Clostridioides difficile Infections in Children: What Is the Optimal Laboratory Diagnostic Method?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Study Design
2.2. C. difficile Laboratory Diagnostic Tests
2.3. Statistical Analysis
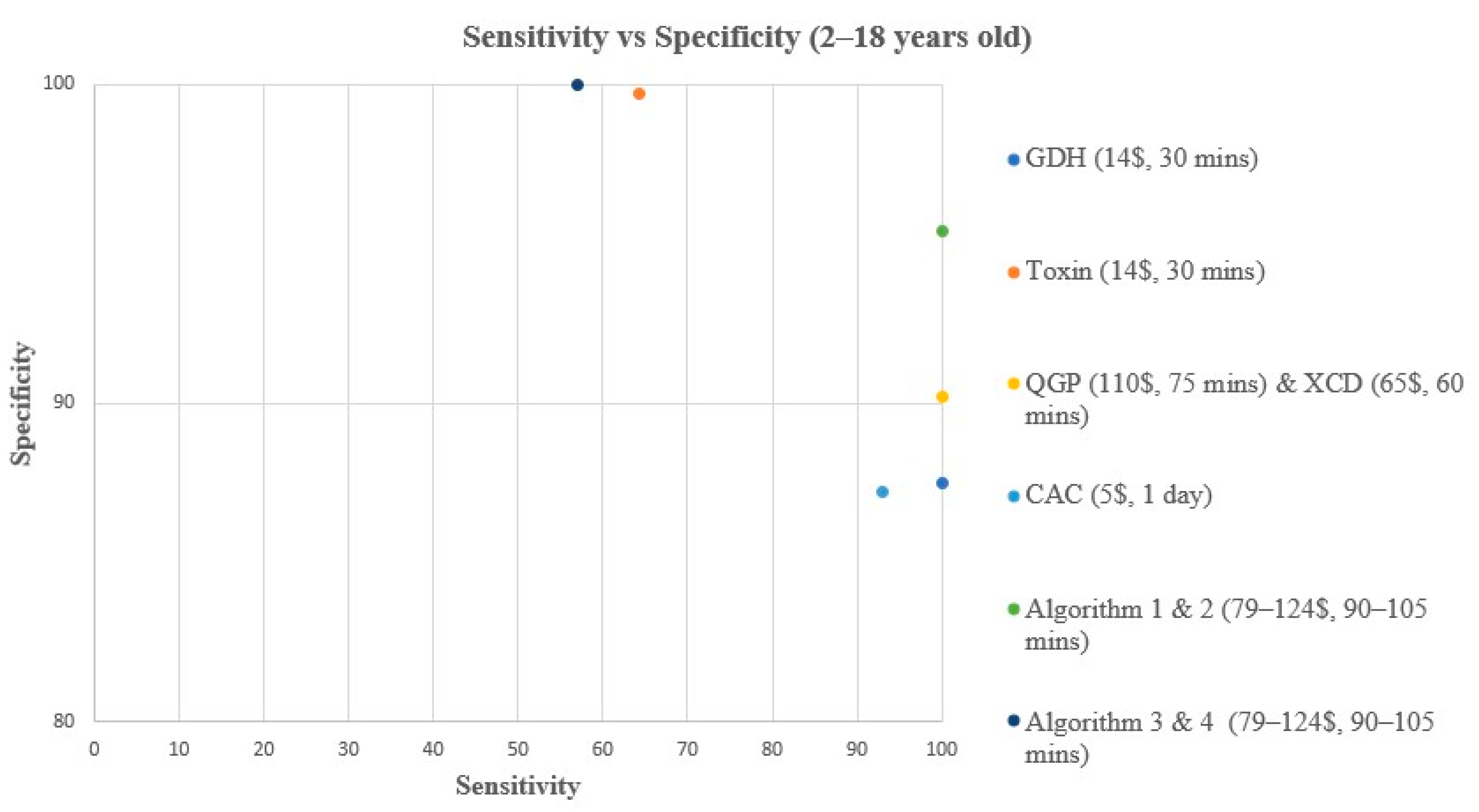
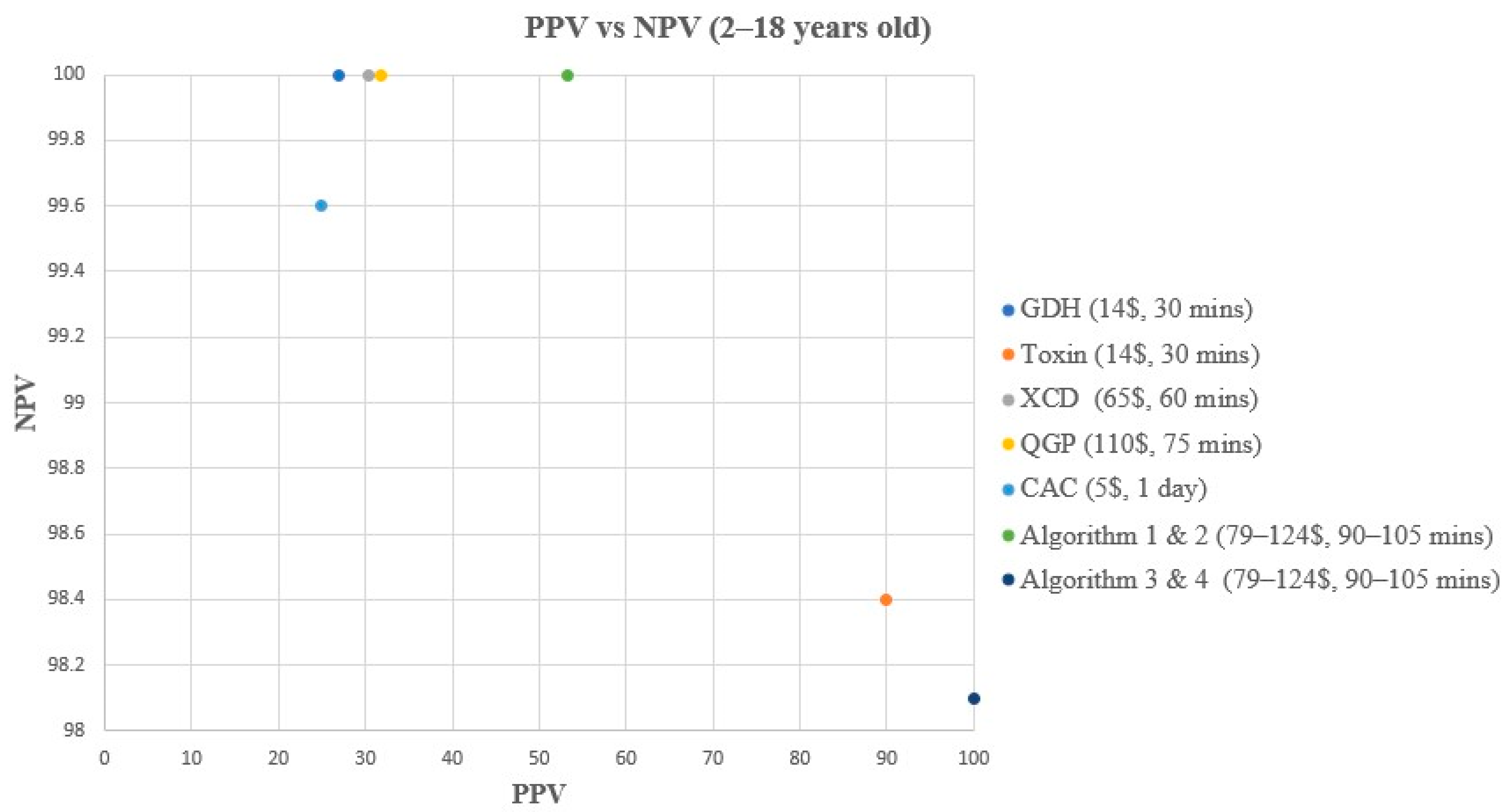
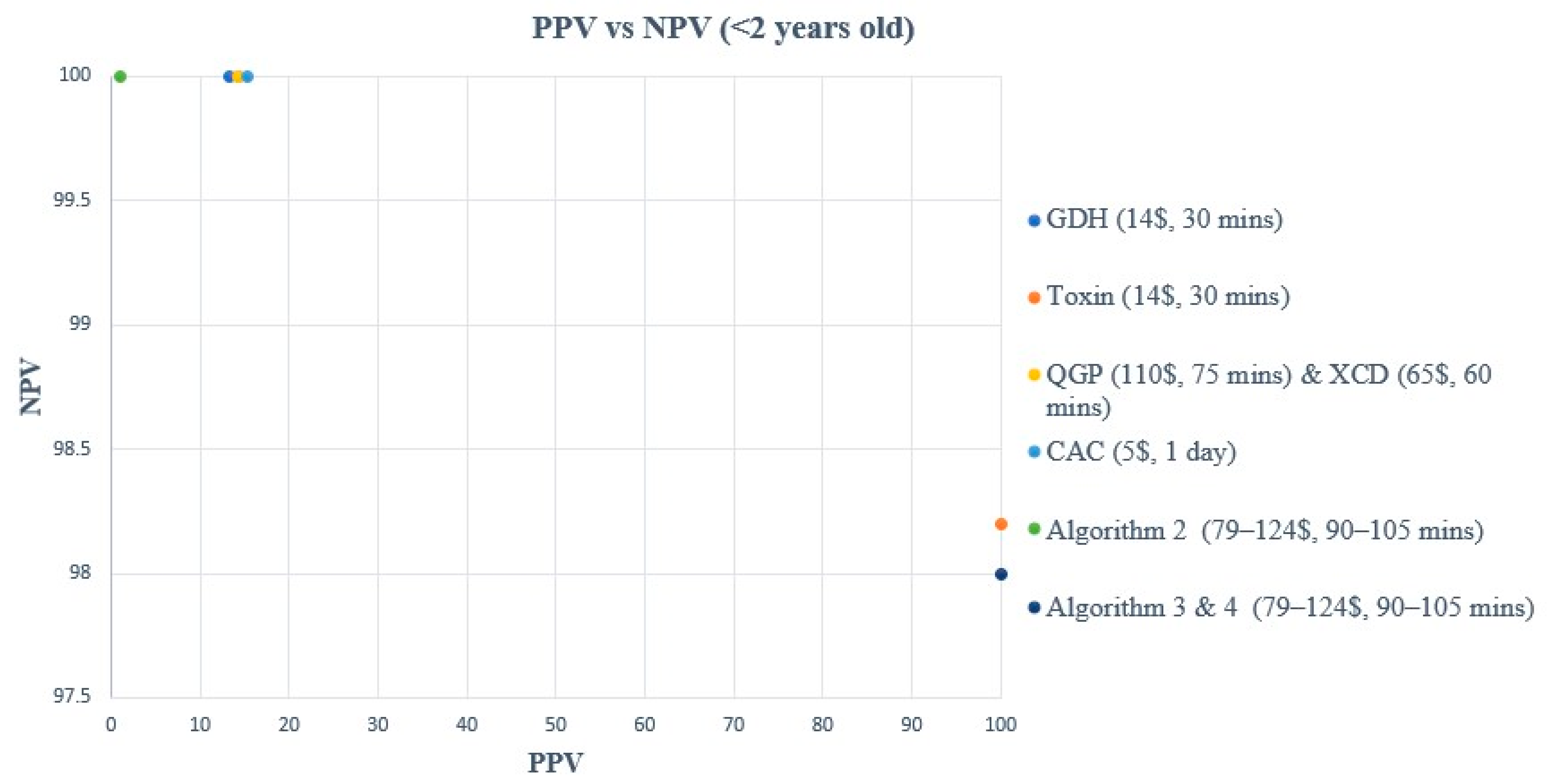
3. Results
3.1. Samples and Demographics
3.2. Performance Characteristics Analysis

4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartlett, J.G.; Chang, T.W.; Gurwith, M.; Gorbach, S.L.; Onderdonk, A.B. Antibiotic-Associated Pseudomembranous Colitis Due to Toxin-Producing Clostridia. N. Engl. J. Med. 1978, 298, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Gnocchi, M.; Gagliardi, M.; Gismondi, P.; Gaiani, F.; De’ Angelis, G.L.; Esposito, S. Updated Management Guidelines for Clostridioides Difficile in Paediatrics. Pathogens 2020, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- CDC. Clostridioides difficile Infection (CDI) Tracking. Available online: https://www.cdc.gov/healthcare-associated-infections/php/haic-eip/cdiff.html (accessed on 7 February 2023).
- Clayton, J.A.; Toltzis, P. Recent Issues in Pediatric Clostridium difficile Infection. Curr. Infect. Dis. Rep. 2017, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile Infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Dubberke, E.R.; Olsen, M.A. Burden of Clostridium difficile on the Healthcare System. Clin. Infect. Dis. 2012, 55, S88–S92. [Google Scholar] [CrossRef]
- Marra, F.; Ng, K. Controversies Around Epidemiology, Diagnosis and Treatment of Clostridium difficile Infection. Drugs 2015, 75, 1095–1118. [Google Scholar] [CrossRef]
- Tougas, S.R.; Lodha, N.; Vandermeer, B.; Lorenzetti, D.L.; Tarr, P.I.; Tarr, G.A.M.; Chui, L.; Vanderkooi, O.G.; Freedman, S.B. Prevalence of Detection of Clostridioides difficile among Asymptomatic Children: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2021, 175, e212328. [Google Scholar] [CrossRef]
- Tullus, K.; Aronsson, B.; Marcus, S.; Möllby, R. Intestinal Colonization with Clostridium Difficile in Infants up to 18 Months of Age. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 390–393. [Google Scholar] [CrossRef]
- Rousseau, C.; Lemée, L.; Le Monnier, A.; Poilane, I.; Pons, J.-L.; Collignon, A. Prevalence and Diversity of Clostridium difficile Strains in Infants. J. Med. Microbiol. 2011, 60, 1112–1118. [Google Scholar] [CrossRef]
- Humphries, R.M. Laboratory Tests for the Diagnosis of Clostridium difficile Infections. Clin. Microbiol. Newsl. 2012, 34, 151–157. [Google Scholar] [CrossRef]
- Schwenk, H.T.; Pollock, N.R.; Vaughan-Malloy, A.M. Pediatric Clostridioides difficile Infection: Diagnosis and Diagnostic Stewardship. J. Pediatr. Infect. Dis. Soc. 2021, 10, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wang, C.; Xia, Y.; Tang, J.; Wang, J.; Shen, L. Clostridioides difficile Infection in Inflammatory Bowel Disease: A Clinical Review. Expert Rev. Anti-Infect. Ther. 2024, 22, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.V.; McGowan, K.L. Clostridium difficile Testing Algorithms Using Glutamate Dehydrogenase Antigen and C. difficile Toxin Enzyme Immunoassays with C. difficile Nucleic Acid Amplification Testing Increase Diagnostic Yield in a Tertiary Pediatric Population. J. Clin. Microbiol. 2012, 50, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.; Putsathit, P.; Knight, D.R.; Sammels, L.; Riley, T.V.; Keil, A. Clostridium difficile Infection Diagnosis in a Paediatric Population: Comparison of Methodologies. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Boyanton, B.L., Jr.; Mehta, S.; Courtney, E.M.; Webb, C.R.; Revell, P.A.; Versalovic, J. Rapid Stool-Based Diagnosis of Clostridium difficile Infection by Real-Time PCR in a Children’s Hospital. J. Clin. Microbiol. 2011, 49, 851–857. [Google Scholar] [CrossRef]
- Gomez, E.J.; Montgomery, S.; Alby, K.; Robinson, D.P.; Roundtree, S.S.; Blecker-Shelly, D.; Sullivan, K.V. Poor Yield of Clostridium difficile Testing Algorithms Using Glutamate Dehydrogenase Antigen and C. Difficile Toxin Enzyme Immunoassays in a Pediatric Population with Declining Prevalence of Clostridium difficile Strain BI/NAP1/027. Diagn. Microbiol. Infect. Dis. 2018, 91, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Toltzis, P.; Kim, J.; Dul, M.; Zoltanski, J.; Smathers, S.; Zaoutis, T. Presence of the Epidemic North American Pulsed Field Type 1 Clostridium difficile Strain in Hospitalized Children. J. Pediatr. 2009, 154, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, S.B.; Gripka, M.; Estes, K.; Nguyen, A.; Jackson, M.A.; Selvarangan, R. Detection of Toxigenic Clostridium difficile in Pediatric Stool Samples: An Evaluation of Quik Check Complete Antigen Assay, BD GeneOhm Cdiff PCR, and ProGastro Cd PCR Assays. Diagn. Microbiol. Infect. Dis. 2011, 71, 224–229. [Google Scholar] [CrossRef]
- Kociolek, L.K.; Kutty, P.K.; Polgreen, P.M.; Beekmann, S.E. Healthcare Provider Diagnostic Testing Practices for Identification of Clostridioides (Clostridium) difficile in Children: An Emerging Infections Network Survey. Infect. Control Hosp. Epidemiol. 2019, 40, 276–280. [Google Scholar] [CrossRef]
- Tenover, F.C.; Novak-Weekley, S.; Woods, C.W.; Peterson, L.R.; Davis, T.; Schreckenberger, P.; Fang, F.C.; Dascal, A.; Gerding, D.N.; Nomura, J.H.; et al. Impact of Strain Type on Detection of Toxigenic Clostridium difficile: Comparison of Molecular Diagnostic and Enzyme Immunoassay Approaches. J. Clin. Microbiol. 2010, 48, 3719–3724. [Google Scholar] [CrossRef] [PubMed]
- Kociolek, L.K.; Patel, S.J.; Shulman, S.T.; Gerding, D.N. Molecular Epidemiology of Clostridium difficile Infections in Children: A Retrospective Cohort Study. Infect. Control Hosp. Epidemiol. 2015, 36, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.N.; Leber, A.; Chapin, K.; Fey, P.D.; Bankowski, M.J.; Jones, M.K.; Rogatcheva, M.; Kanack, K.J.; Bourzac, K.M. Multicenter Evaluation of the BioFire FilmArray Gastrointestinal Panel for Etiologic diagnosis of Infectious Gastroenteritis. J. Clin. Microbiol. 2015, 53, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zhu, H.; Rodriguez, A.; Mhaissen, M.; Schultz-Cherry, S.; Adderson, E.; Hayden, R.T. Comparative Evaluation of Broad-Panel PCR Assays for the Detection of Gastrointestinal Pathogens in Pediatric Oncology Patients. J. Mol. Diagn. 2015, 17, 715–721. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, X.; Zhu, M.; Yin, H.; Tang, J.; Huang, Y.; Zheng, B.; Jin, Y.; Liu, Z. The Application Research of xTAG GPP Multiplex PCR in the Diagnosis of Persistent and Chronic Diarrhea in Children. BMC Pediatr. 2020, 20, 309. [Google Scholar] [CrossRef] [PubMed]
- Buchan, B.W.; Mackey, T.L.A.; Daly, J.A.; Alger, G.; Denys, G.A.; Peterson, L.R.; Kehl, S.C.; Ledeboer, N.A. Multicenter Clinical Evaluation of the Portrait Toxigenic C. difficile Assay for Detection of Toxigenic Clostridium difficile Strains in Clinical Stool Specimens. J. Clin. Microbiol. 2012, 50, 3932–3936. [Google Scholar] [CrossRef]
- Elgendy, S.G.; Aly, S.A.; Fathy, R.; Deaf, E.A.E.; Abu Faddan, N.H.; Hameed, M.R.A. Clinical and Microbial Characterization of Toxigenic Clostridium difficile Isolated from Antibiotic Associated Diarrhea in Egypt. Iran. J. Microbiol. 2020, 12, 296–304. [Google Scholar] [CrossRef]
- Beckmann, C.; Heininger, U.; Marti, H.; Hirsch, H.H. Gastrointestinal Pathogens Detected by Multiplex Nucleic Acid Amplification Testing in Stools of Pediatric Patients and Patients Returning from the Tropics. Infection 2014, 42, 961–970. [Google Scholar] [CrossRef]
- Koyuncu-Ozyurt, O.; Ozhak, B.; Ogunc, D.; Ongut, G.; Gunseren, F.; Donmez, L.; Colak, D. Evaluation of a Nucleic Acid Amplification Assay for the Diagnosis of Clostridioides difficile Infection. Anaerobe 2019, 59, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, J.; Soma, V.L.; Rosen, L.; Ginocchio, C.C.; Rubin, L.G. Similar Proportions of Stool Specimens from Hospitalized Children with and without Diarrhea Test Positive for Clostridium difficile. Pediatr. Infect. Dis. J. 2015, 34, 261–266. [Google Scholar] [CrossRef]
- Mashock, M.J.; Faron, M.L.; Carroll, K.C.; Dang, C.; Lewis, S.; Salimnia, H.; Lephart, P.; Loo, V.G.; Schmitt, B.H.; Young, S.; et al. A Multicenter Study of the Revogene C. difficile System for Detection of the Toxin B Gene from Unformed Stool Specimens. J. Clin. Microbiol. 2020, 58, e01510-19. [Google Scholar] [CrossRef] [PubMed]
- Alghounaim, M.; Longtin, Y.; Gonzales, M.; Merckx, J.; Winters, N.; Quach, C. Clostridium difficile Infections in Children: Impact of the Diagnostic Method on Infection Rates. Infect. Control Hosp. Epidemiol. 2016, 37, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, H.; Morin, S.B.N.; Lloyd, C.D.; Parsons, B.; Stokowski, T.; Xie, J.; Zhuo, R.; Lee, B.E.; Pang, X.L.; Freedman, S.B.; et al. Comparative Evaluation of Luminex xTAG® Gastrointestinal Pathogen Panel and Direct-From-Stool Real-Time PCR for Detection of C. difficile Toxin tcdB in Stool Samples from a Pediatric Population. Microorganisms 2022, 10, 2214. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, C.; Rogatcheva, M.; Harrel, B.; Vaughn, M.; Crisp, R.; Poritz, M.; Thatcher, S.; Korgenski, E.K.; Barney, T.; Daly, J.; et al. How Well Does Physician Selection of Microbiologic Tests Identify Clostridium difficile and Other Pathogens in Paediatric Diarrhoea? Insights Using Multiplex PCR-Based Detection. Clin. Microbiol. Infect. 2015, 21, 179.e9–179.e15. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.M.; Fazili, I.; Bloch, S.C.; Lacy, D.B.; Garcia-Lopez, V.A.; Bernard, R.; Skaar, E.P.; Edwards, K.M.; Nicholson, M.R. Two-Step Testing for Clostridioides difficile Is Inadequate in Differentiating Infection from Colonization in Children. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Planche, T.; Wilcox, M. Reference Assays for Clostridium difficile Infection: One or Two Gold Standards? J. Clin. Pathol. 2011, 64, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Brotons, P.; Saucedo, J.; Simó, S.; Gené, A.; Muñoz-Almagro, C. Performance Comparison of a Novel Rapid Stand-Alone Molecular Test and a 2-Step Diagnostic Algorithm for Clostridioides difficile Detection in Children. Pediatr. Infect. Dis. J. 2021, 40, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Sandora, T.J.; Williams, D.N.; Daugherty, K.; Geer, C.; Cuddemi, C.; Kociolek, L.K.; Chen, X.; Xu, H.; Savage, T.J.; Banz, A.; et al. Stool Toxin Concentration does not Distinguish Clostridioides difficile Infection from Colonization in Children less than 3 Years of Age. J. Pediatr. Infect. Dis. Soc. 2022, 11, 454–458. [Google Scholar] [CrossRef]
- Pahud, B.A.; Hassan, F.; Harrison, C.J.; Halasa, N.B.; Chappell, J.D.; Englund, J.A.; Klein, E.J.; Szilagyi, P.G.; Weinberg, G.A.; Sherman, A.K.; et al. Detection of Clostridioides difficile by Real-Time Pcr in Young Children does not Predict Disease. Hosp. Pediatr. 2020, 10, 555–562. [Google Scholar] [CrossRef]
- Guh, A.Y.; Hatfield, K.M.; Winston, L.G.; Martin, B.; Johnston, H.; Brousseau, G.; Farley, M.M.; Wilson, L.; Perlmutter, R.; Phipps, E.C.; et al. Toxin Enzyme Immunoassays Detect Clostridioides difficile Infection with Greater Severity and Higher Recurrence Rates. Clin. Infect. Dis. 2019, 69, 1667–1674. [Google Scholar] [CrossRef]
- Falces-Romero, I.; Troyano-Hernáez, P.; García-Bujalance, S.; Baquero-Artigao, F.; Mellado-Peña, M.J.; García-Rodríguez, J. Detection of Toxigenic Clostridium difficile in Paediatric Patients. Enferm. Infecc. Microbiol. Clin. 2018, 36, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Lamousé-Smith, E.S.; Weber, S.; Rossi, R.F.; Neinstedt, L.J.; Mosammaparast, N.; Sandora, T.J.; McAdam, A.J.; Bousvaros, A. Polymerase Chain Reaction Test for Clostridium difficile Toxin B Gene Reveals Similar Prevalence Rates in Children with and without Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, H.T.; Bio, L.L.; Kruger, J.F.; Banaei, N. Clinical Impact of Clostridium difficile PCR Cycle Threshold-Predicted Toxin Reporting in Pediatric Patients. J. Pediatr. Infect. Dis. Soc. 2019, 9, 44–50. [Google Scholar] [CrossRef]
- Senchyna, F.; Gaur, R.L.; Gombar, S.; Truong, C.Y.; Schroeder, L.F.; Banaei, N. Clostridium difficile PCR Cycle Threshold Predicts Free Toxin. J. Clin. Microbiol. 2017, 55, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
| Method | TAT | Ease of Use | Cost |
|---|---|---|---|
| GDH | 30 min | Simple, manual procedure | 11–20 USD |
| Toxin | 30 min | Simple, manual procedure | 11–20 USD |
| XCD | 60 min | Simple, automated procedure | 21–100 USD |
| QGP | 75 min | Simple, automated procedure | >100 USD |
| CAC | 24 h | Simple, manual procedure | <10 USD |
| Algorithm 1 | 90 min | Simple, manual, and automated procedure | 21–100 USD |
| Algorithm 2 | 105 min | Simple, manual, and automated procedure | >100 USD |
| Algorithm 3 | 90 min | Simple, manual, and automated procedure | 21–100 USD |
| Algorithm 4 | 105 min | Simple, manual, and automated procedure | >100 USD |
| Method | Reference | TP | TN | FP | FN | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| GDH | Composite | 14 | 267 | 38 | 0 | 100 (78.5–100) | 87.5 (83.3–90.8) | 26.9 (16.8–40.3) | 100 (98.6–100) |
| Toxin | Composite | 9 | 304 | 1 | 5 | 64.3 (38.8–83.7) | 99.7 (98.2–100) | 90 (59.6–98.2) | 98.4 (96.3–99.3) |
| XCD | Composite | 14 | 273 | 32 | 0 | 100 (78.5–100) | 89.5 (85.6–92.5) | 30.4 (19–44.8) | 100 (98.6–100) |
| QGP | Composite | 14 | 275 | 30 | 0 | 100 (78.5–100) | 90.2 (86.3–93) | 31.8 (20–45.6) | 100 (98.6–100) |
| CAC | Composite | 13 | 266 | 39 | 1 | 92.9 (68.5–98.7) | 87.2 (83–90.5) | 25 (15.2–38.2) | 99.6 (97.9–99.9) |
| Algorithm 1 | Composite | 16 | 289 | 14 | 0 | 100 (80.6–100) | 95.4 (92.4–97.2) | 53.3 (36.1–69.8) | 100 (98.7–100) |
| Algorithm 2 | Composite | 14 | 290 | 15 | 0 | 100 (78.5–100) | 95.1 (92.1–97) | 48.3 (31.4–65.6) | 100 (98.7–100) |
| Algorithm 3 | Composite | 8 | 305 | 0 | 6 | 57.1 (32.6–78.6) | 100 (98.8–100) | 100 (67.6–100) | 98.1 (95.9–99.1) |
| Algorithm 4 | Composite | 8 | 305 | 0 | 6 | 57.1 (32.6–78.6) | 100 (98.8–100) | 100 (67.6–100) | 98.1 (95.9–99.1) |
| Method | Reference | TP | TN | FP | FN | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| GDH | Composite | 2 | 40 | 13 | 0 | 100 (34.2–100) | 75.5 (62.4–85.1) | 13.3 (3.7–37.9) | 100 (91.2–100) |
| Toxin | Composite | 1 | 53 | 0 | 1 | 50 (9.5–90.6) | 100 (93.2–100) | 100 (20.7–100) | 98.2 (90.2–99.7) |
| XCD | Composite | 2 | 41 | 12 | 0 | 100 (34.2–100) | 77.4 (64.5–86.6) | 14.3 (4–39.9) | 100 (91.4–100) |
| QGP | Composite | 2 | 41 | 12 | 0 | 100 (34.2–100) | 77.4 (64.5–86.6) | 14.3 (4–39.9) | 100 (91.4–100) |
| CAC | Composite | 2 | 42 | 11 | 0 | 100 (34.2–100) | 79.3 (66.5–88) | 15.4 (4.3–42.2) | 100 (91.4–100) |
| Algorithm 1 | Composite | 0 | 45 | 10 | 0 | NA | 81.8 (69.7–89.8) | NA | 100 (92.1–100) |
| Algorithm 2 | Composite | 2 | 45 | 8 | 0 | 100 (34.2–100) | 84.9 (73–92.1) | 20 (5.7–51) | 100 (92.1–100) |
| Algorithm 3 | Composite | 1 | 53 | 0 | 1 | 50 (9.5–90.6) | 100 (93.2–100) | 100 (20.7–100) | 98.2 (90.2–99.7) |
| Algorithm 4 | Composite | 1 | 53 | 0 | 1 | 50 (9.5–90.6) | 100 (93.2–100) | 100 (20.7–100) | 98.2 (90.2–99.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleiman, M.; Tang, P.; Imam, O.; Morales, P.; Altrmanini, D.; Roberts, J.C.; Pérez-López, A. Clostridioides difficile Infections in Children: What Is the Optimal Laboratory Diagnostic Method? Microorganisms 2024, 12, 1785. https://doi.org/10.3390/microorganisms12091785
Suleiman M, Tang P, Imam O, Morales P, Altrmanini D, Roberts JC, Pérez-López A. Clostridioides difficile Infections in Children: What Is the Optimal Laboratory Diagnostic Method? Microorganisms. 2024; 12(9):1785. https://doi.org/10.3390/microorganisms12091785
Chicago/Turabian StyleSuleiman, Mohammed, Patrick Tang, Omar Imam, Princess Morales, Diyna Altrmanini, Jill C. Roberts, and Andrés Pérez-López. 2024. "Clostridioides difficile Infections in Children: What Is the Optimal Laboratory Diagnostic Method?" Microorganisms 12, no. 9: 1785. https://doi.org/10.3390/microorganisms12091785





