Volatile-Mediated Inhibitory Activity of Rhizobacteria as a Result of Multiple Factors Interaction: The Case of Lysobacter capsici AZ78
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Fungi, Growth Media and Conditions
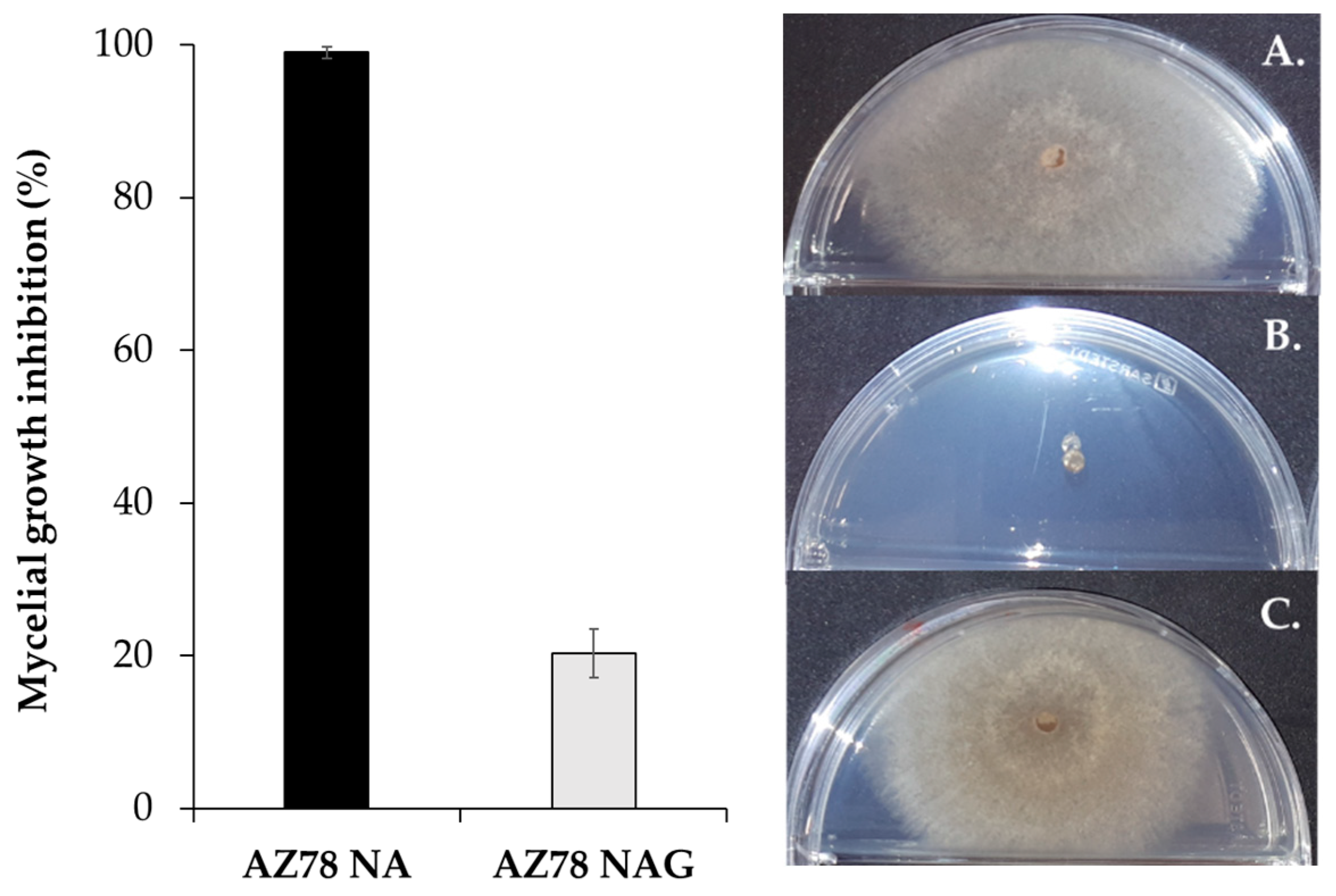
2.2. Inhibition Assay against Rhizoctonia solani with Lysobacter capsici AZ78 Grown on Protein and Sugar-Rich Growth Media
2.3. Analysis of Volatile Organic Compounds Produced by Lysobacter capsici AZ78 on Protein-Rich and Sugar-Rich Growth Media Using Gas Chromatography-Mass Spectrometry
2.3.1. Preparation of Split Petri Dishes for GC–MS Profiling
2.3.2. DHS-TD-GC-MS Analysis
2.4. Measurement of Ammonia in the Headspace of Bacterial Cultures
2.5. Targeted Gas Chromatography-Flame Ionization Analysis of Volatile Methyl Amines Produced by Lysobacter capsici AZ78
2.6. Inhibition Assay Using Phosphoric Acid “Trap”
2.7. Inhibition Assay with Ammonia in the Vapor Phase
2.8. pH Measurement of PDA Medium
2.9. Inhibition Assay Using Alkalinized PDA
2.10. Assay Testing Multiple Factors Hypothesis
2.11. Statistical Analysis
3. Results
3.1. Lysobacter Capsici AZ78 Volatiles Produced on Protein-Rich Medium Significantly Inhibit the Growth of Rhizoctonia solani
3.2. Pyrazines Are Involved in the Inhibition of Rhizoctonia solani Growth by Lysobacter capsici AZ78 Grown on Protein-Rich Medium
3.3. Ammonia Contributes to the Inhibition of Rhizoctonia solani Growth by Lysobacter capsici AZ78 Grown on Protein-Rich Medium
3.4. Alkaline PDA Medium Impedes the Growth of Rhizoctonia solani
3.5. Alkaline PDA, Ammonia and VOC May Jointly Contribute to the Inhibition of Rhizoctonia solani Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De la Pena, C.; Lei, Z.; Watson, B.S.; Sumner, L.W.; Vivanco, J.M. Root microbe communication through protein secretion. J. Biol. Chem. 2008, 283, 25247–25255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, R.; Cordovez, V.; De Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 2329–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyc, O.; Song, X.; Dickschat, S.; Vos, M.; Garbeva, P. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Fiddaman, P.; Rossall, S. Effect of substrate on the production of antifungal volatiles from Bacillus subtilis. J. Appl. Microbiol. 1994, 76, 395–405. [Google Scholar] [CrossRef]
- Weise, T.; Kai, M.; Gummesson, A.; Troeger, A.; von Reuss, S.; Piepenborn, S.; Kosterka, F.; Sklorz, M.; Zimmermann, R.; Francke, W.; et al. Volatile organic compounds produced by the phytopathogenic bacterium Xanthomonas campestris pv. vesicatoria 85–10. Beilstein J. Org. Chem. 2012, 8, 579–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazazzara, V.; Perazzolli, M.; Pertot, I.; Biasioli, F.; Puopolo, G.; Cappellin, L. Growth media affect the volatilome and antimicrobial activity against Phytophthora infestans in four Lysobacter type strains. Microbiol. Res. 2017, 201, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zareian, M.; Silcock, P.; Bremer, P. Effect of medium compositions on microbially-mediated volatile organic compounds release profile. J. Appl. Microbiol. 2018, 125, 813–827. [Google Scholar] [CrossRef]
- Garbeva, P.; Hordijk, C.; Gerards, S.; de Boer, W. Volatiles produced by the mycophagous soil bacterium Collimonas. FEMS Microbiol. Ecol. 2014, 87, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Brescia, F.; Marchetti-Deschmann, M.; Musetti, R.; Perazzolli, M.; Pertot, I.; Puopolo, G. The rhizosphere signature on the cell motility, biofilm formation and secondary metabolite production of a plant-associated Lysobacter strain. Microbiol. Res. 2020, 234, 126424. [Google Scholar] [CrossRef]
- Veselova, M.A.; Plyutaa, V.A.; Khmela, I.A. Volatile compounds of bacterial origin: Structure, biosynthesis, and biological activity. Microbiology 2019, 88, 272–287. [Google Scholar] [CrossRef]
- Agisha, V.N.; Kumarb, A.; Eapen, S.J.; Sheoranb, N.; Suseelabhai, R. Broad-spectrum antimicrobial activity of volatile organic compounds from endophytic Pseudomonas putida BP25 against diverse plant pathogens. Biocontrol Sci. Technol. 2019, 29, 1069–1089. [Google Scholar] [CrossRef]
- Mülner, P.; Bergna, A.; Wagner, P.; Sarajliæ, D.; Gstöttenmayr, B.; Dietel, K.; Grosch, R.; Cernava, T.; Berg, G. Microbiota associated with sclerotia of soilborne fungal pathogens—A novel source of biocontrol agents producing bioactive volatiles. Phytobiomes J. 2019, 3, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Smiley, R.W.; Cook, R.J.; Papendick, R.I. Anhydrous ammonia as a soil fungicide against Fusarium and fungicidal activity in the ammonia retention zone. Phytopathology 1970, 60, 1227–1232. [Google Scholar] [CrossRef]
- Strock, J.S. Ammonification. In Encyclopedia of Ecology; Elsevier Inc.: Amsterdam, The Netherlands, 2008; Volume 5, pp. 162–165. [Google Scholar] [CrossRef]
- Katilie, C.J.; Simon, A.G.; De Greeff, L.E. Quantitative analysis of vaporous ammonia by online derivatization with gas chromatography—Mass spectrometry with applications to ammonium nitrate-based explosives. Talanta 2019, 193, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Crespo, E.; Cristescu, S.M.; Harren, F.J.M.; Francke, W.; Piechulla, B. Serratia odorifera: Analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 2010, 88, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Weise, T.; Kai, M.; Piechulla, B. Bacterial ammonia causes significant plant growth inhibition. PLoS ONE 2013, 8, e63538. [Google Scholar] [CrossRef]
- Avalos, M.; Garbeva, P.; Raaijmakers, J.M.; van Wezel, G.P. Production of ammonia as a low-cost and long-distance antibiotic strategy by Streptomyces species. ISME J. 2019, 1–15. [Google Scholar] [CrossRef]
- Philippot, L.; Germon, J.C. Contribution of bacteria to initial input and cycling of nitrogen in soils. In Microorganisms in Soils: Roles in Genesis and Functions; Buscot, F., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3, pp. 159–176. [Google Scholar]
- Ko, W.H.; Hora, F.K.; Herlicska, E. Isolation and identification of a volatile fungistatic substance from alkaline soil. Phytopathology 1974, 64, 1398–1400. [Google Scholar] [CrossRef]
- Pavlica, D.A.; Hora, T.S.; Bradshaw, J.J.; Skogerboe, R.K.; Baker, R. Volatile compounds from soil influencing activities of soil fungi. Phytopathology 1978, 68, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Schippers, B.; Meijer, J.W.; Liem, J. Effect of ammonia and other soil volatiles on germination and growth of soil fungi. Trans. Br. Mycol. Soc. 1982, 79, 253–259. [Google Scholar] [CrossRef]
- Tsao, P.H.; Oster, J.J. Relation of ammonia and nitrous acid to suppression of Phytophthora in soils amended with nitrogenous organic substances. Phytopathology 1981, 71, 53–59. [Google Scholar] [CrossRef]
- Huang, H.C.; Erickson, R.S.; Chang, C.; Moye, J.R.; Larney, F.J.; Huang, J.W. Organic soil amendments for control of apothecial production of Sclerotinia sclerotiorum. Plant Pathol. Bull. 2002, 11, 207–214. [Google Scholar]
- Howell, C.R.; Beier, C.W.; Stipanovic, R.D. Production of ammonia by Enterobacter cloacae and its possible role in biological control of Pythium pre-emergence damping-off by the bacterium. Phytopathology 1988, 78, 1075–1078. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Handelsman, J.; Parke, J.L. Role of ammonia and calcium in lysis of zoospores of Phytophthora cactorum by Bacillus cereus strain UW85. Exp. Mycol. 1990, 14, 1–8. [Google Scholar] [CrossRef]
- Baligh, M.; Conway, K.E.; Delgado, M.A. Production of ammonia by Pseudomonas cepacia and Pseudomonas aeruginosa: Quantification and effect on host and pathogen. In Recent Research Developments in Plant Pathology; Pandalai, S.G., Ed.; Research Signpost: Trivandrum, India, 1996; Volume 1, pp. 7–19. [Google Scholar]
- Trivedi, P.; Pandy, A.; Palni, L.M. In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol. Res. 2008, 163, 329–336. [Google Scholar] [CrossRef]
- Jeong, J.H.; Park, K.H.; Hwang, D.Y.; Kim, H.S.; Lee, C.Y.; Son, H.J. Keratinolytic enzyme-mediated biodegradation of recalcitrant feather by a newly isolated Xanthomonas sp. P5. Polym. Degrad. Stab. 2010, 95, 1969–1977. [Google Scholar] [CrossRef]
- Kim, B.H.; Gadd, G.M. Bacterial Physiology and Metabolism; Cambridge University Press: New York, NY, USA, 2008; pp. 214–220. [Google Scholar] [CrossRef]
- Lockwood, J.L. Fungistasis in soils. Biol. Rev. 1977, 52, 1–43. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Baath, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blagodatskaya, E.V.; Anderson, T.H. Interactive effects of pH and substrate quality on the fungal-to-bacterial ratio and qCO2 of microbial communities in forest soils. Soil Biol. Biochem. 1998, 30, 1269–1274. [Google Scholar] [CrossRef]
- Baath, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Ingold, C.T.; Hudson, H.J. The Biology of Fungi, 6th ed.; Chapman and Hall: Devon, UK, 1993; Available online: https://books.google.com (accessed on 6 March 2020).
- Moore-Landecker, E.; Stotzky, G. Inhibition of fungal growth and sporulation by volatile metabolites from bacteria. Can. J. Microbiol. 1972, 18, 957–962. [Google Scholar] [CrossRef]
- Reichenbach, H. The Genus Lysobacter. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 6, pp. 939–957. [Google Scholar] [CrossRef]
- Postma, J.; Schilder, M.T.; Bloem, J.; van Leeuwen-Haagsma, W.K. Soil suppressiveness and functional diversity of the soil microflora in organic farming systems. Soil Biol. Biochem. 2008, 40, 2394–2406. [Google Scholar] [CrossRef] [Green Version]
- Iwata, K.; Azlan, A.; Yamakawa, H.; Omori, T. Ammonia accumulation in culture broth by the novel nitrogen-fixing bacterium, Lysobacter sp. E4. J. Biosci. Bioeng. 2010, 110, 415–418. [Google Scholar] [CrossRef]
- Puopolo, G.; Tomada, S.; Pertot, I. The impact of the omics era on the knowledge and use of Lysobacter species to control phytopathogenic microorganisms. J. Appl. Microbiol. 2018, 124, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Puopolo, G.; Sonego, P.; Engelen, K.; Pertot, I. Draft genome sequence of Lysobacter capsici AZ78, a bacterium antagonistic to plant-pathogenic oomycetes. Genome Announc. 2014, 2, e00325-14. [Google Scholar] [CrossRef] [Green Version]
- Tomada, S.; Sonego, P.; Moretto, M.; Engelen, K.; Pertot, I.; Perazzolli, M.; Puopolo, G. Dual RNA-Seq of Lysobacter capsici AZ78 - Phytophthora infestans interaction shows the implementation of attack strategies by the bacterium and unsuccessful oomycete defense responses. Environ. Microbiol. 2017, 19, 4113–4125. [Google Scholar] [CrossRef]
- Puopolo, G.; Cimmino, A.; Palmieri, M.C.; Giovannini, O.; Evidente, A.; Pertot, I. Lysobacter capsici AZ78 produces cyclo(L-Pro- L-Tyr), a 2,5-diketopiperazine with toxic activity against sporangia of Phytophthora infestans and Plasmopara viticola. J. Appl. Microbiol. 2014, 117, 1168–1180. [Google Scholar] [CrossRef]
- Puopolo, G.; Tomada, S.; Sonego, P.; Moretto, M.; Engelen, K.; Perazzolli, M.; Pertot, I. The Lysobacter capsici AZ78 genome has a gene pool enabling it to interact successfully with phytopathogenic microorganisms and environmental factors. Front. Microbiol. 2016, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Vlassi, A.; Nesler, A.; Perazzolli, M.; Lazazzara, V.; Büschl, C.; Parich, A.; Puopolo, G.; Schuhmacher, R. Volatile organic compounds from Lysobacter capsici AZ78 as potential candidates for biological control of soilborne plant pathogens. Front. Microbiol. 2020, 11, 1748. [Google Scholar] [CrossRef]
- Hiller, K.; Hangebrauk, J.; Jäger, C.; Spura, J.; Schreiber, K.; Schomburg, D. MetaboliteDetector: Comprehensive analysis tool for targeted and nontargeted GC/MS based metabolome analysis. Anal. Chem. 2009, 8, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Gallois, A.; Grimont, P. Pyrazines responsible for the potatolike odor produced by some Serratia and Cedecea strains. Appl. Environ. Microbiol. 1985, 50, 1048–1051. [Google Scholar] [CrossRef] [Green Version]
- Blazenovic, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabololites 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Neyer, P.; Bernasconi, L.; Fuchs, J.A.; Allenspach, M.D.; Steuer, C. Derivatization-free determination of short-chain volatile amines in human plasma and urine by headspace gas chromatography-mass spectrometry. J. Clin. Lab. Anal. 2020, 34, e23062. [Google Scholar] [CrossRef]
- Létoffé, S.; Audrain, B.; Bernier, S.P.; Delepierre, M.; Ghigo, J.-M. Aerial exposure to the bacterial volatile compound trimethylamine modifies antibiotic resistance of physically separated bacteria by raising culture medium pH. mBio 2014, 5, e00944-13. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, F.; Bain, R.; Mc Quilken, M. Effects of nutrient status, temperature and pH on mycelial growth, sclerotial production and germination of Rhizoctonia solani from potato. J. Plant Pathol. 2009, 91, 589–596. [Google Scholar]
- Jurtshuk, P., Jr. Bacterial Metabolism. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. Available online: https://www.ncbi.nlm.nih.gov/books/NBK7919/ (accessed on 27 May 2020).
- Barker, H.A. Amino acid degradation by anaerobic bacteria. Annu. Rev. Biochem. 1981, 50, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Niven, C.F., Jr.; Smnley, K.L.; Sherma, J.M. The hydrolysis of arginine by streptococci. J. Bacteriol. 1942, 43, 651–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özogul, F.; Özogul, Y. The ability of biogenic amines and ammonia production by single bacterial cultures. Eur. Food Res. Technol. 2007, 225, 385–394. [Google Scholar] [CrossRef]
- Bernier, S.P.; Létoffé, S.; Delepierre, M.; Ghigo, J.-M. Biogenic ammonia modifies antibiotic resistance at a distance in physically separated bacteria. Mol. Microbiol. 2011, 81, 705–716. [Google Scholar] [CrossRef]
- Fan, T.W.-M.; Lane, A.N.; Shenker, M.; Bartley, J.P.; Crowley, D.; Higashi, R.M. Comprehensive chemical profiling of gramineous plant root exudates using high-resolution NMR and MS. Phytochemistry 2001, 57, 209–221. [Google Scholar] [CrossRef]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Azarova, T.; Makarova, N.; Lugtenberg, B. Organic acids, sugars and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol. Plant Microbe Interact. 2006, 9, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef] [Green Version]
- Raaijmakers, J.M.; Paulitz, C.T.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Moe, L.A. Amino acids in the rhizosphere: From plants to microbes. Am. J. Bot. 2013, 100, 1692–1705. [Google Scholar] [CrossRef]
- Winkler, F.K. Amt/MEP/Rh proteins conduct ammonia. Pflug. Arch. Eur. J. Physiol. 2006, 451, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Plant, P.J.; Manolson, M.F.; Grinstein, S.; Demaurex, N. Alternative mechanisms of vacuolar acidification in H+-ATPase-deficient yeast. J. Biol. Chem. 1999, 274, 37270–37279. [Google Scholar] [CrossRef] [Green Version]
- Hesse, S.J.A.; Ruijter, G.J.G.; Dijkema, C.; Visser, J. Intracellular pH homeostasis in the filamentous fungus Aspergillus niger. Eur. J. Biochem. 2002, 269, 3485–3494. [Google Scholar] [CrossRef]
- Kane, P.M. Proton transport and pH control in fungi. In Advances in Experimental Medicine and Biology, Yeast Membrane Transport; Ramos, J., Sychrová, H., Kschischo, M., Eds.; Springer: Dordrecht, Switzerland, 2016; Volume 892, pp. 33–68. [Google Scholar] [CrossRef] [Green Version]
- Marini, A.M.; Soussi-Boudekou, S.; Vissers, S.; Andre, B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997, 17, 4282–4293. [Google Scholar] [CrossRef] [Green Version]
- Teichert, S.; Rutherford, J.C.; Wottawa, M.; Heitman, J.; Tudzynski, B. The impact of the ammonium permeases MepA, MepB, and MepC on nitrogen-regulated secondary metabolism in Fusarium fujikuroi. Eukaryot. Cell 2008, 7, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Monahan, B.J.; Unkles, S.E.; Tsing, I.T.; Kinghorn, J.R.; Hynes, M.J.; Davis, M.A. Mutation and functional analysis of the Aspergillus nidulans ammonium permease MeaA and evidence for interaction with itself and MepA. Fungal Genet. Biol. 2002, 36, 35–46. [Google Scholar] [CrossRef]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.M.; Kronzucker, H.J. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef] [Green Version]
- Rush, C.M.; Lyda, S.D. Effects of anhydrous ammonia on mycelium and sclerotia of Phymatotrichum omnivorum. Phytopathology 1982, 72, 1085–1089. [Google Scholar] [CrossRef]
- De Pasquale, D.A.; Montville, T.J. Mechanism by which ammonium bicarbonate and ammonium sulfate inhibit mycotoxigenic fungi. Appl. Environ. Microbiol. 1990, 56, 3711–3717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenuta, M.; Lazarovits, G. Ammonia and nitrous acid from nitrogenous amendments kill the microsclerotia of Verticillium dahliae. Phytopathology 2002, 92, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil. Plant Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef]
- Thorn, K.A.; Mikita, M.A. Ammonia fixation by humic substances: A nitrogen-15 and carbon-13 NMR-study. Sci. Total Environ. 1992, 113, 67–87. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Nye, P.H. Changes of pH across the rhizosphere induced by roots. Plant Soil 1981, 61, 7–26. [Google Scholar] [CrossRef]

| Factor | Condition | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| NH3 a | + | + | - | - | + | + | - | - |
| VOC b | + | - | + | - | + | - | + | - |
| pH | 5.6 c | 5.6 | 5.6 | 5.6 | 8.2 d | 8.2 | 8.2 | 8.2 |
| Treatment | Mycelial Growth Inhibition (%) |
|---|---|
| AZ78 on NA medium | (99.0 ± 0.8) |
| AZ78 on NAG medium | (20.4 ± 3.2) b |
| 50 μmol NH3 | (33.0 ± 2.7) a |
| 5 μmol NH3 | (3.0 ± 0.6) c |
| Treatment | Mycelial Growth Inhibition (%) |
|---|---|
| AZ78 on NA medium | (99.0 ± 0.8) * |
| PDA pH 8.2 by NaOH | (55.4 ± 1.4) |
| PDA pH 8.2 by NH3 | 100 * |
| AZ78 on NAG medium | (20.4 ± 3.2) b |
| PDA pH 7.6 by NaOH | (21.3 ± 1.4) b |
| PDA pH 7.6 by NH3 | (35.8 ± 2.3) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlassi, A.; Nesler, A.; Parich, A.; Puopolo, G.; Schuhmacher, R. Volatile-Mediated Inhibitory Activity of Rhizobacteria as a Result of Multiple Factors Interaction: The Case of Lysobacter capsici AZ78. Microorganisms 2020, 8, 1761. https://doi.org/10.3390/microorganisms8111761
Vlassi A, Nesler A, Parich A, Puopolo G, Schuhmacher R. Volatile-Mediated Inhibitory Activity of Rhizobacteria as a Result of Multiple Factors Interaction: The Case of Lysobacter capsici AZ78. Microorganisms. 2020; 8(11):1761. https://doi.org/10.3390/microorganisms8111761
Chicago/Turabian StyleVlassi, Anthi, Andrea Nesler, Alexandra Parich, Gerardo Puopolo, and Rainer Schuhmacher. 2020. "Volatile-Mediated Inhibitory Activity of Rhizobacteria as a Result of Multiple Factors Interaction: The Case of Lysobacter capsici AZ78" Microorganisms 8, no. 11: 1761. https://doi.org/10.3390/microorganisms8111761






