Dissecting the Simultaneous Extracellular/Intracellular Contributions to Cr(VI) Reduction under Aerobic and Anaerobic Conditions Using the Newly Isolating Cr(VI)-Reducing Bacterium of Pseudomonas sp. HGB10
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening and Identification of a Cr(VI)-Resistant Bacterium of Strain HGB10
2.2. Cultivation
2.3. Minimum Inhibitory Concentration (MIC) Test for Cr(VI)
2.4. Determining the Cr(VI) Reduction Rate and Extracellular and Intracellular Contribution under Aerobic and Anaerobic Conditions
2.5. Scanning Electron Microscope (SEM)
2.6. Data Analysis
3. Results and Discussion
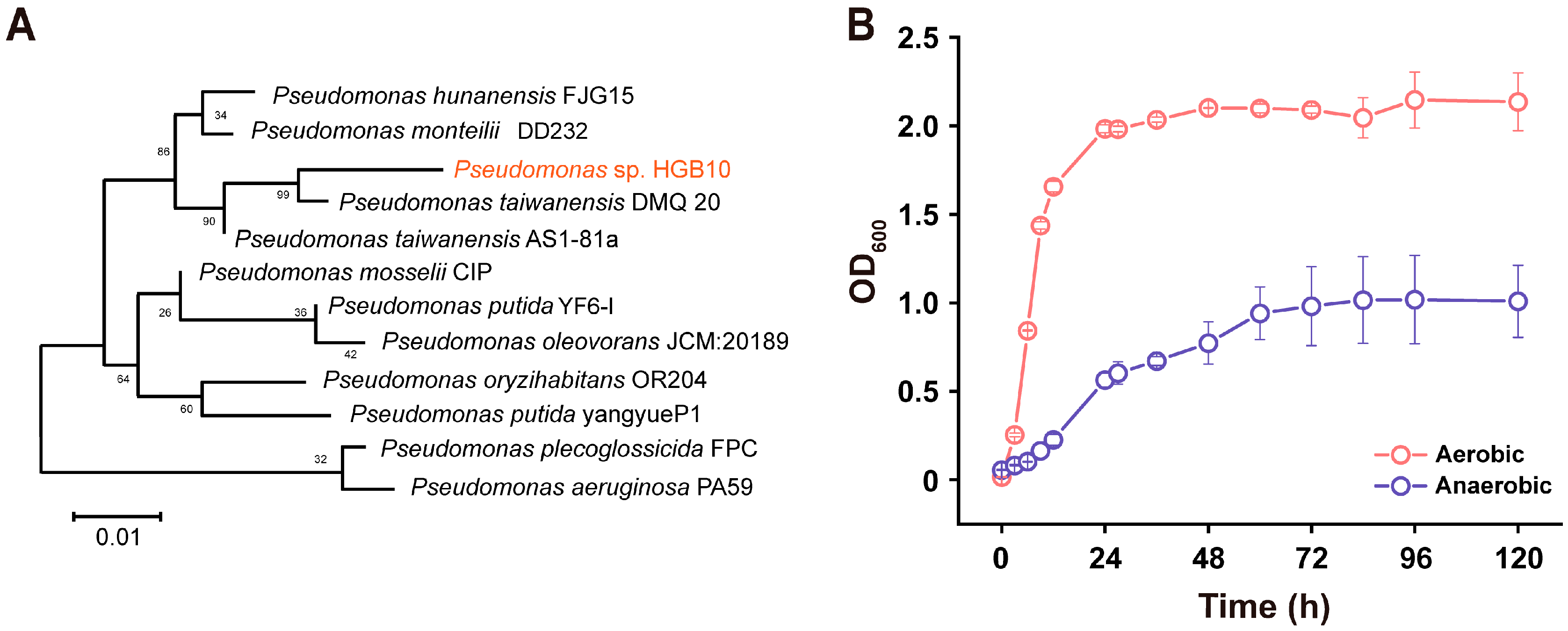
3.1. Isolation Pseudomonas sp. HGB10
3.2. The Growth Characteristics of Pseudomonas sp. HGB10 under Aerobic/Anaerobic Conditions
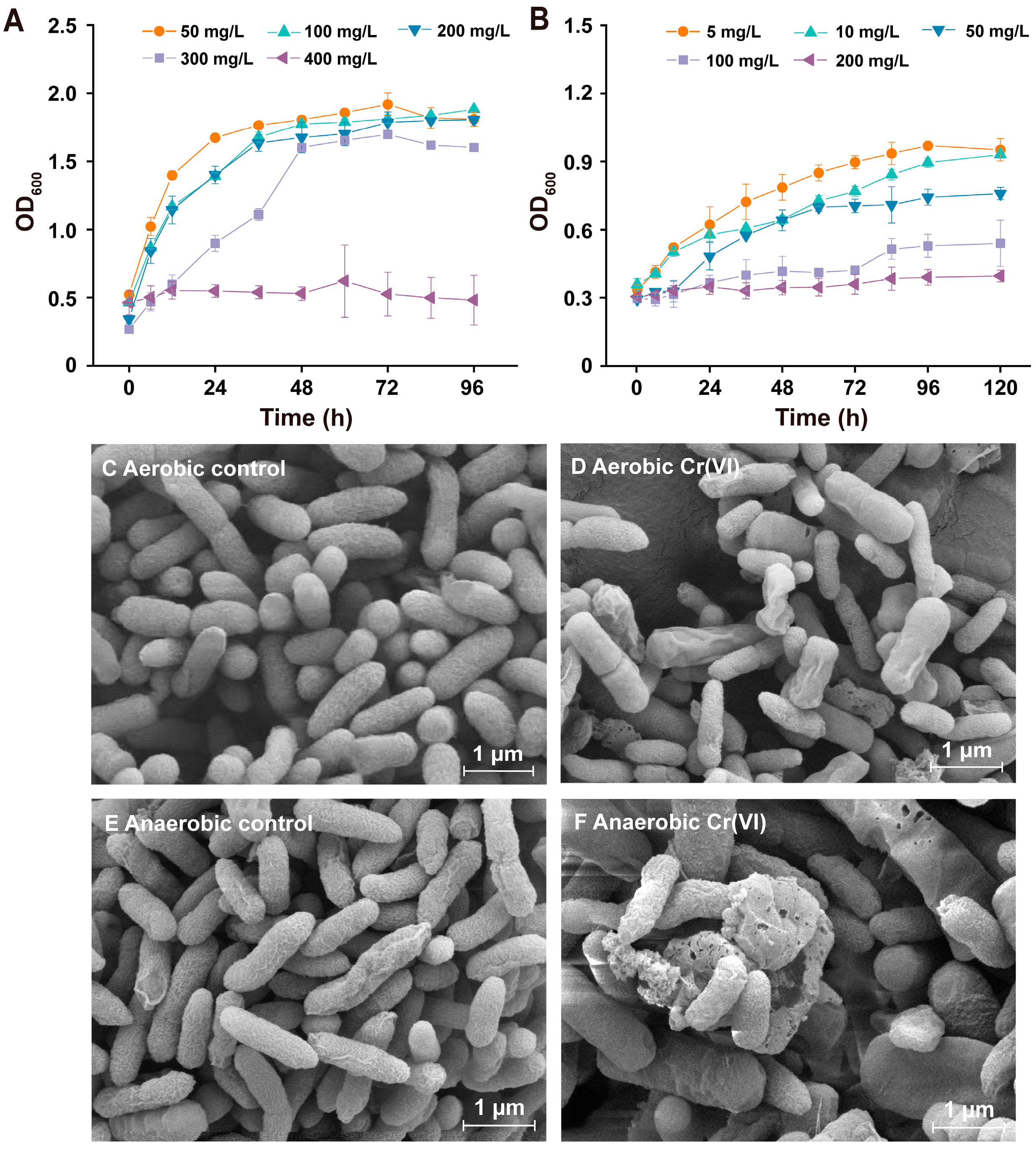
3.3. Resistance to Cr(VI) of Pseudomonas sp. HGB10 under Aerobic/Anaerobic Conditions
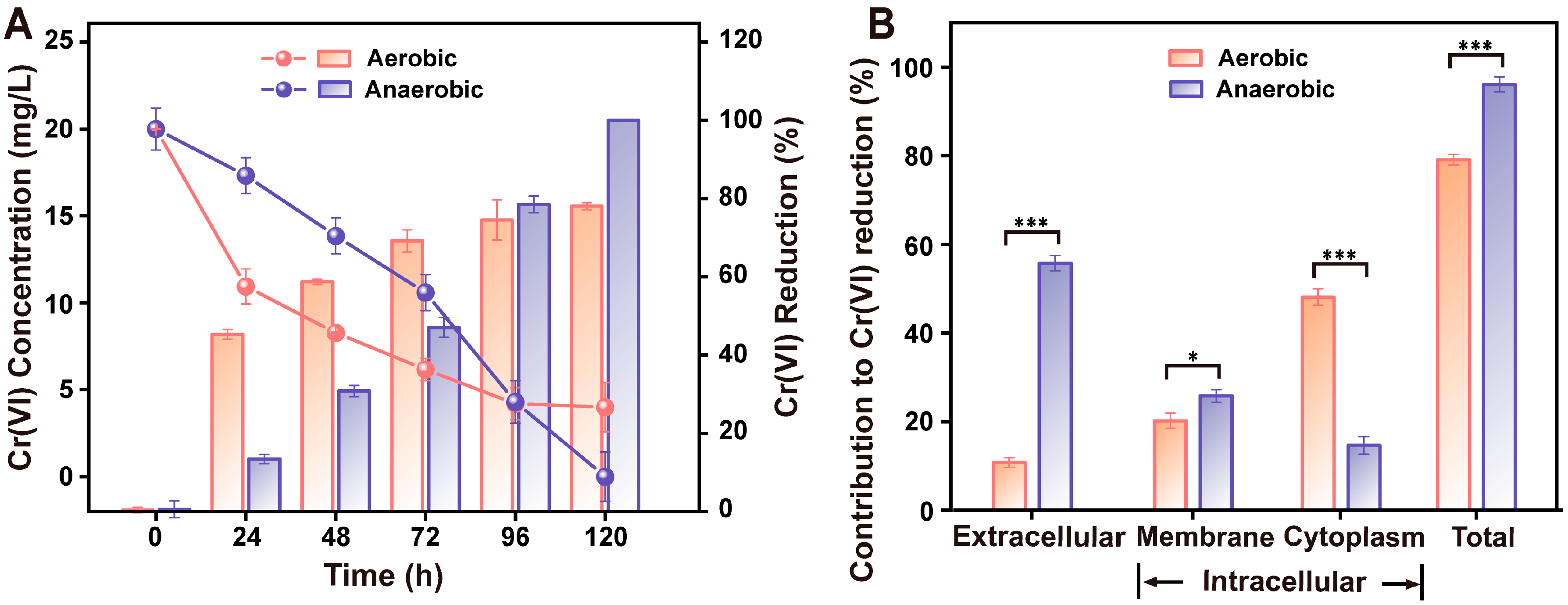
3.4. The Anaerobic Cr(VI)-Reducing Rate of Pseudomonas sp. HGB10 Preponderates over the Aerobic Counterpart
3.5. The Extracellular/Intracellular Contribution to Cr(VI) Reduction under Aerobic/Anaerobic Condition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen uptake rate in microbial processes: An overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Brune, A. Life at the oxic–anoxic interface: Microbial activities and adaptations. FEMS Microbiol. Rev. 2000, 24, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Shi, H.; Qing, Y.; Chen, C.; Shen, L.; Zhou, M.; Li, B.; Lin, H. Molecular oxygen activation: Innovative techniques for environmental remediation. Water Res. 2024, 250, 121075. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yin, Q. Microbial niche nexus sustaining biological wastewater treatment. NPJ Clean Water 2020, 3, 33. [Google Scholar] [CrossRef]
- Ramli, N.N.; Othman, A.R.; Kurniawan, S.B.; Abdullah, S.R.S.; Hasan, H.A. Metabolic pathway of Cr(VI) reduction by bacteria: A review. Microbiol. Res. 2023, 268, 127288. [Google Scholar] [CrossRef] [PubMed]
- Viti, C.; Marchi, E.; Decorosi, F.; Giovannetti, L. Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Microbiol. Rev. 2014, 38, 633–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Y.; Xi, H.; Yu, Y.; Song, Y.; Wu, C.; Zhou, Y. Evaluation methods of inhibition to microorganisms in biotreatment processes: A review. Water Cycle 2023, 4, 70–78. [Google Scholar] [CrossRef]
- Bae, W.; Kang, T.; Kang, I.; Won, Y.; Jeong, B. Reduction of hexavalent chromium by Escherichia coli ATCC 33456 in batch and continuous cultures. J. Microbiol. 2000, 38, 36–39. [Google Scholar]
- Liu, T.; Luo, X.; Wu, Y.; Reinfelder, J.R.; Yuan, X.; Li, X.; Chen, D.; Li, F. Extracellular electron shuttling mediated by soluble c -type cytochromes produced by Shewanella Oneidensis MR-1. Environ. Sci. Technol. 2020, 54, 10577–10587. [Google Scholar] [CrossRef]
- Liu, X.; Chu, G.; Du, Y.; Li, J.; Si, Y. The role of electron shuttle enhances Fe(III)-mediated reduction of Cr(VI) by Shewanella Oneidensis MR-1. World J. Microbiol. Biotechnol. 2019, 35, 64. [Google Scholar] [CrossRef]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiao, C.; Zhao, F. Long-term adaptive evolution of Shewanella Oneidensis MR-1 for establishment of high concentration Cr(VI) tolerance. Front. Environ. Sci. Eng. 2020, 14, 3. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Zhang, B.; Long, Z.-E.; Ni, H.; Fu, X.; Zou, L. Influencing factors and mechanism of Cr(VI) reduction by facultative anaerobic Exiguobacterium Sp. PY14. Front. Microbiol. 2023, 14, 1242410. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, Q.; Hu, L.; Zhong, H.; He, Z. Bioreduction performances and mechanisms of Cr(VI) by Sporosarcina Saromensis W5, a novel Cr(VI)-reducing facultative anaerobic bacteria. J. Hazard. Mater. 2021, 413, 125411. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, M.; Xiao, J.; Hao, L.; Crowley, D.E.; Zhang, Z.; Yu, J.; Huang, N.; Huo, M.; Wu, J. Genome sequence analysis of the naphthenic acid degrading and metal resistant bacterium Cupriavidus gilardii CR3. PLoS ONE 2015, 10, e0132881. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, B.; Chi, Y.; Li, J.; Chi, Y.; Ji, M.; Zhai, H.; Wang, R.; Yuan, T.; Yu, H. Study on the treatment of simulated dye wastewater containing FMPs using the CW-MFC system. J. Water Process Eng. 2024, 66, 105810. [Google Scholar] [CrossRef]
- Mao, H.-T.; Chen, L.-X.; Zhang, M.-Y.; Shi, Q.-Y.; Xu, H.; Zhang, D.-Y.; Zhang, Z.-W.; Yuan, M.; Yuan, S.; Zhang, H.-Y.; et al. Melatonin improves the removal and the reduction of Cr(VI) and alleviates the chromium toxicity by antioxidative machinery in Rhodobacter sphaeroides. Environ. Pollut. 2023, 319, 120973. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, X.; Peng, C.; Shao, P.; Wei, F.; Li, S.; Liu, T.; Yang, L.; Ding, L.; Luo, X. Bioreduction performance of Cr(VI) by microbial extracellular polymeric substances (EPS) and the overlooked role of tryptophan. J. Hazard. Mater. 2022, 433, 128822. [Google Scholar] [CrossRef]
- Tan, H.; Wang, C.; Zeng, G.; Luo, Y.; Li, H.; Xu, H. Bioreduction and biosorption of Cr(VI) by a novel Bacillus sp. CRB-B1 strain. J. Hazard. Mater. 2020, 386, 121628. [Google Scholar] [CrossRef] [PubMed]
- Abo-Alkasem, M.I.; Maany, D.A.; El-Abd, M.A.; Ibrahim, A.S.S. Bioreduction of hexavalent chromium by a novel haloalkaliphilic Salipaludibacillus agaradhaerens strain NRC-R isolated from hypersaline soda lakes. 3 Biotech 2022, 12, 7. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, M.; Zhou, D.; Fan, W.; Wang, X.; Huo, M. Bioremoval of Cu 2+ from CMP wastewater by a novel copper-resistant bacterium Cupriavidus gilardii CR3: Characteristics and mechanisms. RSC Adv. 2017, 7, 18793–18802. [Google Scholar] [CrossRef]
- Schloss, P.D.; Handelsman, J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 2005, 71, 1501–1506. [Google Scholar] [CrossRef]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef]
- Diggle, S.P.; Whiteley, M. Microbe profile: Pseudomonas Aeruginosa: Opportunistic pathogen and lab rat. Microbiology 2020, 166, 30–33. [Google Scholar] [CrossRef]
- Outten, F.W.; Huffman, D.L.; Hale, J.A.; O’Halloran, T.V. The independent cue and cussystems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 2001, 276, 30670–30677. [Google Scholar] [CrossRef]
- Cheung, K.H.; Gu, J.-D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. Int. Biodeterior. Biodegrad. 2007, 59, 8–15. [Google Scholar] [CrossRef]
- Ahemad, M. Bacterial Mechanisms for Cr(VI) Resistance and reduction: An overview and recent advances. Folia Microbiol. 2014, 59, 321–332. [Google Scholar] [CrossRef]
- Bopp, L.H.; Ehrlich, H.L. Chromate resistance and reduction in Pseudomonas fluorescens strain LB300. Arch. Microbiol. 1988, 150, 426–431. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef]
- Chen, J.; Tian, Y. Hexavalent chromium reducing bacteria: Mechanism of reduction and characteristics. Environ. Sci. Pollut. Res. 2021, 28, 20981–20997. [Google Scholar] [CrossRef]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.A. Bacterial reduction of Cr(VI): Operational challenges and feasibility. Curr. Pollut. Rep. 2021, 7, 42. [Google Scholar] [CrossRef]
- Xia, X.; Wu, S.; Zhou, Z.; Wang, G. Microbial Cd(II) and Cr(VI) resistance mechanisms and application in bioremediation. J. Hazard. Mater. 2021, 401, 123685. [Google Scholar] [CrossRef] [PubMed]
- Belchik, S.M.; Kennedy, D.W.; Dohnalkova, A.C.; Wang, Y.; Sevinc, P.C.; Wu, H.; Lin, Y.; Lu, H.P.; Fredrickson, J.K.; Shi, L. Extracellular reduction of hexavalent chromium by cytochromes MtrC and OmcA of Shewanella Oneidensis MR-1. Appl. Environ. Microbiol. 2011, 77, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Manefield, M.; Lee, M.; Kouzuma, A. Electron shuttles in biotechnology. Curr. Opin. Biotech. 2009, 20, 633–641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, X.; Zhao, Q.; Xu, Q.; Zhang, Y. Dissecting the Simultaneous Extracellular/Intracellular Contributions to Cr(VI) Reduction under Aerobic and Anaerobic Conditions Using the Newly Isolating Cr(VI)-Reducing Bacterium of Pseudomonas sp. HGB10. Microorganisms 2024, 12, 1958. https://doi.org/10.3390/microorganisms12101958
Chen S, Wang X, Zhao Q, Xu Q, Zhang Y. Dissecting the Simultaneous Extracellular/Intracellular Contributions to Cr(VI) Reduction under Aerobic and Anaerobic Conditions Using the Newly Isolating Cr(VI)-Reducing Bacterium of Pseudomonas sp. HGB10. Microorganisms. 2024; 12(10):1958. https://doi.org/10.3390/microorganisms12101958
Chicago/Turabian StyleChen, Shenglei, Xiaoyu Wang, Qinyi Zhao, Qiao Xu, and Yini Zhang. 2024. "Dissecting the Simultaneous Extracellular/Intracellular Contributions to Cr(VI) Reduction under Aerobic and Anaerobic Conditions Using the Newly Isolating Cr(VI)-Reducing Bacterium of Pseudomonas sp. HGB10" Microorganisms 12, no. 10: 1958. https://doi.org/10.3390/microorganisms12101958





