Assessment of Cardiotoxicity after a Single Dose of Combretastatin A4-Phosphate in Dogs Using Two-Dimensional Speckle-Tracking Echocardiography
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Echocardiographic Measurements
2.3. Intra- and Inter-Observer Measurement Variability
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Two-Dimensional Speckle Tracking Echocardiographic Measurements
3.3. Inter- and Intra-Observer Measurement Variability
3.4. Blood Pressure and ECG Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA4P | Combretastatin A4-phosphate |
| CTRCD | Cancer therapy-related cardiac dysfunction |
| CV | Coefficient of variation |
| 2D-STE | Two-dimensional speckle tracking echocardiography |
| cTnI | Cardiac troponin I |
| GCS | Global circumferential strain |
| GLS | Global longitudinal strain |
| GRS | Global radial strain |
| LSt | Peak systolic regional longitudinal strain |
| CSt | Peak systolic regional circumferential strain |
| RSt | Peak systolic regional radial strain |
References
- Abma, E.; Daminet, S.; Smets, P.; Ni, Y.; de Rooster, H. Combretastatin A4-phosphate and its potential in veterinary oncology: A review. Vet. Comp. Oncol. 2015, 15, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abma, E.; De Spiegelaere, W.; Vanderperren, K.; Stock, E.; Van Brantegem, L.; Cornelis, I.; Daminet, S.; Ni, Y.; Vynck, M.; Verstraete, G.; et al. A single dose of intravenous combretastatin A4-phosphate is reasonably well tolerated and significantly reduces tumour vascularization in canine spontaneous cancers. Vet. Comp. Oncol. 2018, 16, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Abma, E.; Smets, P.; Daminet, S.; Cornelis, I.; De Clercq, K.; Ni, Y.; Vlerick, L.; de Rooster, H. A dose-escalation study of combretastatin A4-phosphate in healthy dogs. Vet. Comp. Oncol. 2018, 16, E16–E22. [Google Scholar] [CrossRef] [PubMed]
- Griggs, J.; Metcalfe, J.C.; Hesketh, R. Targeting tumour vasculature: The development of combretastatin A4. Lancet Oncol. 2001, 2, 82–87. [Google Scholar] [CrossRef]
- Vincent, L.; Kermani, P.; Young, L.M.; Cheng, J.; Zhang, F.; Shido, K.; Lam, G.; Bompais-Vincent, H.; Zhu, Z.; Hicklin, D.J.; et al. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J. Clin. Investig. 2005, 115, 2992–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Singh, S.B.; Hamel, E.; Lin, C.M.; Alberts, D.S.; Garcia-Kendall, D. Isolation and structure of the stong cell growth and tubulin inhibitor combretastatin A-4. Experientia 1989, 45, 209–210. [Google Scholar] [CrossRef]
- Liu, L.; O’kelly, D.; Schuetze, R.; Carlson, G.; Zhou, H.; Trawick, M.L.; Pinney, K.G.; Mason, R.P. Non-invasive evaluation of acute effects of tubulin binding agents: A review of imaging vascular disruption in tumors. Molecules 2021, 26, 2551. [Google Scholar] [CrossRef]
- Hamel, E. Antimitotic natural products and their interactions with tubulin. Med. Res. Rev. 1996, 16, 207–231. [Google Scholar] [CrossRef]
- Tozer, G.M.; Kanthou, C.; Baguley, B.C. Disrupting tumour blood vessels. Nat. Rev. Cancer 2005, 5, 423–435. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Do, D.V.; Sepah, Y.J.; Shah, S.M.; Van Anden, E.; Hafiz, G.; Donahue, J.K.; Rivers, R.; Balkissoon, J.; Handa, J.T.; et al. Vascular disrupting agent for neovascular age related macular degeneration: A pilot study of the safety and efficacy of intravenous combretastatin a-4 phosphate. BMC Pharmacol. Toxicol. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, J.P.; Rosen, M.; Sun, W.; Gallagher, M.; Haller, D.G.; Vaughn, D.; Giantonio, B.; Zimmer, R.; Petros, W.P.; Stratford, M.; et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day scedule to patients with cancer: Magnetic resonance imaging evidence for altered tumor blood flow. J. Clin. Oncol. 2003, 21, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, A.; Robertson, K.; Cooney, M.; Petros, W.P.; Stratford, M.; Jesberger, J.; Rafie, N.; Overmoyer, B.; Makkar, V.; Stambler, B.; et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin A-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002, 62, 3408–3416. [Google Scholar] [PubMed]
- Rustin, G.J.S.; Galbraith, S.M.; Anderson, H.; Stratford, M.; Folkes, L.K.; Sena, L.; Gumbrell, L.; Price, P.M. Phase I clinical trial of weekly combretastatin A4 phosphate: Clinical and pharmacokinetic results. J. Clin. Oncol. 2003, 21, 2815–2822. [Google Scholar] [CrossRef]
- Tochinai, R.; Nagata, Y.; Ando, M.; Hata, C.; Suzuki, T.; Asakawa, N.; Yoshizawa, K.; Uchida, K.; Kado, S.; Kobayashi, T.; et al. Combretastatin A4 disodium phosphate-induced myocardial injury. J. Toxicol. Pathol. 2016, 29, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Tochinai, R.; Komatsu, K.; Murakami, J.; Nagata, Y.; Ando, M.; Hata, C.; Suzuki, T.; Kado, S.; Kobayashi, T.; Kuwahara, M. Histopathological and functional changes in a single-dose model of combretastatin A4 disodium phosphate-induced myocardial damage in rats. J. Toxicol. Pathol. 2018, 31, 307–313. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Li, S.; Huang, H.; Li, Z.; Chen, L.; Ye, S.; Huang, J.; Zhan, J.; Lin, T. A pharmacokinetic and safety study of single dose intravenous combretastatin A4 phosphate in Chinese patients with refractory solid tumours. Br. J. Clin. Pharmacol. 2011, 71, 860–870. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, I.M.; Lenihan, D.J.; Tsimberidou, A.M. Cardiovascular Toxicity Profiles of Vascular-Disrupting Agents. Oncologist 2011, 16, 1120–1130. [Google Scholar] [CrossRef] [Green Version]
- Bhakta, S.; Flick, S.M.; Cooney, M.M.; Greskovich, J.F.; Gilkeson, R.C.; Remick, S.C.; Ortiz, J. Myocardial stunning following combined modality combretastatin-based chemotherapy: Two case reports and review of the literature. Clin. Cardiol. 2009, 32, E80–E84. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 23, E333–E465. [Google Scholar] [CrossRef]
- Hamabe, L.; Mandour, A.S.; Shimada, K.; Uemura, A.; Yilmaz, Z.; Nagaoka, K.; Tanaka, R. Role of two-dimensional speckle-tracking echocardiography in early detection of left ventricular dysfunction in dogs. Animals 2021, 11, 2361. [Google Scholar] [CrossRef] [PubMed]
- Blessberger, H.; Binder, T. Two dimensional speckle tracking echocardiography: Basic principles. Heart 2010, 96, 716–722. [Google Scholar] [CrossRef]
- Bijnens, B.H.; Cikes, M.; Claus, P.; Sutherland, G.R. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur. J. Echocardiogr. 2009, 10, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Dandel, M.; Hetzer, R. Echocardiographic strain and strain rate imaging—Clinical applications. Int. J. Cardiol. 2009, 132, 11–24. [Google Scholar] [CrossRef]
- Biswas, M.; Sudhakar, S.; Nanda, N.C.; Buckberg, G.; Pradhan, M.; Roomi, A.U.; Gorissen, W.; Houle, H. Two- and three-dimensional speckle tracking echocardiography: Clinical applications and future directions. Echocardiography 2013, 30, 88–105. [Google Scholar] [CrossRef]
- Chetboul, V. Advanced techniques in echocardiography in small animals. Vet. Clin. N. Am.-Small Anim. Pract. 2010, 40, 529–543. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC CardioOncol. 2020, 2, 677–689. [Google Scholar] [CrossRef]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM consensus statement: Guidelines for identification, evaluation and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 2018, 32, 1803–1822. [Google Scholar] [CrossRef] [PubMed]
- Chetboul, V.; Serres, F.; Gouni, V.; Tissier, R.; Pouchelon, J.L. Radial strain and strain rate by two-dimensional speckle tracking echocardiography and the tissue velocity based technique in the dog. J. Vet. Cardiol. 2007, 9, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wess, G.; Keller, L.J.M.; Klausnitzer, M.; Killich, M.; Hartmann, K. Comparison of longitudinal myocardial tissue velocity, strain, and strain rate measured by two-dimensional speckle tracking and by color tissue Doppler imaging in healthy dogs. J. Vet. Cardiol. 2011, 13, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, B.H.; Helle-Valle, T.; Edvardsen, T.; Torp, H.; Crosby, J.; Lyseggen, E.; Støylen, A.; Ihlen, H.; Lima, J.A.C.; Smiseth, O.A.; et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 2006, 47, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandel, M.; Lehmkuhl, H.; Knosalla, C.; Suramelashvili, N.; Hetzer, R. Strain and Strain Rate Imaging by Echocardiography—Basic Concepts and Clinical Applicability. Curr. Cardiol. Rev. 2009, 5, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Payne, E.E.; Roberts, B.K.; Schroeder, N.; Burk, R.L.; Schermerhorn, T. Assessment of a point-of-care cardiac troponin I test to differentiate cardiac from noncardiac causes of respiratory distress in dogs. J. Vet. Emerg. Crit. Care 2011, 21, 217–225. [Google Scholar] [CrossRef]
- Siemann, D.W.; Chaplin, D.J.; Walicke, P.A. A review and update of the current status of the Vasculature Disabling Agent Combretastatin-a4 phosphate (CA4P). Expert Opin. Investig. Drugs 2013, 18, 189–197. [Google Scholar] [CrossRef]
- Gould, S.; Westwood, F.R.; Curwen, J.O.; Ashton, S.E.; Roberts, D.W.; Lovick, S.C.; Ryan, A.J. Effect of pretreatment with atenolol and nifedipine on ZD6126-induced cardiac toxicity in rats. J. Natl. Cancer Inst. 2007, 99, 1724–1728. [Google Scholar] [CrossRef] [Green Version]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.; Elkind, M.S.V.; Rundek, T.; Homma, S.; Sacco, R.L.; Di Tullio, M.R. Prevalence and Prognostic Value of Subclinical Left Ventricular Systolic Dysfunction by Global Longitudinal Strain in a Community-Based Cohort. Eur. J. Heart Fail. 2014, 16, 1301–1309. [Google Scholar] [CrossRef]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mandoli, G.E.; Sciaccaluga, C.; Mondillo, S. More than 10 years of speckle tracking echocardiography: Still a novel technique or a definite tool for clinical practice? Echocardiography 2019, 36, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2021, 77, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Reant, P.; Labrousse, L.; Lafitte, S.; Bordachar, P.; Pillois, X.; Tariosse, L.; Bonoron-Adele, S.; Padois, P.; Deville, C.; Roudaut, R.; et al. Experimental Validation of Circumferential, Longitudinal, and Radial 2-Dimensional Strain During Dobutamine Stress Echocardiography in Ischemic Conditions. J. Am. Coll. Cardiol. 2008, 51, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Steeds, R.P. Echocardiography: Frontier imaging in cardiology. Br. J. Radiol. 2011, 84, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Negishi, T.; Thavendiranathan, P.; Negishi, K.; Marwick, T.H.; Aakhus, S.; Murbræch, K.; Massey, R.; Bansal, M.; Fukuda, N.; Hristova, K.; et al. Rationale and Design of the Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes: The SUCCOUR Trial. JACC Cardiovasc. Imaging 2018, 11, 1098–1105. [Google Scholar] [CrossRef]
- Surachetpong, S.D.; Teewasutrakul, P.; Rungsipipat, A. Serial measurements of cardiac troponin I (cTnI) in dogs treated with doxorubicin. Jpn. J. Vet. Res. 2016, 64, 221–233. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Luis, S.A.; Chan, J.; Pellikka, P.A. Echocardiographic Assessment of Left Ventricular Systolic Function: An Overview of Contemporary Techniques, Including Speckle-Tracking Echocardiography. Mayo Clin. Proc. 2019, 94, 125–138. [Google Scholar] [CrossRef]
- Kusunose, K.; Zhang, Y.; Mazgalev, T.N.; Thomas, J.D.; Popovic, Z.B. Left ventricular strain distribution in healthy dogs and in dogs with tachycardia-induced dilated cardiomyopathy. Cardiovasc. Ultrasound 2013, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Matsumoto, H.; Teshima, T.; Koyama, H. Clinical assessment of systolic myocardial deformations in dogs with chronic mitral valve insufficiency using two-dimensional speckle-tracking echocardiography. J. Vet. Cardiol. 2013, 15, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, S.; Mirea, O.; Bézy, S.; Duchenne, J.; Pagourelias, E.D.; Bogaert, J.; Thomas, J.D.; Badano, L.P.; Voigt, J.U.; Hamilton, J.; et al. Inter-vendor variability in strain measurements depends on software rather than image characteristics. Int. J. Cardiovasc. Imaging 2021, 37, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wright, L.; Negishi, T.; Negishi, K.; Liu, J.; Marwick, T.H. Research to Practice: Assessment of Left Ventricular Global Longitudinal Strain for Surveillance of Cancer Chemotherapeutic-Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1196–1201. [Google Scholar] [CrossRef]
- Suzuki, R.; Matsumoto, H.; Teshima, T.; Koyama, H. Effect of age on myocardial function assessed by two-dimensional speckle-tracking echocardiography in healthy beagle dogs. J. Vet. Cardiol. 2013, 15, 243–252. [Google Scholar] [CrossRef]
- Pedro, B.; Stephenson, H.; Linney, C.; Cripps, P.; Dukes-McEwan, J. Assessment of left ventricular function in healthy Great Danes and in Great Danes with dilated cardiomyopathy using speckle tracking echocardiography. J. Vet. Cardiol. 2017, 19, 363–375. [Google Scholar] [CrossRef]
- Westrup, U.; McEvoy, F.J. Speckle tracking echocardiography in mature Irish Wolfhound dogs: Technical feasibility, measurement error and reference intervals. Acta Vet. Scand. 2013, 55, 41. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Suarez, D.F.; Lopez-Menendez, F.; Roche-Lima, A.; Lopez-Candales, A. Assessment of Mitral Annular Plane Systolic Excursion in Patients With Left Ventricular Diastolic Dysfunction. Cardiol. Res. 2019, 10, 83–88. [Google Scholar] [CrossRef]
- Hu, K.; Liu, D.; Herrmann, S.; Niemann, M.; Gaudron, P.D.; Voelker, W.; Ertl, G.; Bijnens, B.; Weidemann, F. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur. Heart J. 2013, 14, 205–212. [Google Scholar] [CrossRef]
- Santarelli, G.; Talavera López, J.; Fernández del Palacio, J. Evaluation of the right parasternal four-chamber view for the assessment of left ventricular longitudinal strain and strain rate by two-dimensional speckle tracking echocardiography in dogs. Res. Vet. Sci. 2018, 120, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zois, N.E.; Tidholm, A.; Nägga, K.M.; Moesgaard, S.G.; Rasmussen, C.E.; Falk, T.; Häggström, J.; Pedersen, H.D.; Åblad, B.; Nilsen, H.Y.; et al. Radial and Longitudinal Strain and Strain Rate Assessed by Speckle-Tracking Echocardiography in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 2012, 26, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, G.; Baron Toaldo, M.; Bouvard, J.; Glaus, T.M.; Fernandez del Palacio, J. Variability among strain variables derived from two-dimensional speckle tracking echocardiography in dogs by use of varioius software. Am. J. Vet. Res. 2019, 80, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Corda, A.; Pinna Parpaglia, M.L.; Sotgiu, G.; Zobba, R.; Gomez Ochoa, P.; Prieto Ramos, J.; French, A. Use of 2-dimensional speckle-tracking echocardiography to assess left ventricular systolic function in dogs with systemic inflammatory response syndrome. J. Vet. Intern. Med. 2019, 33, 423–431. [Google Scholar] [CrossRef]
- Baron Toaldo, M.; Guglielmini, C.; Diana, A.; Sarcinella, F.; Cipone, M. Feasibility and reproducibility of echocardiographic assessment of regional left atrial deformation and synchrony by tissue doppler ultrasonographic imaging in healthy dogs. Am. J. Vet. Res. 2014, 75, 59–66. [Google Scholar] [CrossRef]
- Abou, R.; Van Der Bijl, P.; Bax, J.J.; Delgado, V. Global longitudinal strain: Clinical use and prognostic implications in contemporary practice. Heart 2020, 106, 1438–1444. [Google Scholar] [CrossRef]
- Johnson, C.; Kuyt, K.; Oxborough, D.; Stout, M. Practical tips and tricks in measuring strain, strain rate and twist for the left and right ventricles. Echo Res. Pract. 2019, 6, R87–R98. [Google Scholar] [CrossRef]
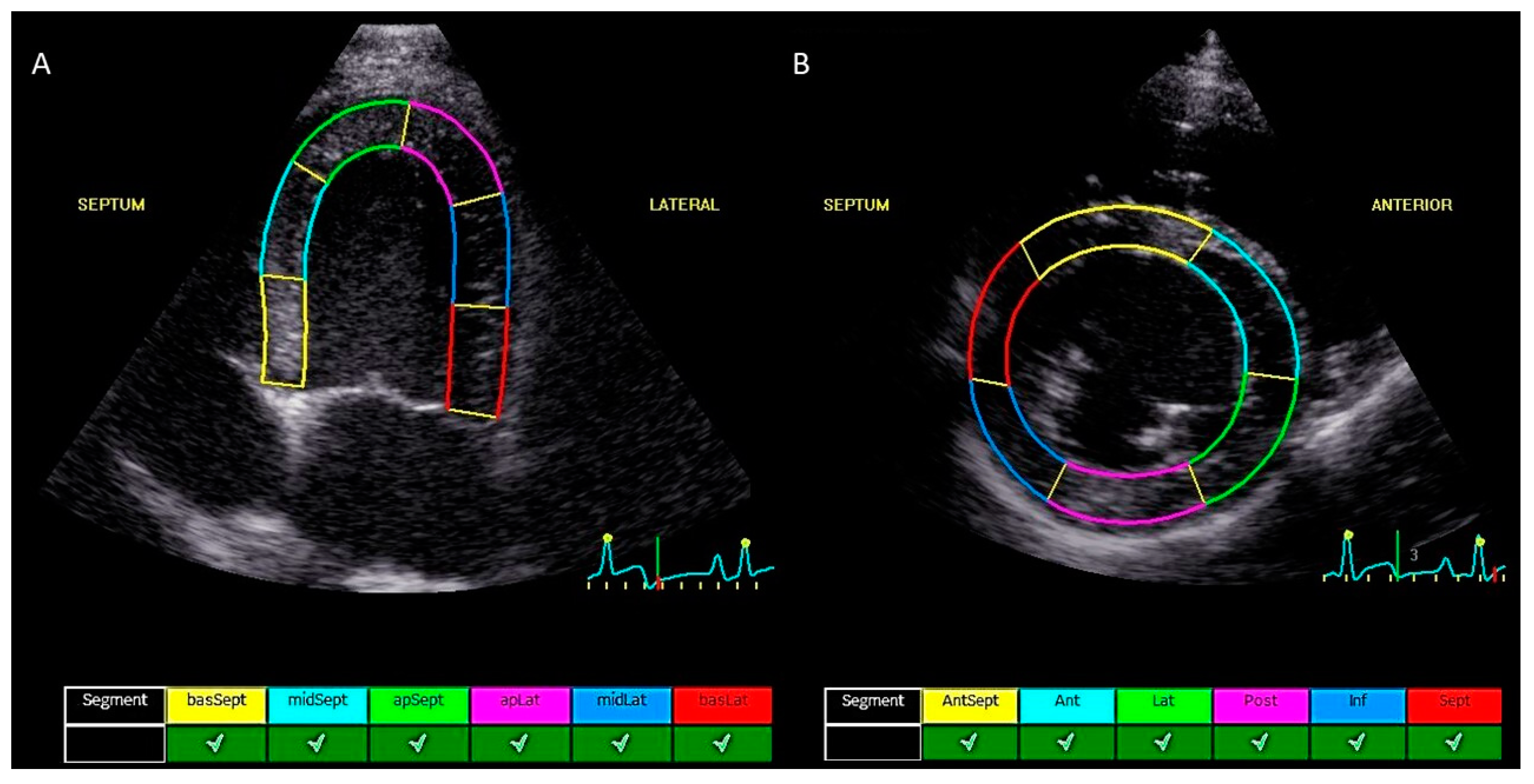


| Dog | Breed | Age (Years) | Sex | Weight (kg) | Dose CA4P (mg/m2) | 2D-STE Parameters | cTnI (µg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSt | CSt | RSt | Pre | Post | |||||||||
| Pre | Post | Pre | Post | Pre | Post | ||||||||
| 1 | Beagle | 7.5 | Mn | 17.0 | 50 | 22.8 | 17.8 | 20.7 | 16.4 | 54.1 | 59.8 | 0.04 | 0.01 |
| 2 | Beagle | 7.5 | Mn | 13.0 | 50 | 21.3 | 18.2 | 20.4 | 16.7 | 55.8 | 54.7 | 0.05 | 0.04 |
| 3 | Beagle | 8 | Fn | 8.0 | 75 | 17.6 | 14.2 | 17.1 | 16.5 | 57.3 | 55.0 | 0.02 | 0.03 |
| 4 | Beagle | 8.5 | Fn | 7.3 | 100 | 24.3 | 19.4 | 18.1 | / | 55.7 | / | 0.03 | 0.40 |
| 5 | Beagle | 7 | Mn | 17.8 | 100 | 22.9 | 17.8 | 20.1 | 16.9 | 64.0 | 72.1 | 0.02 | 4.30 |
| 6 | Beagle | 7.5 | Mn | 19.8 | 75 | 21.7 | 17.2 | 18.7 | 18.4 | 60.0 | 54.2 | 0.02 | 0.16 |
| 7 | Beagle | 7.5 | Mn | 14.5 | 75 | 21.3 | 18.0 | 19.2 | / | 63.5 | / | 0.03 | 0.02 |
| 8 | Am. Staff. | 14 | Mn | 14.0 | 75 | 16.9 | 15.4 | 16.1 | 13.0 | 53.5 | 62.5 | 0.08 | 0.08 |
| 9 | Golden Retriever | 10 | Mn | 35.0 | 75 | 18.4 | 12.4 | 18.8 | 17.6 | 46.4 | 57.7 | 0.05 | 1.77 |
| 10 | Münsterländer | 4 | Fn | 22.0 | 75 | 17.5 | 16.8 | 17.7 | 16.5 | 58.3 | 62.5 | 0.04 | 0.04 |
| 11 | Am. Staff. | 10 | Mn | 35.0 | 75 | 20.3 | 14.1 | 18.2 | 16.5 | 59.5 | 52.5 | 0.05 | 0.95 |
| 12 | Whippet | 7 | Fn | 13.1 | 75 | 19.9 | 15.4 | 14.0 | 11.2 | 50.2 | 41.1 | 0.08 | 0.46 |
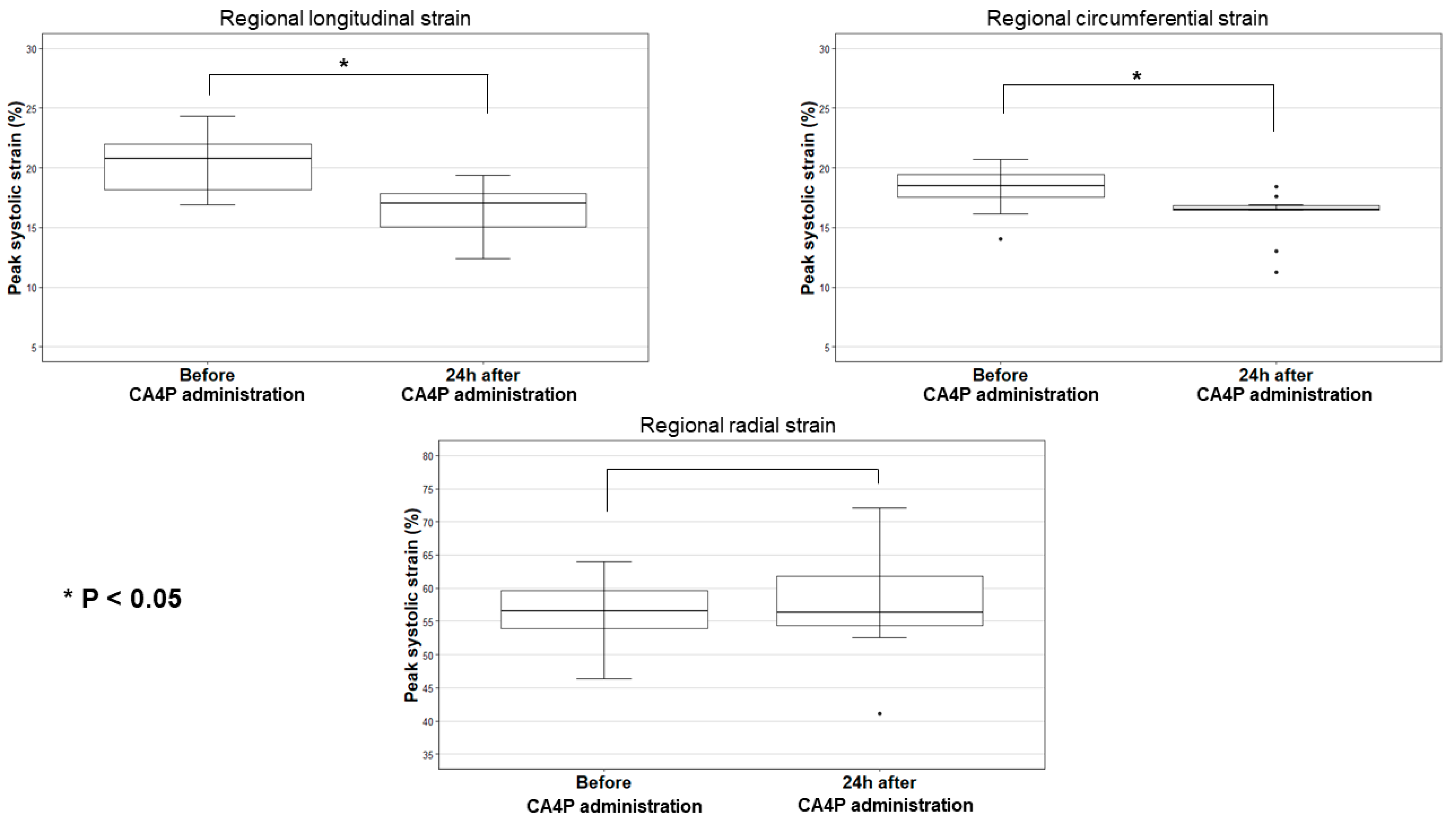
| 2D-STE Parameter | Median Value before CA4P Administration | Range | Median Absolute Change 24 h after CA4P Administration | Range | p-Value |
|---|---|---|---|---|---|
| LSt | 20.8 | 16.9–24.0 | 4.5 | 0.7–6.2 | <0.001 |
| CSt | 18.5 | 14.0–20.7 | 2.3 | 0.3–4.3 | 0.002 |
| RSt | 56.7 | 46.4–64.0 | 1.6 | −9.1–11.4 | 0.70 |
| 2D-STE Parameter | Spearman Rho Value for Correlation with cTnI | p-Value |
|---|---|---|
| LSt | −0.64 | 0.02 |
| CSt | 0.31 | 0.38 |
| RSt | −0.02 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mampaey, G.; Hellemans, A.; de Rooster, H.; Schipper, T.; Abma, E.; Broeckx, B.J.G.; Daminet, S.; Smets, P. Assessment of Cardiotoxicity after a Single Dose of Combretastatin A4-Phosphate in Dogs Using Two-Dimensional Speckle-Tracking Echocardiography. Animals 2022, 12, 3005. https://doi.org/10.3390/ani12213005
Mampaey G, Hellemans A, de Rooster H, Schipper T, Abma E, Broeckx BJG, Daminet S, Smets P. Assessment of Cardiotoxicity after a Single Dose of Combretastatin A4-Phosphate in Dogs Using Two-Dimensional Speckle-Tracking Echocardiography. Animals. 2022; 12(21):3005. https://doi.org/10.3390/ani12213005
Chicago/Turabian StyleMampaey, Gitte, Arnaut Hellemans, Hilde de Rooster, Tom Schipper, Eline Abma, Bart J. G. Broeckx, Sylvie Daminet, and Pascale Smets. 2022. "Assessment of Cardiotoxicity after a Single Dose of Combretastatin A4-Phosphate in Dogs Using Two-Dimensional Speckle-Tracking Echocardiography" Animals 12, no. 21: 3005. https://doi.org/10.3390/ani12213005





