Dry Stamping Coral Powder: An Effective Method for Isolating Coral Symbiotic Actinobacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Actinobacteria Isolation by Dry Stamping (DS)
2.3. Actinobacteria Isolation by Heat Shock (HS)
2.4. Actinobacteria Strain Purification
2.5. Strain Morphotypes
2.6. Phylogenetic Analysis of Actinobacteria
2.7. Statistical Analysis
2.8. Functional Analysis and Annotation
3. Results
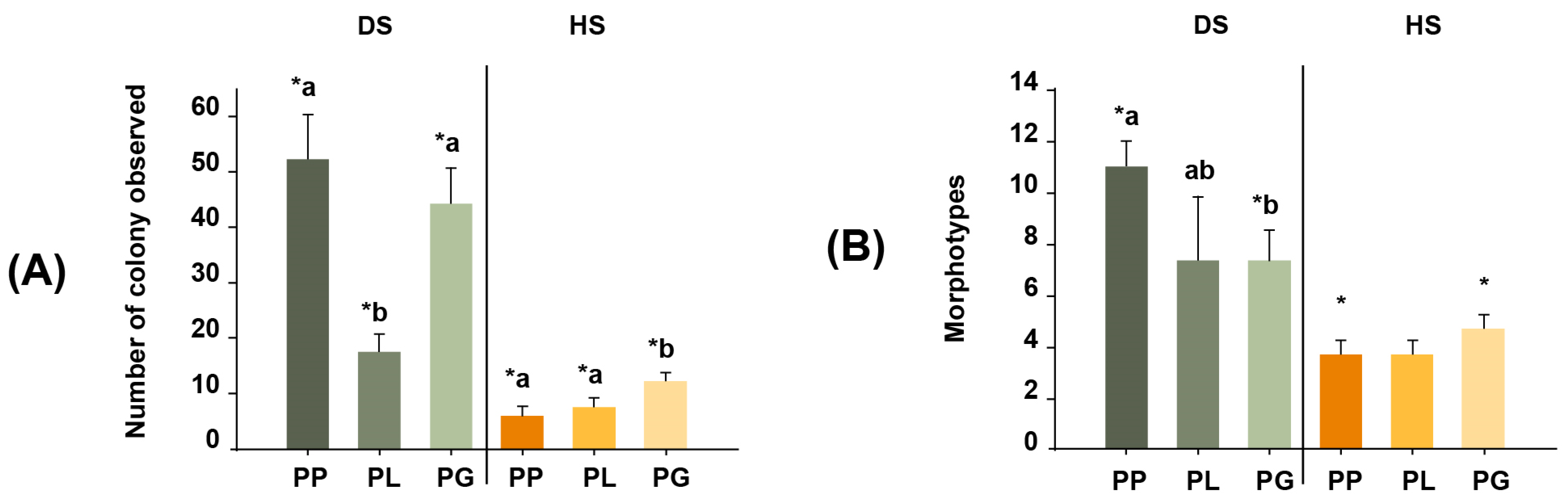
3.1. Recovery of Total Coral Actinobacteria Colonies in the DS and HS Treatments
3.2. Actinobacteria Morphotypes
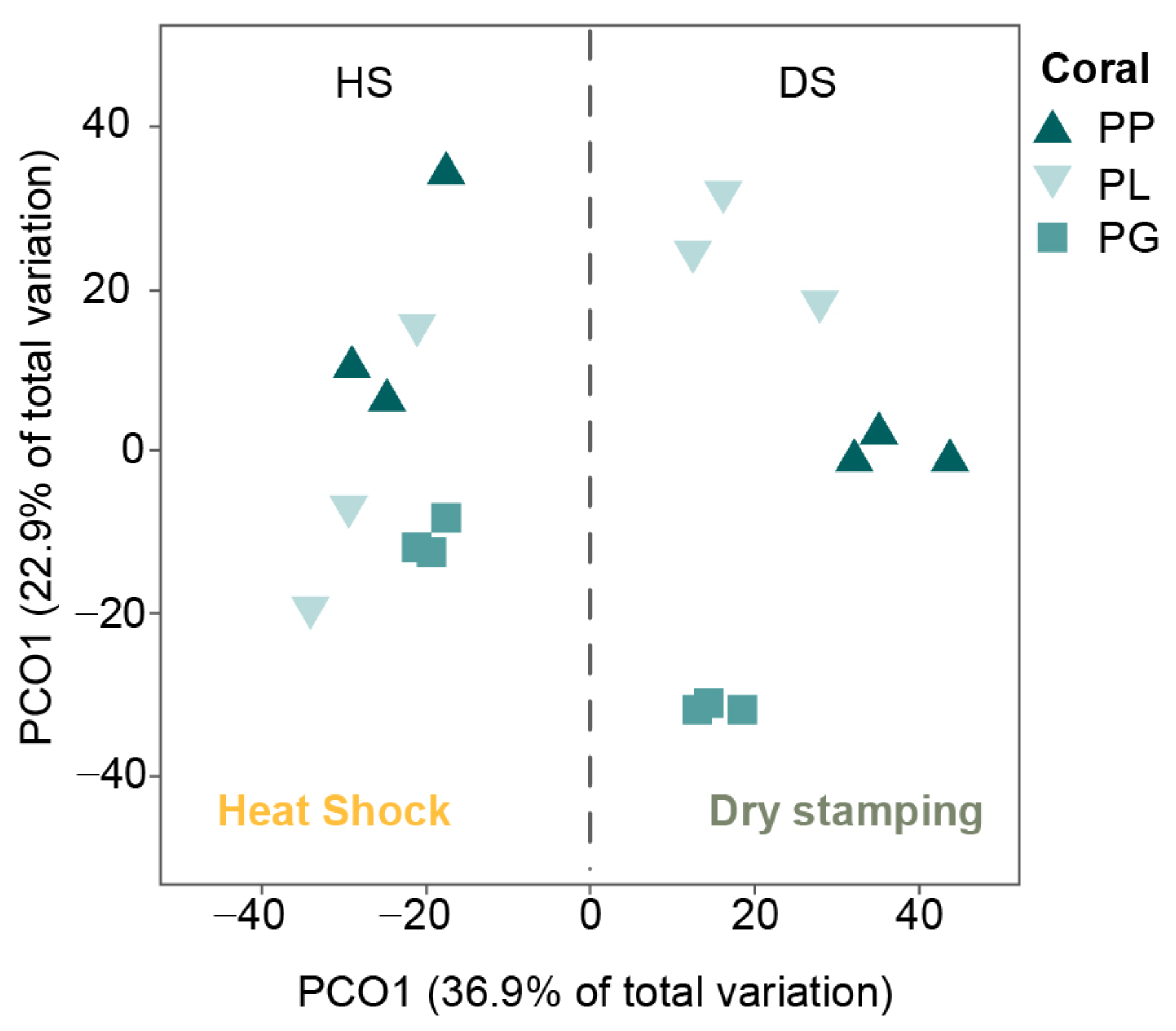
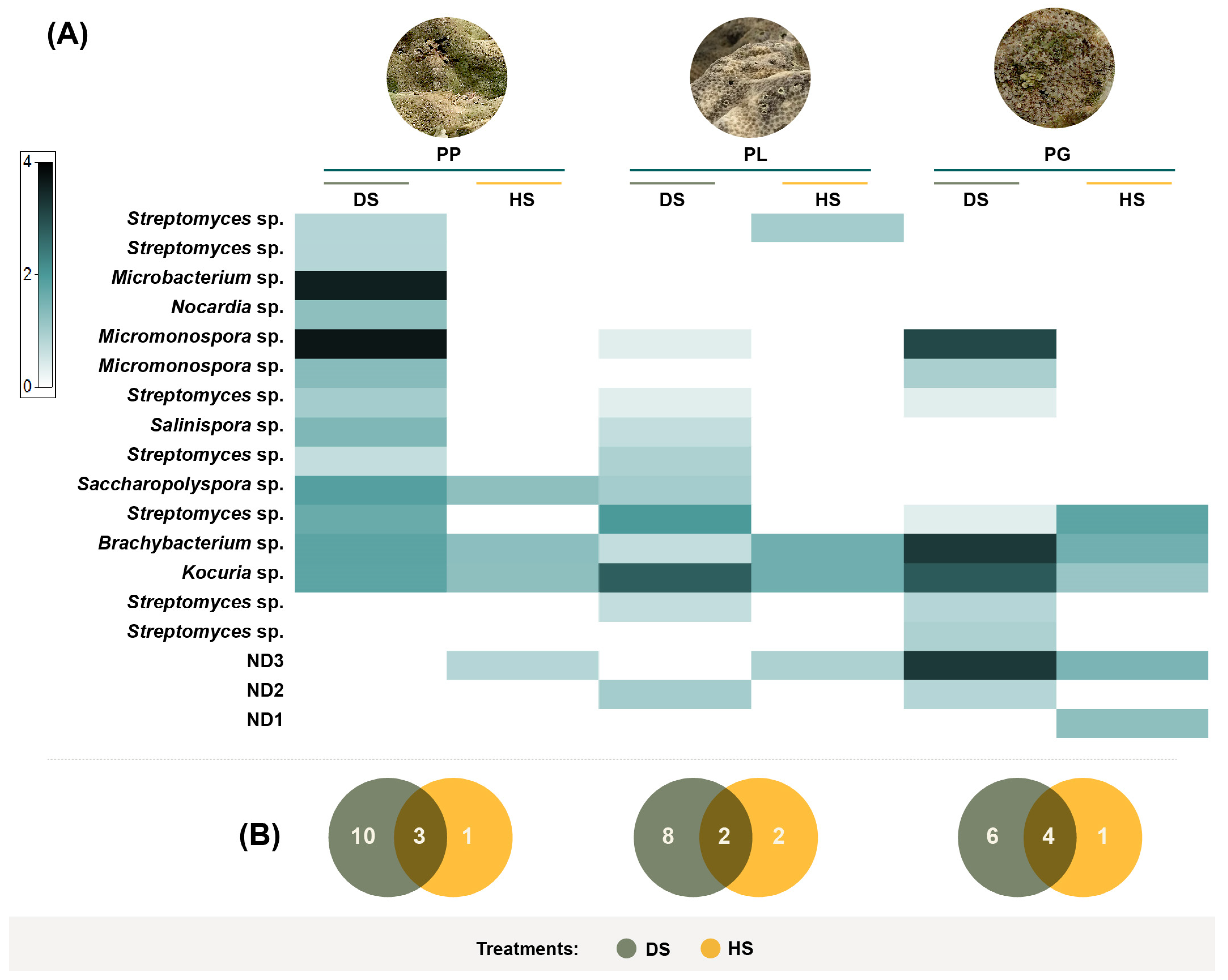
3.3. Actinobacteria Phylogeny
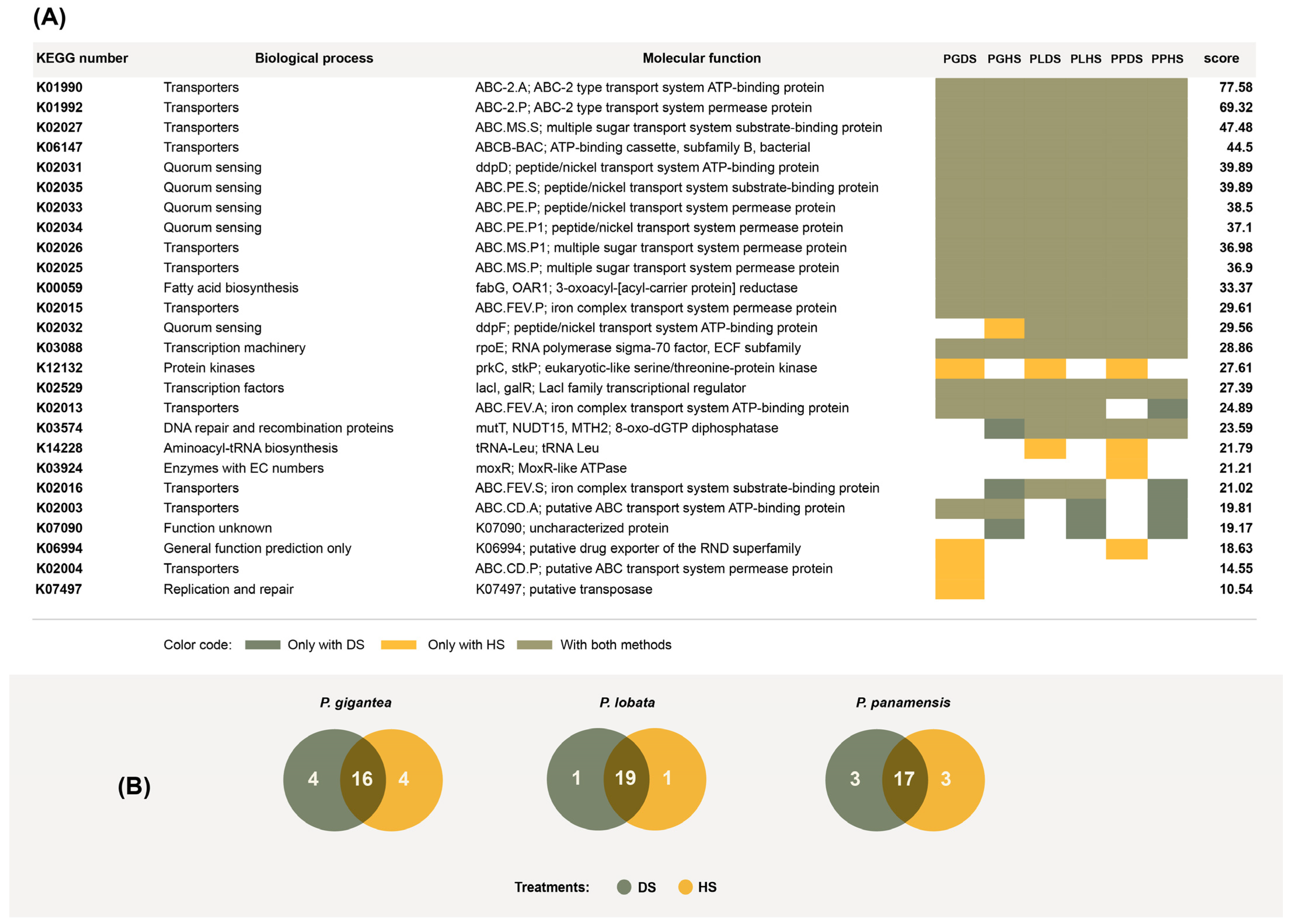
3.4. Overrepresented Functions in Actinobacteria Genomes Recovered Using the HS and DS Treatments
4. Discussion
4.1. Recovery of Actinobacteria by Heat Shock and Dry Stamping
4.2. Dry Stamping Facilitates the Recovery of Coral Microbiome Actinobacteria
4.3. Molecular Processes Necessary for Symbiosis Were Identified Based on the Functional Genome Annotation of the Actinobacteria Isolates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Girão, M.; Ribeiro, I.; Carvalho, M.D.F. Actinobacteria from marine environments: A unique source of natural products. In Natural Products from Actinomycetes: Diversity, Ecology and Drug Discovery; Rai, R.V., Bai, J.A., Eds.; Springer: Singapore, 2022; pp. 1–45. [Google Scholar] [CrossRef]
- Kuang, W.; Li, J.; Zhang, S.; Long, L. Diversity and distribution of Actinobacteria associated with reef coral Porites lutea. Front. Microbiol. 2015, 6, 1094. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Tseng, C.H.; Huang, C.R.; Chen, C.P.; Tandon, K.; Lee, S.T.M.; Chiang, P.W.; Shiu, J.U.; Chen, A.C.; Tang, S.L. Long-term survey is necessary to reveal various shifts of microbial composition in corals. Front. Microbiol. 2017, 8, 1094. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, T.D.; Krause, L.; Bridge, T.; Torda, G.; Raina, J.B.; Zakrzewski, M.; Gates, R.D.; Padilla-Gamiño, J.L.; Spalding, H.L.; Smith, C.; et al. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J. 2015, 9, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Q.; Long, L.J.; Dong, J.; De Yang, J.; Zhang, S. Bacterial dynamics within the mucus, tissue, and skeleton of the coral Porites lutea during different seasons. Sci. Rep. 2014, 4, 7320. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, E.; Ben Yosef, D.Z.; Pavlov, V.; Kushamro, A. Corynebacterium maris sp. nov., a marine bacterium isolated from the mucus of the coral Fungia granulosa. Int. J. Syst. Evol. Microbiol. 2009, 59, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, A.; Takahashi, Y.; Yasumoto-hirose, M.; Kasai, H.; Shizuri, Y.; Omura, S. Janibacter corallicola sp. nov., isolated from coral in Palau. J. Gen. Appl. Microbiol. 2007, 53, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ramaprasad, E.V.V.; Mahidhara, G.; Sasikala, C.; Ramana, C.V. Rhodococcus electrodiphilus sp. nov., a marine electro active actinobacterium isolated from coral reef. Int. J. Syst. Evol. Microbiol. 2018, 68, 2644–2649. [Google Scholar] [CrossRef]
- Wu, J.F.F.; Li, J.; You, Z.Q.Q.; Zhang, S. Prauserella coralliicola sp. nov., isolated from the coral Galaxea fascicularis. Int. J. Syst. Evol. Microbiol. 2014, 64, 3341–3345. [Google Scholar] [CrossRef]
- Gong, Y.; Bai, J.L.; Yang, H.T.; Zhang, W.D.; Xiong, Y.W.; Ding, P.; Qin, S. Phylogenetic diversity and investigation of plant growth-promoting traits of actinobacteria in coastal salt marsh plant rhizospheres from Jiangsu, China. Syst. Appl. Microbiol. 2018, 41, 516–527. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.W.; Wang, T.T.; Ding, P.; Xing, K.; Jiang, J.H. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil 2017, 416, 117–132. [Google Scholar] [CrossRef]
- Yandigeri, M.S.; Malviya, N.; Solanki, M.K.; Shrivastava, P.; Sivakumar, G. Chitinolytic Streptomyces vinaceusdrappus S5MW2 isolated from Chilika lake, India enhances plant growth and biocontrol efficacy through chitin supplementation against Rhizoctonia solani. World J. Microbiol. Biotechnol. 2015, 31, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, J.; De Yang, J.; Luo, X.M.; Zhang, S. Detection of polyketide synthase and nonribosomal peptide synthetase biosynthetic genes from antimicrobial coral-associated actinomycetes. Antonie Van Leeuwenhoek 2014, 106, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.M.; Kalendar, A.A. Coral-associated Actinobacteria: Diversity, abundance, and biotechnological potentials. Front. Microbiol. 2016, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Nithyanand, P.; Thenmozhi, R.; Rathna, J.; Pandian, S.K. Inhibition of Streptococcus pyogenes biofilm formation by coral-associated actinomycetes. Curr. Microbiol. 2010, 60, 454–460. [Google Scholar] [CrossRef]
- You, J.L.; Xue, X.L.; Cao, L.X.; Lu, X.; Wang, J.; Zhang, L.X.; Zhou, S. Inhibition of Vibrio biofilm formation by a marine actinomycete strain A66. Appl. Microbiol. Biotechnol. 2007, 76, 1137–1144. [Google Scholar] [CrossRef]
- Zhang, B.H.; Ding, Z.G.; Li, H.Q.; Mou, X.Z.; Zhang, Y.Q.; Yang, J.Y.; Zhou, E.M.; Li, W.J. Algicidal activity of Streptomyces eurocidicus JXJ-0089 metabolites and their effects on Microcystis physiology. Appl. Environ. Microbiol. 2016, 82, 5132–5143. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Alvarez, H.; Meza-Canales, I.D.; Mateos-Salmón, C.; Rios-Jara, E.; Rodríguez-Zaragoza, F.A.; Robles-Murguía, C.; Muñoz-Urias, A.; Hernández-Herrera, R.M.; Choix-Ley, F.J.; Becerril-Espinosa, A. Diving into reef ecosystems for land-agriculture solutions: Coral microbiota can alleviate salt stress during germination and photosynthesis in terrestrial plants. Front. Plant Sci. 2020, 11, 648. [Google Scholar] [CrossRef]
- Becerril-Espinosa, A.; Hernández-Herrera, R.M.; Meza-Canales, I.D.; Perez-Ramirez, R.; Rodríguez-Zaragoza, F.A.; Méndez-Morán, L.; Sánchez-Hernández, C.V.; Palmeros-Suárez, P.A.; Palacios, O.A.; Choix, F.J.; et al. Habitat-adapted heterologous symbiont Salinispora arenicola promotes growth and alleviates salt stress in tomato crop plants. Front. Plant Sci. 2022, 13, 920881. [Google Scholar] [CrossRef]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Damjanovic, K.; Van Oppen, M.J.H.; Menéndez, P.; Blackall, L.L. Experimental inoculation of coral recruits with marine bacteria indicates scope for microbiome manipulation in Acropora tenuis and Platygyra daedalea. Front. Microbiol. 2019, 10, 1702. [Google Scholar] [CrossRef]
- Gontang, E.A.; Fenical, W.; Jensen, P.R. Phylogenetic diversity of gram-positive bacteria cultured from marine sediments. Appl. Environ. Microbiol. 2007, 73, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Thejaswini, S.; Jojy, S.; Vijayan, A.; Martin Paul, A. Isolation of Gut Actinobacteria from Fishes. In Methods in Actinobacteriology; Dharumadurai, D., Ed.; Springer Protocols Handbooks: New York, NY, USA; Humana: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Hayakawa, M.; Yoshida, Y.; Iimura, Y. Selective isolation of bioactive soil actinomycetes belonging to the Streptomyces violaceusniger phenotypic cluster. J. Appl. Microbiol. 2004, 96, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Thomas, J. Isolation and characterization of a novel Actinomycete isolated from marine sediments and its antibacterial activity against fish pathogens. Antibiotics 2022, 11, 1546. [Google Scholar] [CrossRef] [PubMed]
- Ensign, J.C. Formation, properties, and germination of actinomycete spores. Ann. Rev. Microbiol. 1978, 32, 185–219. [Google Scholar] [CrossRef] [PubMed]
- Mincer, T.J.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl. Environ. Microbiol. 2002, 68, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Powers, E.M. Efficacy of the Ryu nonstaining KOH technique for rapidly determining gram reactions of food-borne and waterborne bacteria and yeasts. Appl. Environ. Microbiol. 1995, 61, 3756–3758. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Heberle, H.; Meirelles, V.G.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Iwai, S.; Weinmaier, T.; Schmidt, B.L.; Albertson, D.G.; Poloso, N.J.; Dabbagh, K.; DeSantis, T.Z. Piphillin: Improved prediction of metagenomic content by direct inference from human microbiomes. PLoS ONE 2016, 11, e0166104. [Google Scholar] [CrossRef]
- Tenenbaum, D. KEGGREST: Client-side REST access to KEGG, R Package Version 1.14.1; 2016; 1. Available online: https://bioconductor.org/packages/release/bioc/html/KEGGREST.html (accessed on 21 November 2023).
- Kanehisha, M. The KEGG database. In ‘In Silico’ simulation of Biological Processes: Novartis Foundation Symposium; John Wiley & Sons, Ltd.: Chichester, UK, 2002; Volume 247, pp. 91–103. [Google Scholar]
- Abia, A.L.K.; Alisoltani, A.; Keshri, J.; Ubomba-Jaswa, E. Metagenomic analysis of the bacterial communities and their functional profiles in water and sediments of the Apies River, South Africa, as a function of land use. Sci. Total Environ. 2018, 616, 326–334. [Google Scholar] [CrossRef]
- Bates, K.A.; Clare, F.C.; O’Hanlon, S.; Bosch, J.; Brookes, L.; Hopkins, K.; McLaughlin, E.L.; Daniel, O.; Garner, T.W.J.; Fisher, M.C.; et al. Amphibian chytridiomycosis outbreak dynamics are linked with host skin bacterial community structure. Nat. Commun. 2018, 9, 693. [Google Scholar] [CrossRef]
- Mullish, B.H.; McDonald, J.A.; Pechlivanis, A.; Allegretti, J.R.; Kao, D.; Barker, G.F.; Diya Kapila, D.; Petrof, E.O.; Joyce, S.A.; Gahan, C.G.M.; et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut 2019, 68, 1791–1800. [Google Scholar] [CrossRef]
- Jin, M.; Lu, J.; Chen, Z.; Nguyen, S.H.; Mao, L.; Li, J.; Yuan, Z.; Guo, J. Antidepressant fluoxetine induces multiple antibiotics resistance in Escherichia coli via ROS-mediated mutagenesis. Environ. Int. 2018, 120, 421–430. [Google Scholar] [CrossRef]
- Nandakumar, M.P.; Cheung, A.; Marten, M.R. Proteomic Analysis of Extracellular Proteins from Escherichia coli W3110. J. Proteome Res. 2006, 5, 1155–1161. [Google Scholar] [CrossRef]
- Goodfellow, M. Selective isolation of Actinobacteria. In Manual of Industrial Microbiology and Biotechnology; Baltz, R., Demain, A., Davies, J., Bull, A., Junker, B., Katz, L., Lynd, L., Masurekar, P., Reeves, C., Zhao, H., Eds.; ASM Press: Washington, DC, USA, 2010; pp. 13–27. [Google Scholar] [CrossRef]
- Ribeiro, I.; Girão, M.; Alexandrino, D.A.; Ribeiro, T.; Santos, C.; Pereira, F.; Mucha, A.P.; Urbatzka, R.; Leão, P.N.; Carvalho, M.F. Diversity and bioactive potential of actinobacteria isolated from a coastal marine sediment in northern Portugal. Microorganisms 2020, 8, 1691. [Google Scholar] [CrossRef]
- Liu, T.; Wu, S.; Zhang, R.; Wang, D.; Chen, J.; Zhao, J. Diversity and antimicrobial potential of Actinobacteria isolated from diverse marine sponges along the Beibu Gulf of the South China Sea. FEMS Microbiol. Ecol. 2019, 95, fiz089. [Google Scholar] [CrossRef]
- Gobalakrishnan, R. Phylogenetic diversity of culturable marine actinobacteria isolated from the Havelock island, the Andamans, India. Ecol. Genet. Genom. 2022, 23, 100123. [Google Scholar] [CrossRef]
- Pollock, F.J.; McMinds, R.; Smith, S.; Bourne, D.G.; Willis, B.L.; Medina, M.; Thurber, R.V.; Zaneveld, J.R. Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny. Nat. Commun. 2018, 9, 4921. [Google Scholar] [CrossRef]
- Garlapati, D.; Charankumar, B.; Ramu, K.; Madeswaran, P.; Ramana Murthy, M.V. A review on the applications and recent advances in environmental DNA (eDNA) metagenomics. Rev. Environ. Sci. Biotechnol. 2019, 18, 389–411. [Google Scholar] [CrossRef]
- Sottorff, I.; Wiese, J.; Imhoff, J.F. High diversity and novelty of Actinobacteria isolated from the coastal zone of the geographically remote young volcanic Easter Island, Chile. Int. Microbiol. 2019, 22, 377–390. [Google Scholar] [CrossRef]
- Siro, G.; Pipite, A.; Christi, K.; Srinivasan, S.; Subramani, R. Marine actinomycetes associated with stony corals: A potential hotspot for specialized metabolites. Microorganisms 2022, 10, 1349. [Google Scholar] [CrossRef]
- Buangrab, K.; Sutthacheep, M.; Yeemin, T.; Harunari, E.; Igarashi, Y.; Sripreechasak, P.; Kanchanasin, P.; Tanasupawat, S.; Phongsopitanun, W. Streptomyces corallincola and Kineosporia corallincola sp. nov., two new coral-derived marine actinobacteria. Int. J. Syst. Evol. Microbiol. 2022, 72, 005249. [Google Scholar] [CrossRef]
- Hernández-Vázquez, P.B.; Ocampo-Alvarez, H.; Galván-Villa, C.M.; Ríos-Jara, E.; Becerril-Espinosa, A. Cultivable bacterial microbiota of the sea urchin Hesperocidaris asteriscus, inhabitant of the upper mesophotic zone in the Los Arcos submarine cayon, Puerto Vallarta. e-CUCBA 2023, 20, 141–150. [Google Scholar] [CrossRef]
- Becerril-Espinosa, A.; Freel, K.C.; Jensen, P.R.; Soria-Mercado, I.E. Marine Actinobacteria from the Gulf of California: Diversity, abundance, and secondary metabolite biosynthetic potential. Antonie Van Leeuwenhoek 2013, 103, 809–819. [Google Scholar] [CrossRef]
- Méndez, C.; Salas, J.A. The role of ABC transporters in antibiotic-producing organisms: Drug secretion and resistance mechanisms. Res. Microbiol. 2001, 152, 341–350. [Google Scholar] [CrossRef]
- Kai, K. Bacterial quorum sensing in symbiotic and pathogenic relationships with hosts. Biosci. Biotechnol. Biochem. 2018, 82, 363–371. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerril-Espinosa, A.; Mateos-Salmón, C.; Burgos, A.; Rodríguez-Zaragoza, F.A.; Meza-Canales, I.D.; Juarez-Carrillo, E.; Rios-Jara, E.; Ocampo-Alvarez, H. Dry Stamping Coral Powder: An Effective Method for Isolating Coral Symbiotic Actinobacteria. Microorganisms 2023, 11, 2951. https://doi.org/10.3390/microorganisms11122951
Becerril-Espinosa A, Mateos-Salmón C, Burgos A, Rodríguez-Zaragoza FA, Meza-Canales ID, Juarez-Carrillo E, Rios-Jara E, Ocampo-Alvarez H. Dry Stamping Coral Powder: An Effective Method for Isolating Coral Symbiotic Actinobacteria. Microorganisms. 2023; 11(12):2951. https://doi.org/10.3390/microorganisms11122951
Chicago/Turabian StyleBecerril-Espinosa, Amayaly, Carolina Mateos-Salmón, Asdrubal Burgos, Fabián A. Rodríguez-Zaragoza, Iván D. Meza-Canales, Eduardo Juarez-Carrillo, Eduardo Rios-Jara, and Héctor Ocampo-Alvarez. 2023. "Dry Stamping Coral Powder: An Effective Method for Isolating Coral Symbiotic Actinobacteria" Microorganisms 11, no. 12: 2951. https://doi.org/10.3390/microorganisms11122951







