Adaptive Strategies and Underlying Response Mechanisms of Ciliates to Salinity Change with Note on Fluctuation Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organisms and Cultivation
2.2. Preparation of a Gradient-Concentration Salt Solution
2.3. Experimental Design
2.4. Determination of the Population Growth Dynamics
2.5. Measurement of Enzyme Activity and Energy Storage Substance
2.6. Gene Expression Levels of Energy Metabolism Enzymes
2.7. Statistical Analyses
3. Results
3.1. Effects on the Logistic Growth Dynamics of Euplotes Vannus
3.2. Effects on Energy Storage Substance
3.3. Effects on Energy Metabolism Enzyme Activity
3.4. Gene Expression Levels of Enzymes Related to Energy Metabolism
3.5. Correlations between Growth Dynamic and Energy Metabolism Parameters
4. Discussion
4.1. Salinity Fluctuations on Population Growth Dynamics and Underlying Adaptive Strategies
4.2. Responding Mechanism to Salinity Fluctuations
4.3. Trade-Off in Life-History Strategy Influenced by Salinity Fluctuations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Ota, Y.; Sumaila, U.R.; Cisneros-Montemayor, A.M.; Cheung, W.W. Adaptation strategies to climate change in marine systems. Global Chang. Biol. 2018, 24, e1–e14. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.; Iwamura, T.; Butt, N. Mapping vulnerability and conservation adaptation strategies under climate change. Nat. Clim. Chang. 2013, 3, 989–994. [Google Scholar] [CrossRef]
- Gedan, K.B.; Silliman, B.R.; Bertness, M.D. Centuries of human-driven change in salt marsh ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Myrberg, K.; Post, P.; Chubarenko, I.; Dailidiene, I.; Hinrichsen, H.-H.; Hüssy, K.; Liblik, T.; Meier, H.M.; Lips, U. Salinity dynamics of the Baltic Sea. Earth Syst. Dyn. 2022, 13, 373–392. [Google Scholar] [CrossRef]
- Vuorinen, I.; Hänninen, J.; Rajasilta, M.; Laine, P.; Eklund, J.; Montesino-Pouzols, F.; Corona, F.; Junker, K.; Meier, H.M.; Dippner, J.W. Scenario simulations of future salinity and ecological consequences in the Baltic Sea and adjacent North Sea areas–implications for environmental monitoring. Ecol. Indic. 2015, 50, 196–205. [Google Scholar] [CrossRef]
- Brischoux, F.; Tingley, R.; Shine, R.; Lillywhite, H.B. Salinity influences the distribution of marine snakes: Implications for evolutionary transitions to marine life. Ecography 2012, 35, 994–1003. [Google Scholar] [CrossRef]
- Held, M.B.; Harley, C.D. Responses to low salinity by the sea star Pisaster ochraceus from high- and low-salinity populations. Invertebr. Biol. 2009, 128, 381–390. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Tebbe, C.C.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X.; Yu, L. Salinity controls soil microbial community structure and function in coastal estuarine wetlands. Environ. MicroBiol. 2021, 23, 1020–1037. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.; Davis, R.P.; Nazeer, S.; Ibarra-Castro, L.; Davis, D.A. Effect of salinity on growth, survival, and serum osmolality of red snapper, Lutjanus campechanus. Fish Physiol. Biochem. 2021, 47, 1687–1696. [Google Scholar] [CrossRef]
- Laramore, S.; Laramore, C.R.; Scarpa, J. Effect of low salinity on growth and survival of postlarvae and juvenile Litopenaeus vannamei. J. World Aquacult. Soc. 2001, 32, 385–392. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Zhang, L.; Zhuang, P.; Liu, J. Survival, growth, food conversion efficiency and plasma osmolality of juvenile Siganus guttatus (Bloch, 1787): Experimental analyses of salinity effects. Fish Physiol. Biochem. 2013, 39, 1025–1030. [Google Scholar] [CrossRef]
- Bœuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Arnott, S.E.; Celis-Salgado, M.P.; Valleau, R.E.; DeSellas, A.M.; Paterson, A.M.; Yan, N.D.; Smol, J.P.; Rusak, J.A. Road salt impacts freshwater zooplankton at concentrations below current water quality guidelines. Environ. Sci. Technol. 2020, 54, 9398–9407. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Sun, Y.; Zhang, L.; Lyu, K.; Yang, Z. Size-specific sensitivity of cladocerans to freshwater salinization: Evidences from the changes in life history and population dynamics. Environ. Pollut. 2022, 296, 118770. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, B.; Wang, T.; Song, L. Adaptability of Moina affinis (Crustacea: Cladocera: Moinidae) to seawater salinity. J. Appl. Ecol. [Artic. Chin.] 2006, 17, 1521–1525. Available online: https://pubmed.ncbi.nlm.nih.gov/17066715 (accessed on 1 September 2024).
- Kirwan, M.L.; Megonigal, J.P. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 2013, 504, 53–60. [Google Scholar] [CrossRef]
- Schroeder, K.; Chiggiato, J.; Bryden, H.; Borghini, M.; Ben Ismail, S. Abrupt climate shift in the Western Mediterranean Sea. Sci. Rep. 2016, 6, 23009. [Google Scholar] [CrossRef]
- Verschuren, D.; Laird, K.R.; Cumming, B.F. Rainfall and drought in equatorial east Africa during the past 1100 years. Nature 2000, 403, 410–414. [Google Scholar] [CrossRef]
- Yang, H.; Meng, Y.; Song, Y.; Tan, Y.; Warren, A.; Li, J.; Lin, X. Salinity fluctuation influencing biological adaptation: Growth dynamics and Na+/K+--ATPase activity in a euryhaline bacterium. J. Basic MicroBiol. 2017, 57, 617–624. [Google Scholar] [CrossRef]
- Moll, R.J.; Redilla, K.M.; Mudumba, T.; Muneza, A.B.; Gray, S.M.; Abade, L.; Hayward, M.W.; Millspaugh, J.J.; Montgomery, R.A. The many faces of fear: A synthesis of the methodological variation in characterizing predation risk. J. Anim. Ecol. 2017, 86, 749–765. [Google Scholar] [CrossRef]
- Lancaster, L.; Hazard, L.; Clobert, J.; Sinervo, B. Corticosterone manipulation reveals differences in hierarchical organization of multidimensional reproductive trade-offs in r-strategist and K-strategist females. J. Evol. Biol. 2008, 21, 556–565. [Google Scholar] [CrossRef]
- Sinervo, B.; Svensson, E.; Comendant, T. Density cycles and an offspring quantity and quality game driven by natural selection. Nature 2000, 406, 985–988. [Google Scholar] [CrossRef]
- Ernande, B.; Boudry, P.; Clobert, J.; Haure, J. Plasticity in resource allocation based life history traits in the Pacific oyster, Crassostrea gigas. I. Spatial variation in food abundance. J. Evol. Biol. 2004, 17, 342–356. [Google Scholar] [CrossRef]
- Ruan, Y.; Dou, Y.; Chen, J.; Warren, A.; Li, J.; Lin, X. Evaluation of phenol-induced ecotoxicity in two model ciliate species: Population growth dynamics and antioxidant enzyme activity. Ecotoxicol. Environ. Saf. 2018, 166, 176–185. [Google Scholar] [CrossRef]
- Tankoua, O.F.; Buffet, P.; Amiard, J.; Berthet, B.; Mouneyrac, C.; Amiard-Triquet, C. Integrated assessment of estuarine sediment quality based on a multi-biomarker approach in the bivalve Scrobicularia plana. Ecotoxicol. Environ. Saf. 2013, 88, 117–125. [Google Scholar] [CrossRef]
- Luna-Acosta, A.; Bustamante, P.; Thomas-Guyon, H.; Zaldibar, B.; Izagirre, U.; Marigómez, I. Integrative biomarker assessment of the effects of chemically and mechanically dispersed crude oil in Pacific oysters, Crassostrea gigas. Sci. Total Environ. 2017, 598, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Panzarino, O.; Hyršl, P.; Dobeš, P.; Vojtek, L.; Vernile, P.; Bari, G.; Terzano, R.; Spagnuolo, M.; de Lillo, E. Rank-based biomarker index to assess cadmium ecotoxicity on the earthworm Eisenia andrei. Chemosphere 2016, 145, 480–486. [Google Scholar] [CrossRef]
- Smolders, R.; Bervoets, L.; De Coen, W.; Blust, R. Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ. Pollut. 2004, 129, 99–112. [Google Scholar] [CrossRef]
- Ferreira, N.G.; Morgado, R.; Santos, M.J.; Soares, A.M.; Loureiro, S. Biomarkers and energy reserves in the isopod Porcellionides pruinosus: The effects of long-term exposure to dimethoate. Sci. Total Environ. 2015, 502, 91–102. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, A.; Yuan, C.; Zhao, T.; Chang, H.; Zhang, J. Transcriptome analysis of liver lipid metabolism disorders of the turbot Scophthalmus maximus in response to low salinity stress. Aquaculture 2021, 534, 736273. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Spoelstra, K. Effects of anthropogenic light on species and ecosystems. Science 2023, 380, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Jurdzinski, K.T.; Mehrshad, M.; Delgado, L.F.; Deng, Z.; Bertilsson, S.; Andersson, A.F. Large-scale phylogenomics of aquatic bacteria reveal molecular mechanisms for adaptation to salinity. Sci. Adv. 2023, 9, eadg2059. [Google Scholar] [CrossRef]
- Esteban, G.F.; Fenchel, T.M. Ecology of Protozoa. In The Biology of Free-Living Phagotrophic Protists, 2nd ed.; Springer Nature: Cham, Switzerland, 2020; pp. 107–131. ISBN 978-3-030-59978-2. [Google Scholar]
- Kazmi, S.S.U.H.; Warren, A.; Xu, H. Insights into evaluating the toxic effects of nitrofurazone on ecological integrity in marine ecosystems using periphytic ciliate communities. Ecol. Indic. 2022, 141, 109095. [Google Scholar] [CrossRef]
- Montagnes, D.; Roberts, E.; Lukeš, J.; Lowe, C. The rise of model protozoa. Trends MicroBiol. 2012, 20, 184–191. [Google Scholar] [CrossRef]
- Sikder, M.N.A.; Xu, H.; Warren, A. Colonization features of marine biofilm-dwelling protozoa in Chinese coastal waters of the Yellow Sea. Mar. Life Sci. Technol. 2020, 2, 292–301. [Google Scholar] [CrossRef]
- Chen, Z.; Song, W. Phylogenetic positions of Aspidisca steini and Euplotes vannus within the order Euplotida (Hypotrichia, Ciliophora) inferred from complete small subunit ribosomal RNA gene sequences. Acta Protozool. 2002, 41, 1–10. [Google Scholar]
- Zhou, L.; Li, J.; Lin, X.; Al-Rasheid, K.A. Use of RAPD to detect DNA damage induced by nitrofurazone in marine ciliate, Euplotes vannus (Protozoa, Ciliophora). Aquati. Toxicol. 2011, 103, 225–232. [Google Scholar] [CrossRef]
- Yang, J.; Wei, H.; Yalin, T.; Alan, W.; Xiaofeng, L.; Jiqiu, L. Combined effects of food resources and exposure to ammonium nitrogen on population growth performance in the bacterivorous ciliate Paramecium caudatum. Eur. J. Protistol. 2019, 71, 125631. [Google Scholar] [CrossRef]
- Cabaud, P.G.; Wróblewski, F.; Ok, W.T.T.A.; Ruggiero, V. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am. J. Clin. Pathol. 1958, 30, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Hohorst, H.-J. l-(-)-Malate: Determination with Malic Dehydrogenase and DPN. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1965; pp. 328–334. ISBN 978-0-12-395630-9. [Google Scholar]
- Hess, B.; Wieker, H.-J. Pyruvate kinase from yeast. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 778–783. ISBN 978-0-12-091302-2. [Google Scholar]
- Carroll, N.V.; Longley, R.W.; Roe, J.H. The determination of glycogen in liver and muscle by use of anthrone reagent. J. Biol. Chem. 1956, 220, 583–593. [Google Scholar] [CrossRef]
- Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1985, 1, 302–304. Available online: https://pubmed.ncbi.nlm.nih.gov/2906672 (accessed on 31 August 2024). [PubMed]
- Cheng, Y.-S.; Zheng, Y.; VanderGheynst, J.S. Rapid quantitative analysis of lipids using a colorimetric method in a microplate format. Lipids 2011, 46, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.E.; Krohn, R.I.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Lin, X.; Yi, Z.; Al-Rasheid, K.A. Characterizing dose-responses of catalase to nitrofurazone exposure in model ciliated protozoan Euplotes vannus for ecotoxicity assessment: Enzyme activity and mRNA expression. Ecotoxicol. Environ. Saf. 2014, 100, 294–302. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.-O.; Schulte, P.M.; Wood, C.; Schiemer, F. Niche dimensions in fishes: An integrative view. Physiol. Biochem. Zool. 2010, 83, 808–826. [Google Scholar] [CrossRef]
- Chase, J.M. To grow or to reproduce? The role of life-history plasticity in food web dynamics. Am. Nat. 1999, 154, 571–586. [Google Scholar] [CrossRef]
- Einum, S.; Fleming, I.A. Highly fecund mothers sacrifice offspring survival to maximize fitness. Nature 2000, 405, 565–567. [Google Scholar] [CrossRef]
- Dupont, L.; Thierry, M.; Zinger, L.; Legrand, D.; Jacob, S. Beyond reaction norms: The temporal dynamics of phenotypic plasticity. Trends Ecol. Evol. 2023, 39, 41–51. [Google Scholar] [CrossRef]
- Raynaud-Berton, B.; Gibert, P.; Suppo, C.; Pincebourde, S.; Colinet, H. Modelling thermal reaction norms for development and viability in Drosophila suzukii under constant, fluctuating and field conditions. J. Therm. Biol. 2024, 123, 103891. [Google Scholar] [CrossRef] [PubMed]
- De Jong, G. Covariances between traits deriving from successive allocations of a resource. Funct. Ecol. 1993, 7, 75–83. [Google Scholar] [CrossRef]
- Hadley, N.F. The Adaptive Role of Lipids in Biological Systems; Wiley: New York, NY, USA, 1985; p. 319. [Google Scholar]
- Li, L.; Zhou, H.; Wang, J.; Li, J.; Lyu, X.; Wang, W.; Luo, C.; Huang, H.; Zhou, D.; Chen, X. Metabolic switch from glycogen to lipid in the liver maintains glucose homeostasis in neonatal mice. J. Lipid Res. 2023, 64, 100440. [Google Scholar] [CrossRef] [PubMed]
- Plaistow, S.J.; Troussard, J.-P.; Cézilly, F. The effect of the acanthocephalan parasite Pomphorhynchus laevis on the lipid and glycogen content of its intermediate host Gammarus pulex. Int. J. Parasitol. 2001, 31, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Lampert, W. Veränderungen im Gewicht und der chemischen Zusammensetzung von Daphnia pulex im Hunger. Arch. Hydrobiol. Suppl. 1975, 48, 108–137. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL7650138743 (accessed on 1 September 2024).
- Downer, R.; Matthews, J. Patterns of lipid distribution and utilisation in insects. Am. Zool. 1976, 16, 733–745. [Google Scholar] [CrossRef]
- Sparkes, T.C.; Keogh, D.P.; Pary, R.A. Energetic costs of mate guarding behavior in male stream-dwelling isopods. Oecologia 1996, 106, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Shirly-Lim, Y.L.; Rahmah, S.; Abd Ghaffar, M.; Liang, L.Q.; Chang, Y.M.; Chisti, Y.; Lee, M.-A.; Liew, H.J. Pacific whiteleg shrimps compromize their physiological needs to cope with environmental stress. Environ. Adv. 2024, 15, 100492. [Google Scholar] [CrossRef]
- Guo, T.; Yang, Y.; Meng, F.; Wang, S.; Xia, S.; Qian, Y.; Li, M.; Wang, R. Effects of low salinity on gill and liver glycogen metabolism of great blue-spotted mudskippers (Boleophthalmus pectinirostris). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 230, 108709. [Google Scholar] [CrossRef]
- Berger, V.J.; Kharazova, A. Mechanisms of salinity adaptations in marine molluscs. Hydrobiologia 1997, 355, 115–126. [Google Scholar] [CrossRef]
- Wilson, L.H.; Cho, J.H.; Estrella, A.; Smyth, J.A.; Wu, R.; Chengsupanimit, T.; Brown, L.M.; Weinstein, D.A.; Lee, Y.M. Liver glycogen phosphorylase deficiency leads to profibrogenic phenotype in a murine model of glycogen storage disease type VI. Hepatol. Commun. 2019, 3, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Glycolysis. Cold Spring Harb. Perspect. Biol. 2021, 13, a040535. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. The tricarboxylic acid cycle. In Chemical Pathways of Metabolism; Greenberg, D.M., Ed.; Academic Press: Cambridge, MA, USA, 1954; pp. 109–171. [Google Scholar] [CrossRef]
- Chen, Z.J.; Qu, Y.N.; Li, S.Y.; Wang, H.W.; Ji, C.H.; Shi, X.Z.; Yang, H.; Li, X.S. Insight into the relationship between metabolic enzymes and oxadiazon degradation in Oryza sativa for reducing environmental risks. Ecotoxicol. Environ. Saf. 2024, 274, 116242. [Google Scholar] [CrossRef]
- Schormann, N.; Hayden, K.L.; Lee, P.; Banerjee, S.; Chattopadhyay, D. An overview of structure, function, and regulation of pyruvate kinases. Protein Sci. 2019, 28, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, L.G.; Rossi, M.; Di Stefano, G.; Rossi, V.; Binda, C.; Brigotti, M.; Tomaselli, F.; Pasti, A.P.; Dal Piaz, F.; Cerini, S. Allosteric transitions of rabbit skeletal muscle lactate dehydrogenase induced by pH-dependent dissociation of the tetrameric enzyme. Biochimie 2022, 199, 23–35. [Google Scholar] [CrossRef]
- Musrati, R.; Kollarova, M.; Mernik, N.; Mikulasova, D. Malate dehydrogenase: Distribution, function and properties. Gen. Physiol. Biophys. 1998, 17, 193–210. Available online: https://www.ncbi.nlm.nih.gov/pubmed/9834842 (accessed on 1 September 2024).
- Hong, Y.; Tan, Y.; Meng, Y.; Yang, H.; Zhang, Y.; Warren, A.; Li, J.; Lin, X. Evaluation of biomarkers for ecotoxicity assessment by dose-response dynamic models: Effects of nitrofurazone on antioxidant enzymes in the model ciliated protozoan Euplotes vannus. Ecotoxicol. Environ. Saf. 2017, 144, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Hrabak, E.M.; Chan, C.W.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.R.; Luan, S.; Nimmo, H.G.; Sussman, M.R. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef]
- Ding, S.; Wang, F.; Dong, S.; Li, Y. Comparison of the respiratory metabolism of juvenile Litopenaeus vannamei cultured in seawater and freshwater. J. Ocean Univ. China 2014, 13, 331–337. [Google Scholar] [CrossRef]
- Hong, M.; Li, N.; Li, J.; Li, W.; Liang, L.; Li, Q.; Wang, R.; Shi, H.; Storey, K.B.; Ding, L. Adenosine monophosphate-activated protein kinase signaling regulates lipid metabolism in response to salinity stress in the red-eared slider turtle Trachemys scripta elegans. Front. Physiol. 2019, 10, 962. [Google Scholar] [CrossRef]
- Lovas, A.; Bimbo, A.; Szabó, L.; Bánfalvi, Z. Antisense repression of StubGAL83 affects root and tuber development in potato. Plant J. 2003, 33, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Moreno, D.G.; Valenzuela-Soto, E.M.; Peregrino-Uriarte, A.B.; Leyva-Carrillo, L.; Soñanez-Organis, J.G.; Yepiz-Plascencia, G. The pyruvate kinase of the whiteleg shrimp Litopenaeus vannamei: Gene structure and responses to short term hypoxia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 283, 111468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, S.; Liu, P.; Cui, Y.; Hu, Z.; Liu, C.; Zhang, Z.; Yang, M.; Li, X.; Wu, X. Genome-wide characterization of soybean malate dehydrogenase genes reveals a positive role of GmMDH2 in salt stress response. J. Integr. Agr. 2024, in press. [Google Scholar] [CrossRef]
- Clobert, J.; Garland, T., Jr.; Barbault, R. The evolution of demographic tactics in lizards: A test of some hypotheses concerning life history evolution. J. Evol. Biol. 1998, 11, 329–364. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- Stone, C.; Hamilton, I.; Foster, W.A. A survival and reproduction trade-off is resolved in accordance with resource availability by virgin female mosquitoes. Anim. Behav. 2011, 81, 765–774. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, S.; Lin, X.; Li, J.; Yi, Z.; Al-Rasheid, K.A. Recognizing the importance of exposure–dose–response dynamics for ecotoxicity assessment: Nitrofurazone-induced antioxidase activity and mRNA expression in model protozoan Euplotes vannus. Environ. Sci. Pollut. Res. 2015, 22, 9544–9553. [Google Scholar] [CrossRef]
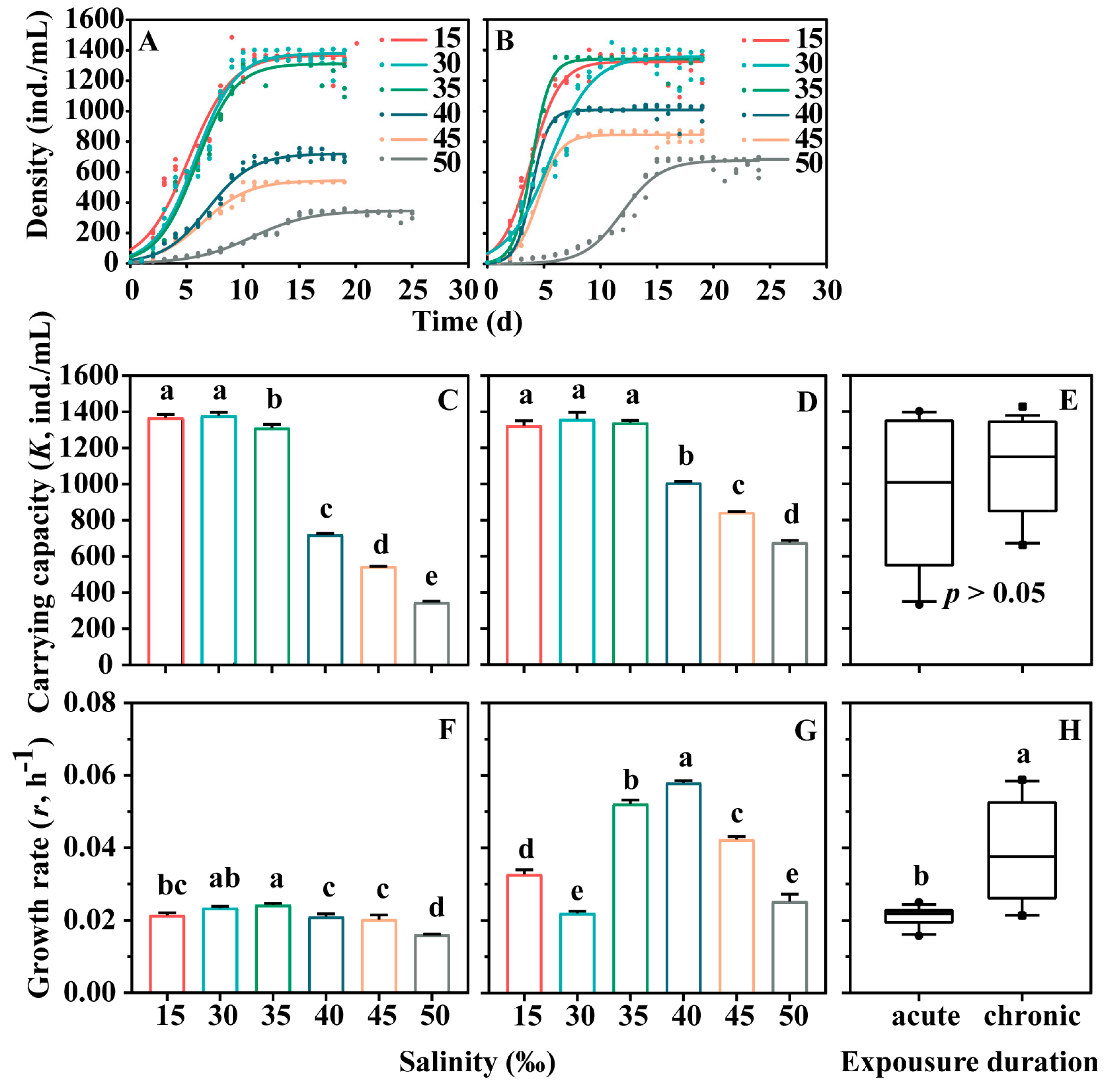
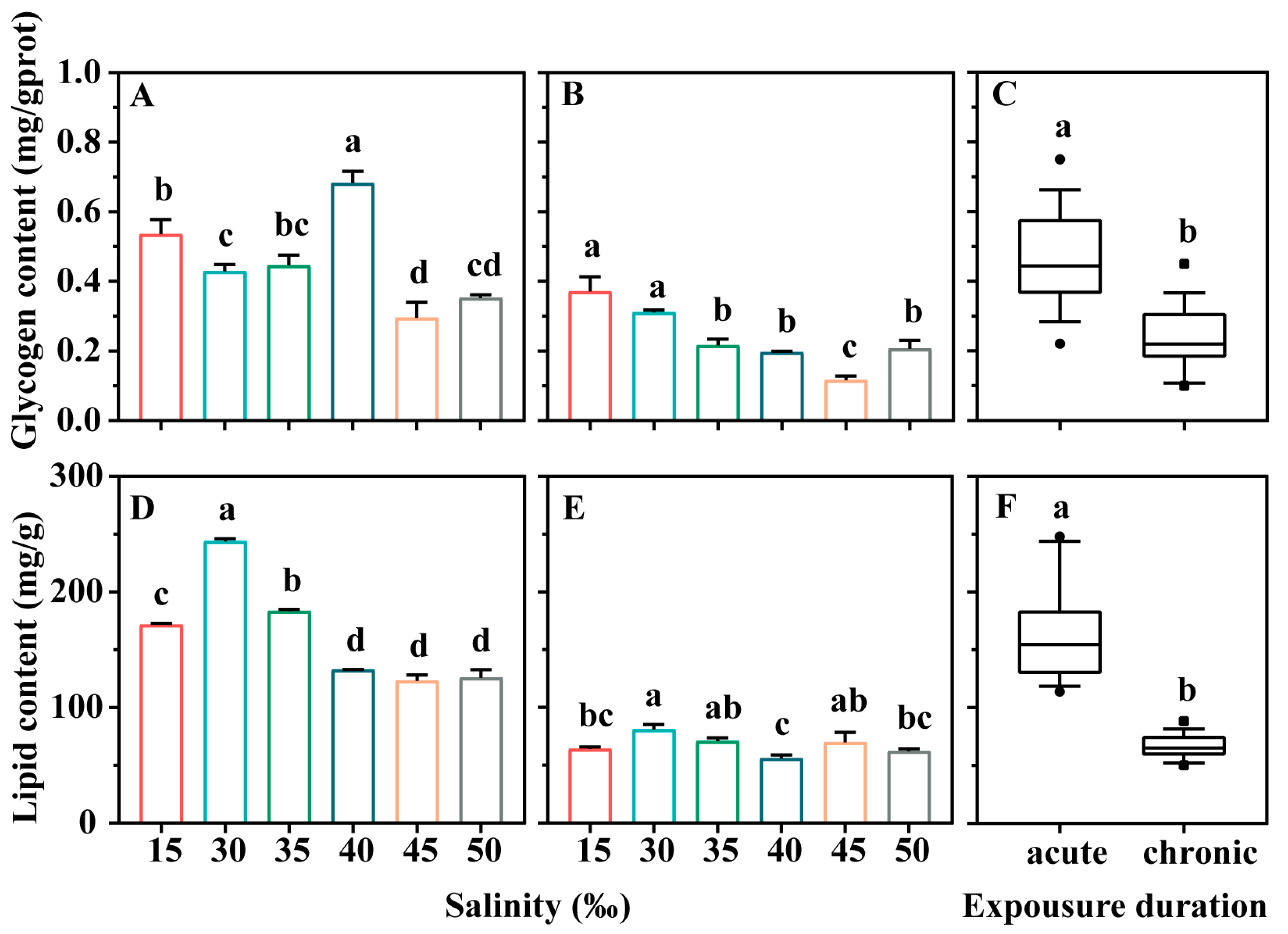

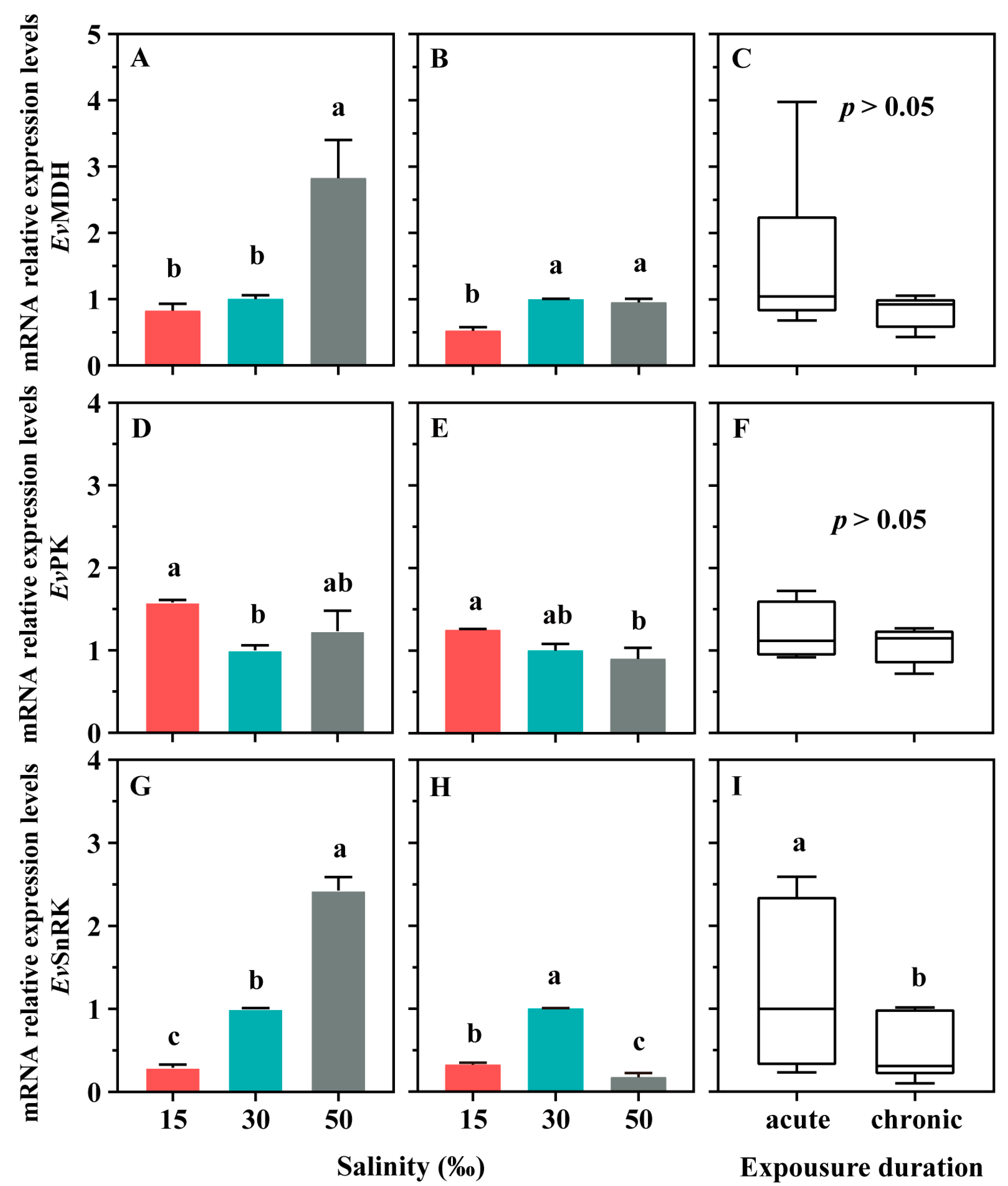
| Variable | Source of Variation | df | F-Values | p-Values |
|---|---|---|---|---|
| K | Time | 1 | 330.813 | 0.000 |
| Salinity | 5 | 674.154 | 0.000 | |
| Time × Salinity | 5 | 79.782 | 0.000 | |
| r | Time | 1 | 827.119 | 0.000 |
| Salinity | 5 | 176.471 | 0.000 | |
| Time × Salinity | 5 | 85.741 | 0.000 | |
| LDH | Time | 1 | 13.721 | 0.003 |
| Salinity | 5 | 25.917 | 0.000 | |
| Time × Salinity | 5 | 4.069 | 0.022 | |
| MDH | Time | 1 | 9.685 | 0.011 |
| Salinity | 5 | 0.588 | 0.710 | |
| Time × Salinity | 5 | 6.646 | 0.006 | |
| PK | Time | 1 | 5.296 | 0.040 |
| Salinity | 5 | 7.642 | 0.002 | |
| Time × Salinity | 5 | 3.012 | 0.055 | |
| Glycogen | Time | 1 | 192.714 | 0.000 |
| Salinity | 5 | 23.664 | 0.000 | |
| Time × Salinity | 5 | 12.163 | 0.000 | |
| Lipid | Time | 1 | 3059.075 | 0.000 |
| Salinity | 5 | 61.116 | 0.000 | |
| Time × Salinity | 5 | 89.660 | 0.000 | |
| EvMDH mRNA expression | Time | 1 | 11.518 | 0.015 |
| Salinity | 2 | 16.000 | 0.004 | |
| Time × Salinity | 2 | 7.408 | 0.024 | |
| EvPK mRNA expression | Time | 1 | 4.845 | 0.070 |
| Salinity | 2 | 6.616 | 0.030 | |
| Time × Salinity | 2 | 1.211 | 0.362 | |
| EvSnRK mRNA expression | Time | 1 | 247.404 | 0.000 |
| Salinity | 2 | 75.237 | 0.000 | |
| Time × Salinity | 2 | 258.763 | 0.000 |
| Factor | Acute Stress Experiment | Chronic Stress Experiment |
|---|---|---|
| r × Glycogen content | 0.180 (−) | −0.448 (+) |
| r × lipid content | 0.678 b (−) | −0.327 (+) |
| r × LDH | −0.297 (+) | 0.461 (−) |
| r × MDH | −0.512 (+) | −0.440 (+) |
| r × PK | 0.006 (−) | −0.166 (+) |
| r × EvMDH mRNA | −0.802 a (+) | −0.921 b (+) |
| r × EvPK mRNA | −0.116 (+) | 0.377 (−) |
| r × EvSnRK mRNA | −0.793 a (+) | −0.549 (+) |
| Glycogen content × LDH | −0.304 (+) | −0.514 a (+) |
| Glycogen content × MDH | 0.002 (−) | 0.590 a (−) |
| Glycogen content × PK | 0.023 (−) | 0.713 b (−) |
| Lipid content × LDH | −0.415 (+) | −0.234 (+) |
| Lipid content × MDH | −0.271 (+) | −0.270 (+) |
| Lipid content × PK | 0.358 (−) | −0.143 (+) |
| Glycogen content × EvMDH mRNA | −0.704 a (+) | −0.485 (+) |
| Glycogen content × EvPK mRNA | 0.466 (−) | 0.698 b (−) |
| Glycogen content ×EvSnRK mRNA | −0.846 b (+) | 0.273 (−) |
| Lipid content × EvMDH mRNA | −0.675 a (+) | 0.420 (−) |
| Lipid content × EvPK mRNA | −0.533 (+) | −0.269 (+) |
| Lipid content × EvSnRK mRNA | −0.317 (+) | 0.891 b (−) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Yang, J.; Li, J.; Lin, X. Adaptive Strategies and Underlying Response Mechanisms of Ciliates to Salinity Change with Note on Fluctuation Properties. Microorganisms 2024, 12, 1957. https://doi.org/10.3390/microorganisms12101957
Li F, Yang J, Li J, Lin X. Adaptive Strategies and Underlying Response Mechanisms of Ciliates to Salinity Change with Note on Fluctuation Properties. Microorganisms. 2024; 12(10):1957. https://doi.org/10.3390/microorganisms12101957
Chicago/Turabian StyleLi, Fenfen, Jing Yang, Jiqiu Li, and Xiaofeng Lin. 2024. "Adaptive Strategies and Underlying Response Mechanisms of Ciliates to Salinity Change with Note on Fluctuation Properties" Microorganisms 12, no. 10: 1957. https://doi.org/10.3390/microorganisms12101957






