Network Analysis of Gut Microbial Communities Reveals Key Reason for Quercetin Protects against Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Assessment of Colitis
2.3. Levels of DAO and D-LA in Serum
2.4. Level of Cytokines in Colon Tissues
2.5. Level of MUC2 in Colon Tissues
2.6. Antioxidant Enzyme Activity Determination
2.7. Alcian Blue and PAS Staining
2.8. RNA Extraction and qRT-PCR
2.9. Immunohistochemistry
2.10. Immunofluorescence
2.11. Gut Microbiota Analysis
2.12. Statistical Analyses
3. Results
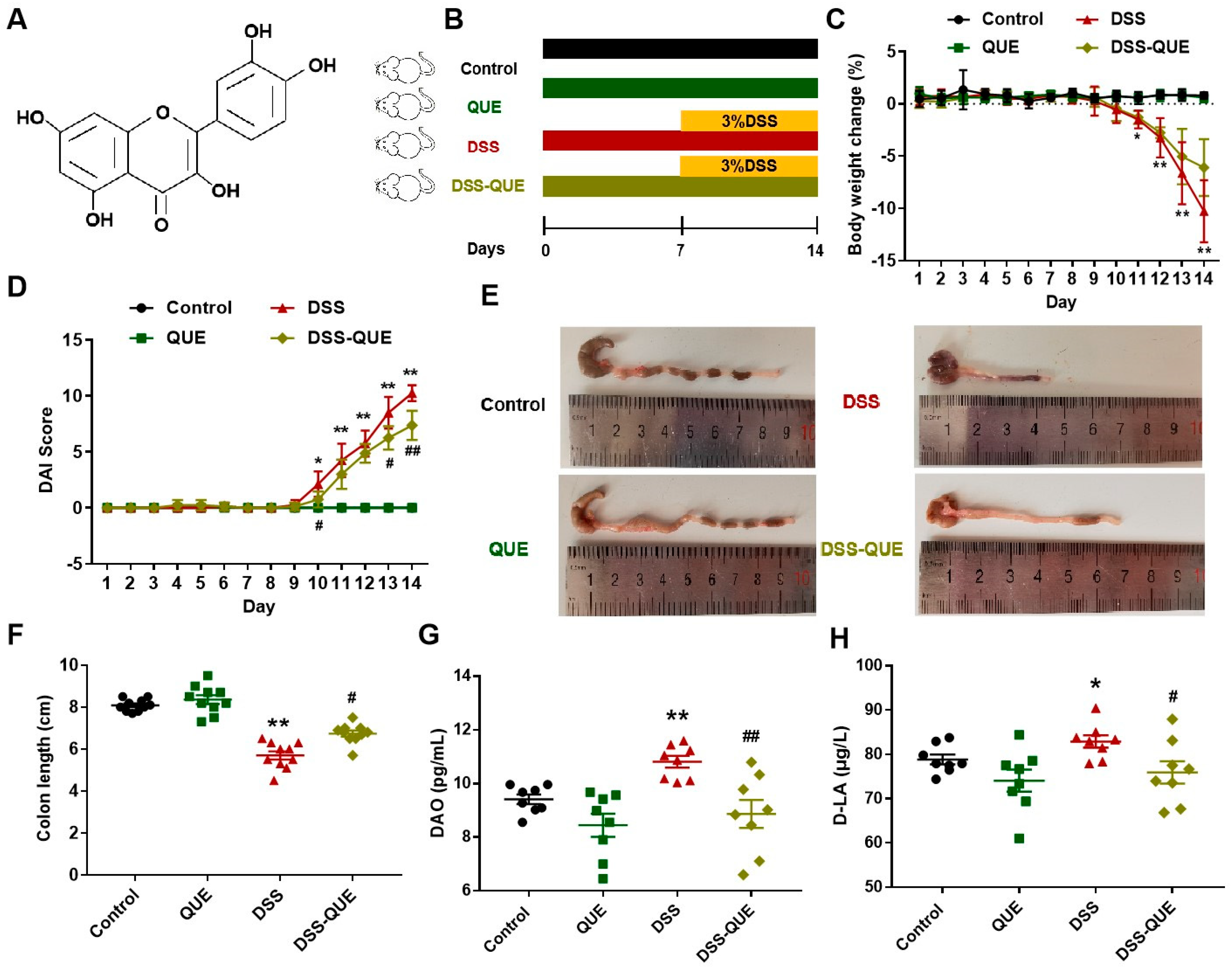
3.1. QUE Treatment Improved the Symptoms of DSS-Induced Colitis
3.2. QUE Alleviated Colonic Tissue Damage Caused by DSS and Protected the Intestinal Barrier
3.3. QUE Could Reduce DSS-Induced Inflammatory Cytokine Secretion and Inhibit Macrophage Polarization
3.4. QUE Ameliorates DSS-Induced Oxidative Stress in the Colon of Mice
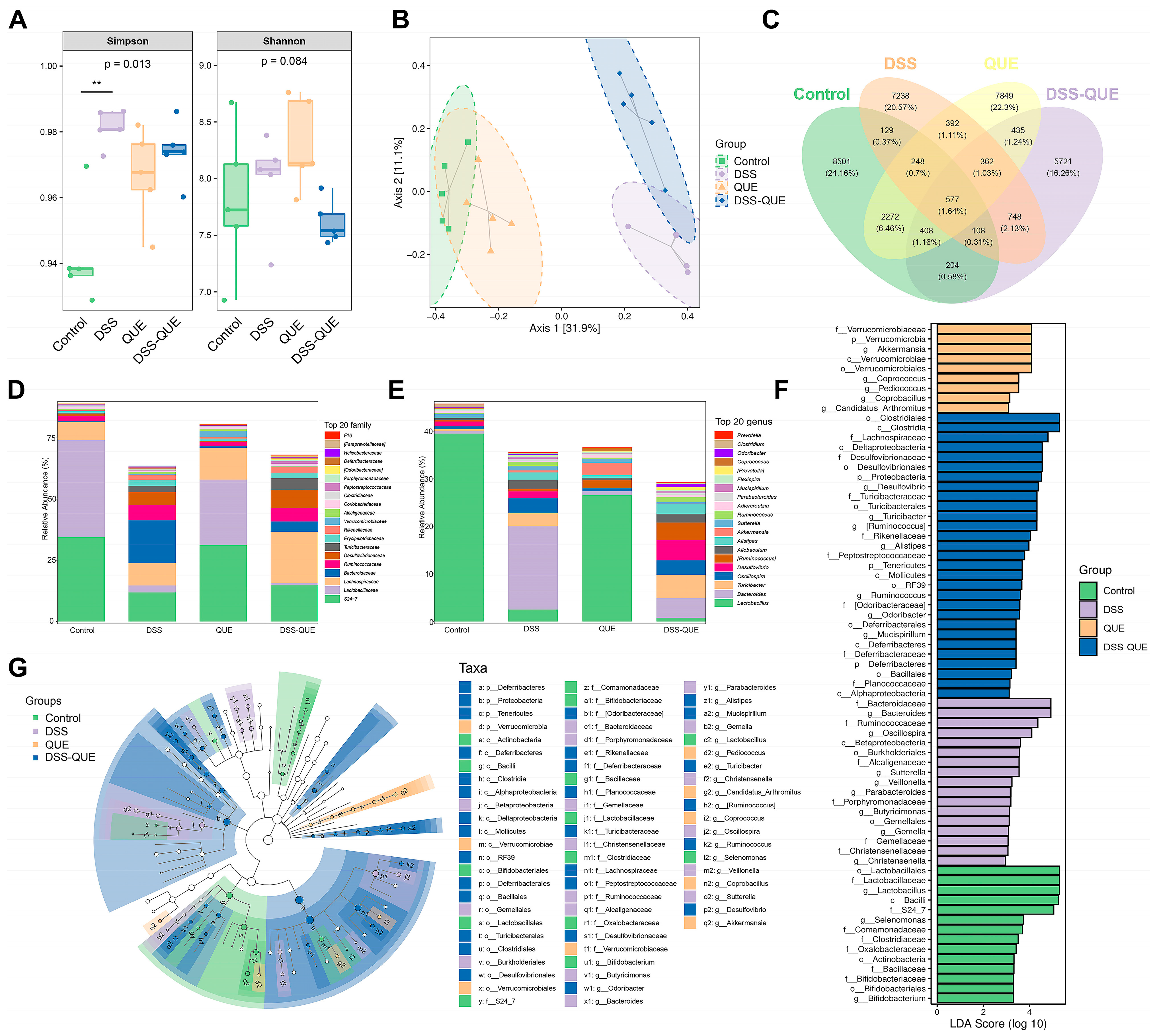
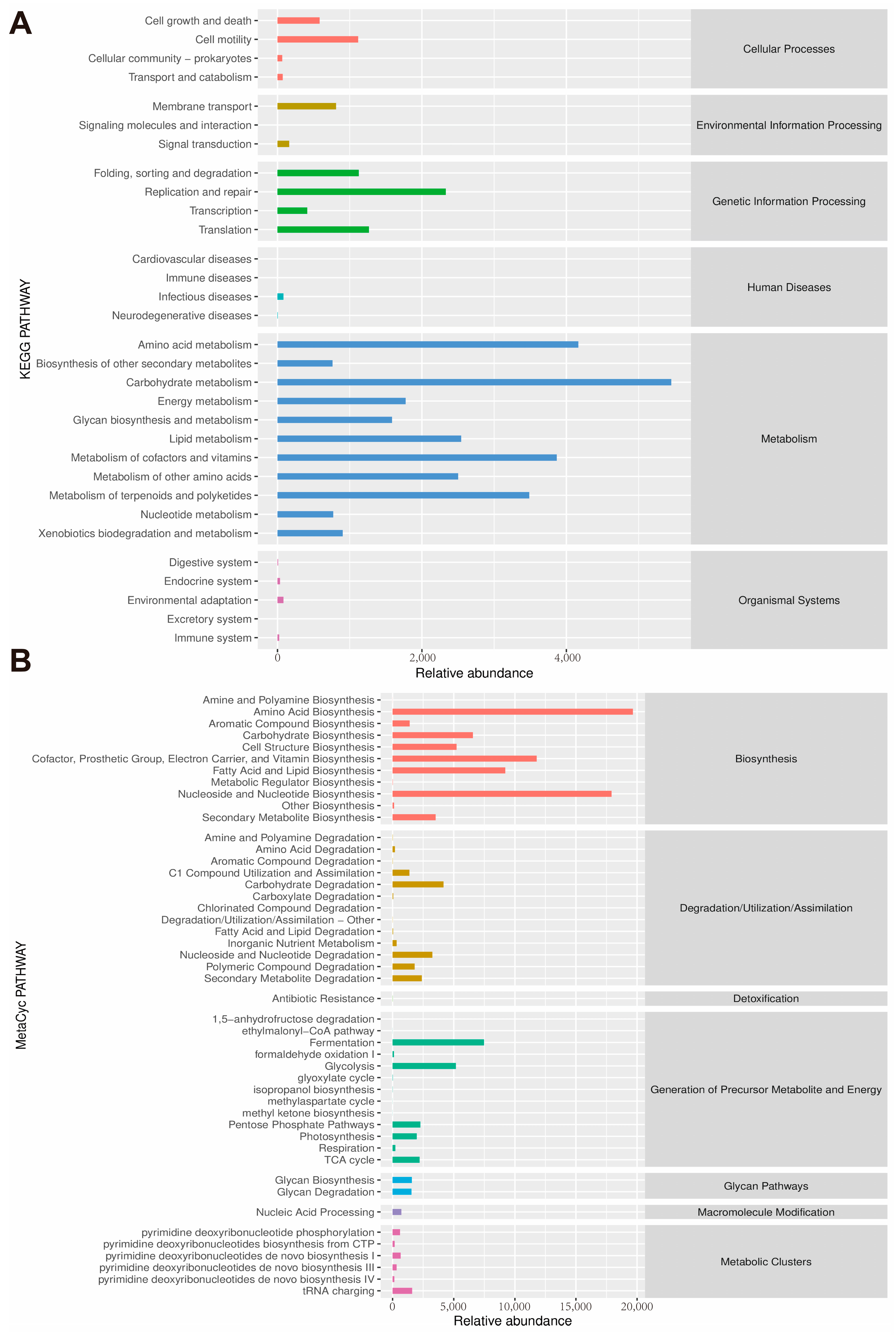
3.5. Regulation of Gut Microbiota Composition by QUE
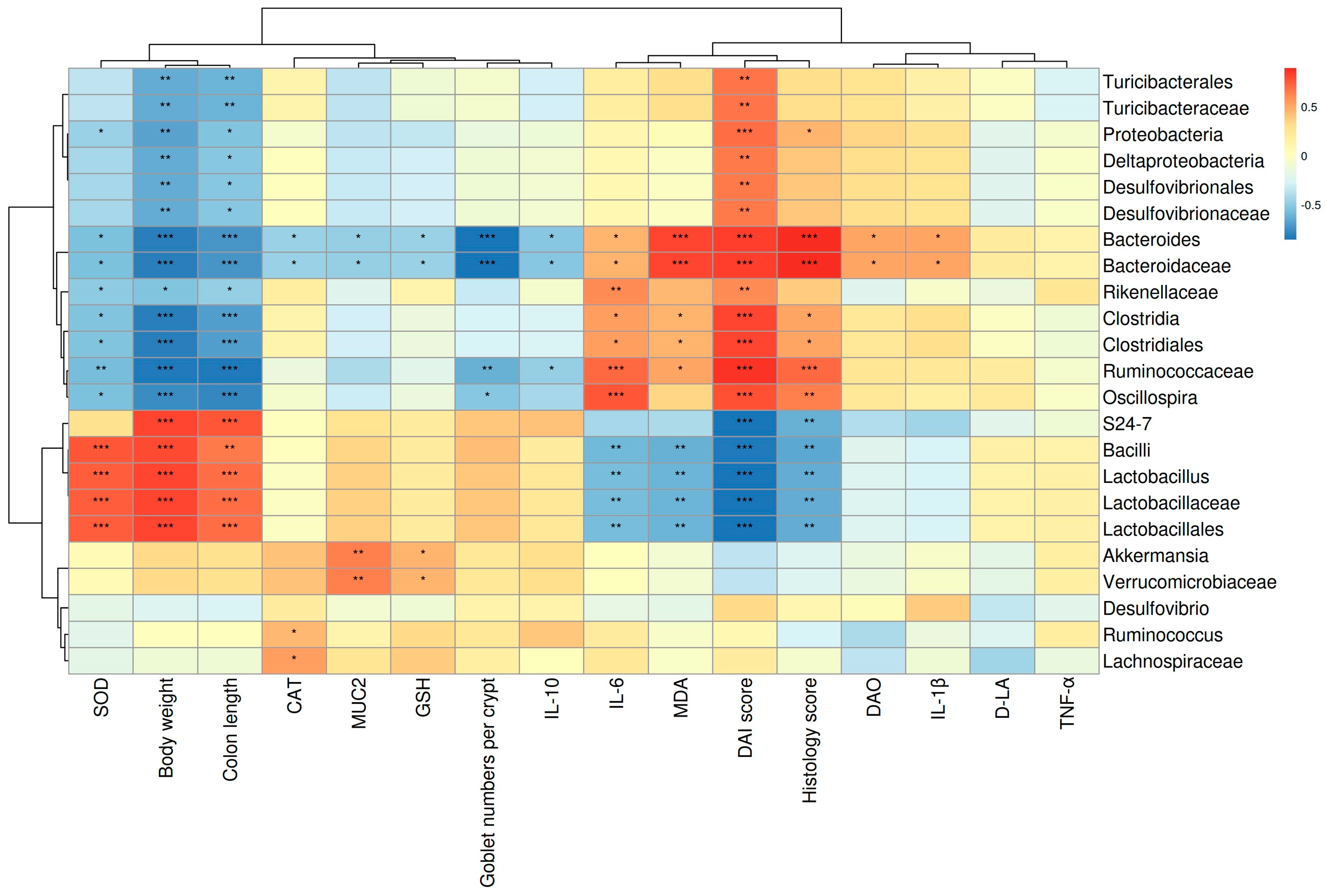
3.6. The Correlations between Gut Microbes and Micro-Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eom, T.; Kim, Y.S.; Choi, C.H.; Sadowsky, M.J.; Unno, T. Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease. J. Microbiol. 2018, 56, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Rana, A.; Ahlawat, A.; Bijjem, K.R.; Kumar, P. Animal models of inflammatory bowel disease: A review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Hou, Y.; Yin, Y.; Qiu, Y.; Wu, G.; Hu, C.A. Roles of amino acids in preventing and treating intestinal diseases: Recent studies with pig models. Amino Acids 2017, 49, 1277–1291. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.; Chan, F.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, B.; Ross, R.P.; Jin, Y.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Orally Administered CLA Ameliorates DSS-Induced Colitis in Mice via Intestinal Barrier Improvement, Oxidative Stress Reduction, and Inflammatory Cytokine and Gut Microbiota Modulation. J. Agric. Food Chem. 2019, 67, 13282–13298. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, D.; Leszczynska, K.; Gorska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)-A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Yuan, Z.W.; Qu, C.; Yu, X.T.; Huang, T.; Chen, P.V.; Su, Z.R.; Dou, Y.X.; Wu, J.Z.; Zeng, H.F.; et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol. Res. 2018, 137, 34–46. [Google Scholar] [CrossRef]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Radulovic, K.; Normand, S.; Rehman, A.; Delanoye-Crespin, A.; Chatagnon, J.; Delacre, M.; Waldschmitt, N.; Poulin, L.F.; Iovanna, J.; Ryffel, B.; et al. A dietary flavone confers communicable protection against colitis through NLRP6 signaling independently of inflammasome activation. Mucosal Immunol. 2018, 11, 811–819. [Google Scholar] [CrossRef]
- Abron, J.D.; Singh, N.P.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS ONE 2018, 13, e199631. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kumar, J.M.; Sistla, R. Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: Relevance of NF-kappaB signaling. J. Nutr. Biochem. 2016, 28, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Wang, F.; Cui, S.X.; Qu, X.J. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol. Ther. 2018, 19, 735–744. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Ahn, H.J.; Ji, G.E. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J. Agric. Food Chem. 2010, 58, 10886–10892. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Dong, Y.S.; Wang, J.L.; Feng, D.Y.; Qin, H.Z.; Wen, H.; Yin, Z.M.; Gao, G.D.; Li, C. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int. J. Med. Sci. 2014, 11, 282–290. [Google Scholar] [CrossRef]
- Dong, K.F.; Huo, M.Q.; Sun, H.Y.; Li, T.K.; Li, D. Mechanism of Astragalus membranaceus in the treatment of laryngeal cancer based on gene co-expression network and molecular docking. Sci. Rep. 2020, 10, 11184. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, Z.; Ma, H.; Yue, Y.; Hao, K.; Li, J.; Xiang, Y.; Min, Y. Quercetin alleviates intestinal inflammation and improves intestinal functions via modulating gut microbiota composition in LPS-challenged laying hens. Poult. Sci. 2023, 102, 102433. [Google Scholar] [CrossRef]
- Li, B.; Yan, Y.; Zhang, T.; Xu, H.; Wu, X.; Yao, G.; Li, X.; Yan, C.; Wu, L.L. Quercetin reshapes gut microbiota homeostasis and modulates brain metabolic profile to regulate depression-like behaviors induced by CUMS in rats. Front. Pharmacol. 2024, 15, 1362464. [Google Scholar] [CrossRef]
- Gao, F.; Zhu, F.; Shuai, B.; Wu, M.; Wei, C.; Yuan, Y.; Gui, Y.; Tian, Y.; Fan, H.; Wu, H. Quercetin ameliorates ulcerative colitis by restoring the balance of M2/M1 and repairing the intestinal barrier via downregulating cGAS–STING pathway. Front. Pharmacol. 2024, 15, 1351538. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Li, Y.; Xie, X.; Huang, S.; Pan, S.; Zou, Y.; Pan, Z.; Wang, Q.; Chen, J.; et al. Quercetin ameliorates ulcerative colitis by activating aryl hydrocarbon receptor to improve intestinal barrier integrity. Phytother. Res. 2024, 38, 253–264. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter rodentium-Infected Mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Kihara, N.; de la Fuente, S.G.; Fujino, K.; Takahashi, T.; Pappas, T.N.; Mantyh, C.R. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 2003, 52, 713–719. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, J.L.; Kang, J.Q.; Guo, S.B.; Zhang, N.; Shang, L.; Zhang, Y.L.; Zhang, J.; Jiang, X.; Lin, Y. Astragaloside IV Alleviates the Experimental DSS-Induced Colitis by Remodeling Macrophage Polarization Through STAT Signaling. Front. Immunol. 2021, 12, 740565. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Hu, M.; Zhang, L.; Gao, Y.; Ma, L.; Xu, Q. Dietary Taxifolin Protects Against Dextran Sulfate Sodium-Induced Colitis via NF-kappaB Signaling, Enhancing Intestinal Barrier and Modulating Gut Microbiota. Front. Immunol. 2020, 11, 631809. [Google Scholar]
- Li, X.; Jin, Q.; Yao, Q.; Xu, B.; Li, Z.; Tu, C. Quercetin attenuates the activation of hepatic stellate cells and liver fibrosis in mice through modulation of HMGB1-TLR2/4-NF-kappaB signaling pathways. Toxicol. Lett. 2016, 261, 1–12. [Google Scholar] [CrossRef]
- Liang, W.; Luo, Z.; Ge, S.; Li, M.; Du, J.; Yang, M.; Yan, M.; Ye, Z.; Luo, Z. Oral administration of quercetin inhibits bone loss in rat model of diabetic osteopenia. Eur. J. Pharmacol. 2011, 670, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Tsukano, K.; Nakatsuji, H.; Suzuki, K. Plasma diamine oxidase activity decline with diarrhea severity in calves indicating systemic dysfunction related to intestinal mucosal damage. Res. Vet. Sci. 2019, 126, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, H.; Weng, M.; Jiang, S.; Gao, J. Diagnostic and Clinical Significance of Serum Levels of D-Lactate and Diamine Oxidase in Patients with Crohn’s Disease. Gastroenterol. Res. Pract. 2019, 2019, 8536952. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Gren, S.T.; Grip, O. Role of Monocytes and Intestinal Macrophages in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1992–1998. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Wang, C.; Baer, H.M.; Gaya, D.R.; Nibbs, R.; Milling, S. Can molecular stratification improve the treatment of inflammatory bowel disease? Pharmacol. Res. 2019, 148, 104442. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar] [CrossRef]
- Gilardi, D.; Fiorino, G.; Genua, M.; Allocca, M.; Danese, S. Complementary and alternative medicine in inflammatory bowel diseases: What is the future in the field of herbal medicine? Expert Rev. Gastroenterol. Hepatol. 2014, 8, 835–846. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Abdollahi, M. Induction of clinical response and remission of inflammatory bowel disease by use of herbal medicines: A meta-analysis. World J. Gastroenterol. 2013, 19, 5738–5749. [Google Scholar] [CrossRef]
- Annese, V.; Rogai, F.; Settesoldi, A.; Bagnoli, S. PPARgamma in Inflammatory Bowel Disease. PPAR Res. 2012, 2012, 620839. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Buller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, S.M.; Qin, X.; Lu, Q.; Feketeova, E.; Palange, D.C.; Dong, W.; Sheth, S.U.; Lee, M.A.; Reino, D.; Xu, D.Z.; et al. Loss of the intestinal mucus layer in the normal rat causes gut injury but not toxic mesenteric lymph nor lung injury. Shock 2010, 34, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, M. Gut reaction. Nature 2018, 563, S34–S35. [Google Scholar] [CrossRef]
- Poritz, L.S.; Harris, L.R.; Kelly, A.A.; Koltun, W.A. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig. Dis. Sci. 2011, 56, 2802–2809. [Google Scholar] [CrossRef]
- Tanaka, H.; Takechi, M.; Kiyonari, H.; Shioi, G.; Tamura, A.; Tsukita, S. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut 2015, 64, 1529–1538. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Madsen, K.; Doyle, J.; Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2009, 58, 41–48. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, Y.; Gao, W.; Jiang, B.; Shi, L. Capparis spinosa Alleviates DSS-Induced Ulcerative Colitis via Regulation of the Gut Microbiota and Oxidative Stress. Evid.-Based Complement. Altern. Med. 2021, 2021, 1227876. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.J.; van Gent, D.; Ferguson, L.R. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J. Gastroenterol. 2013, 19, 3931–3941. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; Alcaide, A.; Avila-Roman, J.; Garcia-Maurino, S.; Vendramini-Costa, D.; Motilva, V. Expression patterns of sirtuin 1-AMPK-autophagy pathway in chronic colitis and inflammation-associated colon neoplasia in IL-10-deficient mice. Int. Immunopharmacol. 2016, 35, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Hamidzadeh, K.; Christensen, S.M.; Dalby, E.; Chandrasekaran, P.; Mosser, D.M. Macrophages and the Recovery from Acute and Chronic Inflammation. Annu. Rev. Physiol. 2017, 79, 567–592. [Google Scholar] [CrossRef]
- Hunter, M.M.; Wang, A.; Parhar, K.S.; Johnston, M.J.; Van Rooijen, N.; Beck, P.L.; McKay, D.M. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 2010, 138, 1395–1405. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, J.; Nie, Y.; Shi, X.; Liu, Y.; Li, F.; Zhang, X.L. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol. Investig. 2014, 43, 638–652. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 5, 992–1006. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 23, 6544–6560. [Google Scholar] [CrossRef]
- El-Akabawy, G.; El-Sherif, N.M. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2019, 111, 841–851. [Google Scholar] [CrossRef]
- Arda-Pirincci, P.; Aykol-Celik, G. Galectin-1 reduces the severity of dextran sulfate sodium (DSS)-induced ulcerative colitis by suppressing inflammatory and oxidative stress response. Bosnian J. Basic Med. Sci. 2020, 20, 319–328. [Google Scholar]
- Cortese-Krott, M.M.; Pullmann, D.; Feelisch, M. Nitrosopersulfide (SSNO(-)) targets the Keap-1/Nrf2 redox system. Pharmacol. Res. 2016, 113, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Choubey, P.; Kwatra, M.; Pandey, S.N.; Kumar, D.; Dwivedi, D.K.; Rajput, P.; Mishra, A.; Lahkar, M.; Jangra, A. Ameliorative effect of fisetin against lipopolysaccharide and restraint stress-induced behavioral deficits via modulation of NF-kappaB and IDO-1. Psychopharmacology 2019, 236, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Kim, N.S.; Kim, Y.R.; Yang, C.; Jung, I.C.; Jang, I.S.; Seo, C.S.; Choi, J.J.; Lee, M.Y. Antidepressant and Anti-Neuroinflammatory Effects of Bangpungtongsung-San. Front. Pharmacol. 2020, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.J. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, H.; Zhang, W.; Wang, J.; Liu, Z.; Jing, J.; Han, L.; Gao, A. Probiotics ameliorate benzene-induced systemic inflammation and hematopoietic toxicity by inhibiting Bacteroidaceae-mediated ferroptosis. Sci. Total Environ. 2023, 899, 165678. [Google Scholar] [CrossRef]
- Jing, W.; Dong, S.; Luo, X.; Liu, J.; Wei, B.; Du, W.; Yang, L.; Luo, H.; Wang, Y.; Wang, S.; et al. Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol. Res. 2021, 164, 105358. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. Ebiomedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker, N.M. Increased Systolic and Diastolic Blood Pressure Is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, M.; Wu, W.; Yang, W.; Huang, X.; Xiao, Y.; Ma, C.; Xu, L.; Yao, S.; Liu, Z.; et al. Microbiota Metabolite Butyrate Differentially Regulates Th1 and Th17 Cells’ Differentiation and Function in Induction of Colitis. Inflamm. Bowel Dis. 2019, 25, 1450–1461. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Peng, J.; Ma, X.; Liang, Z.; Salekdeh, G.H.; Ke, Q.; Shen, W.; Yan, Z.; Li, H.; Wang, S.; et al. Network Analysis of Gut Microbial Communities Reveals Key Reason for Quercetin Protects against Colitis. Microorganisms 2024, 12, 1973. https://doi.org/10.3390/microorganisms12101973
Lv Y, Peng J, Ma X, Liang Z, Salekdeh GH, Ke Q, Shen W, Yan Z, Li H, Wang S, et al. Network Analysis of Gut Microbial Communities Reveals Key Reason for Quercetin Protects against Colitis. Microorganisms. 2024; 12(10):1973. https://doi.org/10.3390/microorganisms12101973
Chicago/Turabian StyleLv, Yanan, Jing Peng, Xiaoyu Ma, Zeyi Liang, Ghasem Hosseini Salekdeh, Qunhua Ke, Wenxiang Shen, Zuoting Yan, Hongsheng Li, Shengyi Wang, and et al. 2024. "Network Analysis of Gut Microbial Communities Reveals Key Reason for Quercetin Protects against Colitis" Microorganisms 12, no. 10: 1973. https://doi.org/10.3390/microorganisms12101973






