Wren, B.W.; Dorrell, N. The Campylobacter jejuni MarR-like Transcriptional Regulators RrpA and RrpB Both Influence Bacterial Responses to Oxidative and Aerobic Stresses. Front. Microbiol. 2015, 6, 145604. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.; Ahn, E.; Mao, Q.; Chen, C.; Ryu, S.; Jeon, B. Stimulation of Surface Polysaccharide Production under Aerobic Conditions Confers Aerotolerance in Campylobacter jejuni. Microbiol. Spectr. 2023, 11, e03761-22. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.; Hong, G.; Davies, C.; Elmi, A.; Sima, F.; Stratakos, A.; Stef, L.; Pet, I.; Hachani, A.; Corcionivoschi, N.; et al. The Campylobacter jejuni Type VI Secretion System Enhances the Oxidative Stress Response and Host Colonization. Front. Microbiol. 2019, 10, 462614. [Google Scholar] [CrossRef]
- He, Y.; Frye, J.G.; Strobaugh, T.P.; Chen, C.Y. Analysis of AI-2/LuxS–Dependent Transcription in Campylobacter jejuni Strain 81-176. Foodborne Pathog. Dis. 2008, 5, 399–415. [Google Scholar] [CrossRef]
- Bronnec, V.; Turoňová, H.; Bouju, A.; Cruveiller, S.; Rodrigues, R.; Demnerova, K.; Tresse, O.; Haddad, N.; Zagorec, M. Adhesion, Biofilm Formation, and Genomic Features of Campylobacter jejuni Bf, an Atypical Strain Able to Grow under Aerobic Conditions. Front. Microbiol. 2016, 7, 1002. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Tang, Y.; Zhang, Q. A Mutator Phenotype Promoting the Emergence of Spontaneous Oxidative Stressresistant Mutants in Campylobacter jejun. Appl. Environ. Microbiol. 2017, 83, e01685-17. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Sun, Y.Q.; Stintzi, A. Cj1386 Is an Ankyrin-Containing Protein Involved in Heme Trafficking to Catalase in Campylobacter jejuni. J. Bacteriol. 2012, 194, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Strauch, K.L.; Johnson, K.; Beckwith, J. Characterization of DegP, a Gene Required for Proteolysis in the Cell Envelope and Essential for Growth of Escherichia Coli at High Temperature. J. Bacteriol. 1989, 171, 2689–2696. [Google Scholar] [CrossRef]
- Laskowska, E.; Kuczyńska-Wiśnik, D.; Skórko-Glonek, J.; Taylor, A. Degradation by Proteases Lon, Clp and HtrA, of Escherichia Coli Proteins Aggregated in Vivo by Heat Shock; HtrA Protease Action in Vivo and in Vitro. Mol. Microbiol. 1996, 22, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Bæk, K.T.; Vegge, C.S.; Skórko-Glonek, J.; Brøndsted, L. Different Contributions of HtrA Protease and Chaperone Activities to Campylobacter jejuni Stress Tolerance and Physiology. Appl. Environ. Microbiol. 2011, 77, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kalmokoff, M.; Lanthier, P.; Tremblay, T.L.; Foss, M.; Lau, P.C.; Sanders, G.; Austin, J.; Kelly, J.; Szymanski, C.M. Proteomic Analysis of Campylobacter jejuni 11168 Biofilms Reveals a Role for the Motility Complex in Biofilm Formation. J. Bacteriol. 2006, 188, 4312–4320. [Google Scholar] [CrossRef]
- Gilbert, M.; Karwaski, M.-F.; Bernatchez, S.; Young, N.M.; Taboada, E.; Michniewicz, J.; Cunningham, A.-M.; Wakarchuk, W.W. The Genetic Bases for the Variation in the Lipo-Oligosaccharide of the Mucosal Pathogen, Campylobacter jejuni. J. Biol. Chem. 2002, 277, 327–337. [Google Scholar] [CrossRef]
- Gundogdu, O.; Mills, D.C.; Elmi, A.; Martin, M.J.; Wren, B.W.; Dorrell, N. The Campylobacter jejuni Transcriptional Regulator Cj1556 Plays a Role in the Oxidative and Aerobic Stress Response and Is Important for Bacterial Survival in Vivo. J. Bacteriol. 2011, 193, 4238–4249. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Pocheron, A.L.; Hernould, M.; Haddad, N.; Tresse, O.; Cappelier, J.M. Description of Campylobacter jejuni Bf, an Atypical Aero-Tolerant Strain. Gut Pathog. 2015, 7, 30. [Google Scholar] [CrossRef]
- Tang, Y.; Quail, M.A.; Artymiuk, P.J.; Guest, J.R.; Green, J. Escherichia Coli Aconitases and Oxidative Stress: Post-Transcriptional Regulation of SodA Expression. Microbiology 2002, 148, 1027–1037. [Google Scholar] [CrossRef]
| Environmental Condition | References |
|---|---|
| Growth Media and Supplements | |
| Iron, iron sources, norepinephrine | [25,33,36] |
| Blood | [30,34,36,56] |
| Iron sulfate, sodium bisulfite, and sodium pyruvate (FBP) | [26,27,30,34,38,56] |
| Charcoal, iron sulfate, sodium pyruvate | [36] |
| C4-dicarboxylates, C3-monocarboxylates, and sodium bicarbonate | [40,53] |
| Pyruvate | [32,52] |
| Hemin | [34] |
| Bisulfite | [28,31,32] |
| Different brands of complex media | [32] |
| Chicken or beef liver juice, meat juice | [39] |
| Beef extract | [40] |
| Formate ‡ | [71] |
| Serine | [57] |
| Dithionite or histidine | [29] |
| Allopurionol, azelaic acid, caffeine, cimetidine, TEMPOL | [32] |
| Enzymes | |
| Superoxide dismutase | [27], [29] *, [30], [32] *, [58] |
| Catalase | [27,29] |
| Oxyrase | [34,50] |
| Polymicrobial Interactions | |
| Co-cultivation with Pseudomonas spp. | [58,62] |
| Co-cultivation with S. aureus | [60] |
| Co-cultivation with B. subtilis | [61] |
| Co-cultivation with Salmonella | [59] |
| Membrane fragments from P. aeruginosa and E. coli | [62] |
| Co-cultivation with amoeba | [63,64] |
| Bacteriophage exposure | [65] |
| Other Environmental Conditions | |
| Biofilm formation | [19,42,43,44,45,55,67,68,69,71,72] |
| Darkness | [27,29] |
| Temperature | [11,28,31,77] |
| Cell density | [12] |
| Subculturing and air-adaptation | [81,82,83,84,85] |
| VBNC state | [54,55,56] |
| >97% carbon dioxide ‡ | [78] |
| Acid stress | [80] |
| Genes/Proteins Involved in Aerotolerance | References |
|---|---|
| Oxidative Stress Response | |
| katA | [54,80,90,95,96,97,98,103,105,107,108,109] |
| ahpC | [17,96,98,105,106,107,108,109] |
| sodB/SOD | [54,90,96,98,100,101,102,103,107] |
| tpx | [98,110] |
| bcp | [110] |
| rrc | [109,111,112] |
| trxB | [98,109] |
| herA | [113] |
| fdxA | [114,115] |
| FldA | [103] |
| msrA and msrB | [116] |
| Iron, Zinc, or Phosphate Regulation | |
| dps | [98,117] |
| cft | [103,118] |
| tonB2 | [111] |
| czcD | [106] |
| pstC | [111] |
| Stress Responses and Virulence | |
| htrA | [119,120] |
| HspR | [121] |
| Cj62 | [122] |
| FusA, CadF, FlaA | [103] |
| cmeG | [88] |
| Transcriptional Regulators | |
| csrA | [123] |
| perR † | [106,108,109,124] |
| cosR † | [125] |
| rrpA and rrpB/cj1556 | [126] |
| acnB | [111] |
| Structural Components | |
| kpsS and waaF/rfaF | [127] |
| htrB | [86] |
| tssD | [128] |
| MreB | [103] |
| Biofilm Formation and Motility | |
| peb4 | [43] |
| flgP, flgI, flgK, flgL, flgR, flhB, flgD, flgH, pseB, motA, motB | [111] |
| Other Enzymes | |
| luxS | [125,129] |
| oorA | [98] |
| oorD | [130] |
| MutY † | [131] |
| Unknown Functions | |
| cj0203, cj0264c, cj0415, cj0425, cj0629, cj0864 | [12] |
| cj1386 | [132] |
| Unidentified catalase-like heme-binding protein | [21] |
| cj1371, cj1476c, cj0012c | [103] |
| cj0062c, cj0344, cj0947c, cj1388 | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delaporte, E.; Karki, A.B.; Fakhr, M.K. Aerotolerancy of Campylobacter spp.: A Comprehensive Review. Pathogens 2024, 13, 842. https://doi.org/10.3390/pathogens13100842
Delaporte E, Karki AB, Fakhr MK. Aerotolerancy of Campylobacter spp.: A Comprehensive Review. Pathogens. 2024; 13(10):842. https://doi.org/10.3390/pathogens13100842
Chicago/Turabian StyleDelaporte, Elise, Anand B. Karki, and Mohamed K. Fakhr. 2024. "Aerotolerancy of Campylobacter spp.: A Comprehensive Review" Pathogens 13, no. 10: 842. https://doi.org/10.3390/pathogens13100842







