Fetal Growth and Osteogenesis Dynamics during Early Development in the Ovine Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Conceptus Collection and Measurements
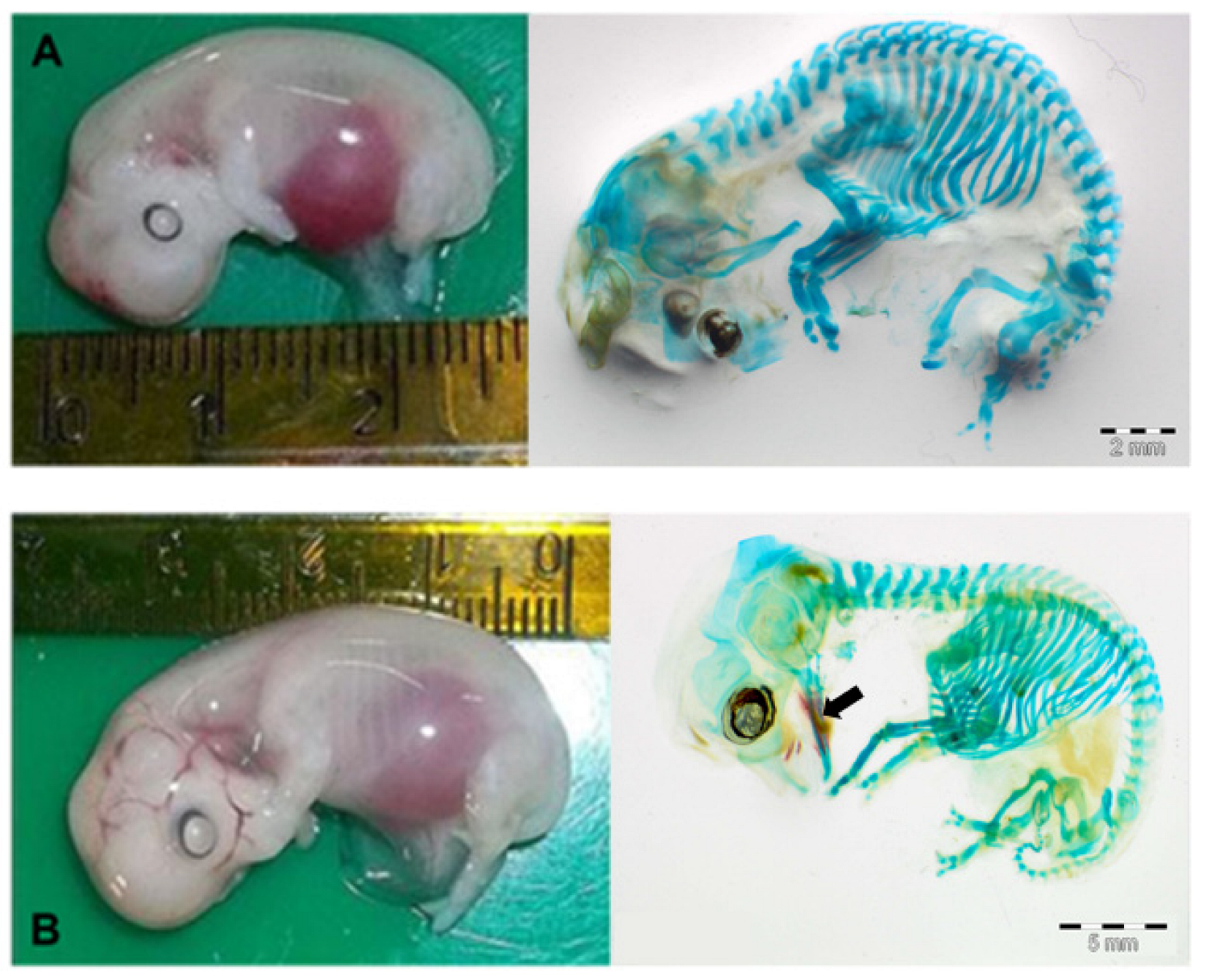
2.2. Conceptus Staining for the Study of Osteogenesis Dynamics
2.3. Statistical Analysis
3. Results
3.1. Concepti Measurements
3.2. Study of Osteogenesis Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMillen, C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.J.; Smith, G.W.; Scheetz, D.; Jimenez-Krassel, F.; Folger, J.K.; Ireland, J.L.H.; Mossa, F.; Lonergan, P.; Evans, A.C.O. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Mullerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod. Fertil. Dev. 2011, 23, 1–14. [Google Scholar] [PubMed]
- Ergaz, Z.; Avgil, M.; Ornoy, A. Intrauterine growth restriction-etiology and consequences: What do we know about the human situation and experimental models? Reprod. Toxicol. 2005, 20, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Flouri, D.; Darby, J.R.T.; Holman, S.L.; Cho, S.K.S.; Dimasi, C.G.; Perumal, S.R.; Ourselin, S.; Aughwane, R.; Mufti, N.; Macgowan, C.K.; et al. Placental MRI Predicts Fetal Oxygenation and Growth Rates in Sheep and Human Pregnancy. Adv. Sci. 2022, 9, 2203738. [Google Scholar] [CrossRef]
- Morrison, J.L.; Riggs, K.W.; Rurak, D.W. Fluoxetine during pregnancy: Impact on fetal development. Reprod. Fertil. Dev. 2005, 17, 641–650. [Google Scholar] [CrossRef]
- Carter, A.M. Animal models of human placentation-a review. Placenta 2007, 28 (Suppl. SA), S41–S47. [Google Scholar] [CrossRef]
- Banstola, A.; Reynolds, J.N.J. The Sheep as a Large Animal Model for the Investigation and Treatment of Human Disorders. Biology 2022, 11, 1251. [Google Scholar] [CrossRef]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy, 4th ed.; Elsevier: St. Louis, MO, USA, 2010. [Google Scholar]
- Barbera, A.; Jones, O.; Zerbi, G.; Hobbins, J.; Battaglia, F.; Meschia, G. Ultrasonographic assessment of fetal growth: Comparison between human and ovine fetus. Am. J. Obstet. Gynecol. 1995, 173, 560–562. [Google Scholar] [CrossRef]
- Saeedipanah Ardakani, M.; Khoramian Toosi, B.; Azizzadeh, M.; Rajabioun, M. Estimation of gestational age using ultrasonography in Baluchi sheep. Vet. Res. Forum 2022, 13, 257–263. [Google Scholar]
- Nieddu, S.M.; Strina, A.; Corda, A.; Furcas, G.; Ledda, M.; Pau, S.; Ledda, S. Comparation of Foetal Growth in Natural Mated and Vitrified/Warmed Ovine Embryos by Ultrasonographic Measurements. Glob. Vet. 2014, 12, 91–97. [Google Scholar]
- Correia Santos, V.J.; Garcia Kako Rodriguez, M.; Del Aguila da Silva, P.; Sitta Gomes Mariano, R.; Taira, A.R.; de Almeida, V.T.; Ramirez Uscategui, R.A.; Nociti, R.P.; Maia Teixeira, P.P.; Rossi Feliciano, M.A.; et al. B-mode ultrasonography and ecobiometric parameters for assessment of embryonic and fetal development in sheep. Anim. Reprod. Sci. 2018, 197, 193–202. [Google Scholar] [CrossRef] [Green Version]
- McGeady, T.A.; Quinin, P.J.; Fizpatrich, E.S.; Rayon, M.T. Veterinary Embryology; Wiley-Blackwell: Oxford, UK, 2006. [Google Scholar]
- Rigueur, D.; Lyons, K.M. Whole-Mount Skeletal Staining. Methods Mol. Biol. 2014, 1130, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Vernunft, A.; Eggert, A.; Brüssow, K.-P. Ultrasonographic Monitoring of Fetal Growth and Fetal Weight Calculation in Sows during Gestation. Agriculture 2023, 13, 16. [Google Scholar] [CrossRef]
- Ogata, Y.; Nakao, T.; Takahashi, K.; Abe, H.; Misawa, T.; Urushiyama, Y. Intrauterine growth retardation as a cause of perinatal mortality in Japanese black beef calves. J. Vet. Med. 1999, 46, 327–334. [Google Scholar] [CrossRef]
- Butt, K.; Lim, K. Diagnostic Imaging Committee. Determination of gestational age by ultrasound. J. Obstet. Gynaecol. Can. 2014, 36, 171–181. [Google Scholar] [CrossRef]
- Becsek, A.; Tzanidakis, N.; Blanco, M.; Bollwein, H. Transrectal three-dimensional fetal volumetry and crown-rump length measurement during early gestation in mares: Intra- and inter-observer reliability and agreement. Theriogenology 2019, 126, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Rotheneder, S.; González-Grajales, L.A.; Beck, H.; Bootz, F.; Bollwein, H. Variability of bovine conceptus-related volumes in early pregnancy measured with transrectal 3-dimensional ultrasonography. J. Dairy Sci. 2022, 105, 4534–4546. [Google Scholar] [CrossRef]
- Ghorayeb, S.R.; Bracero, L.A.; Blitz, M.J.; Rahman, Z.; Lesser, M.L. Quantitative ultrasound texture analysis for differentiating preterm from term fetal lungs. J. Ultrasound Med. 2017, 36, 1437–1443. [Google Scholar] [CrossRef]
- Zamarian, A.C.P.; Caetano, A.C.R.; Grohmann, R.M.; Mazzola, J.B.; Milani, H.J.F.; Passos, J.P.; Araujo Júnior, E.; Nardozza, L.M.M. Prediction of lung maturity in fetuses with growth restriction through quantitative ultrasound analysis. Ultrasound Med. Biol. 2022, 48, 20–26. [Google Scholar] [CrossRef]
- Chacham, S.; Pasi, R.; Chegondi, M.; Ahmad, N.; Mohanty, S.B. Metabolic bone disease in premature neonates: An unmet challenge. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 332–339. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.; Uscategui, R.; Santos, V.; Taira, A.R.; Mariano, R.; Rodrigues, M.; Simões, A.; Maronezi, M.C.; Avante, M.L.; Vicente, W.; et al. Qualitative and quantitative ultrasound attributes of maternal-foetal structures in pregnant ewes. Reprod. Domest. Anim. 2018, 53, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.W.; Newnham, J.P. Estimation of gestational age in Merino ewes by ultrasound measurement of fetal head size. Aust. J. Agric. Res. 1989, 40, 1293–1299. [Google Scholar] [CrossRef]
- Metodiev, N.; Dimov, D.; Ralchev, I.; Raicheva, E. Measurement of foetal growth via transabdominal ultrasonography during first half of pregnancy at ewes from synthetic population Bulgarian milk. Bulg. J. Agric. Sci. 2012, 18, 493–500. [Google Scholar]
- Fowler, D.; Wilkins, J. Diagnosis of pregnancy and number of foetuses in sheep by real- time ultrasonic imaging. Effects of number of foetuses, stage of gestation, operator, and breed of ewe on accuracy of diagnosis. Livest. Prod. Sci. 1984, 11, 437–450. [Google Scholar] [CrossRef]
- Menezes, M.C.; Lega, E.; Coelho, L.A.F. Utilização da ultrassonografia por via transretal em vacas da raça girolando para acompanhamento do desenvolvimento embrionário e/ou fetal 26 a 181 dias de gestacao. Nucl. Anim. 2011, 3, 37–60. [Google Scholar] [CrossRef]
- Wenham, G. Studies on reproduction in prolific ewes. A radiographic study of the primary and secondary ossification centers in the fetus. J. Agric. Sci. 1977, 88, 553–566. [Google Scholar] [CrossRef]
- Dhingra, L.D.; Tyagi, R.P.S. A study of the prenatal ossification centers and epiphyseal ossification in the limb bones of the goat. Vet. Bull. 1972, 42, 52. [Google Scholar]
- Oishi, A.; Yamada, S.; Sakamota, H.; Kamlya, S.; Yanagida, K.; Kubota, C.; Watanabe, Y.; Shimizu, R. Radiographical evaluation of bone maturation in Japanese black beef cattle. J. Vet. Med. Sci. 1996, 58, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Wenham, G.; Fowler, V.R.; Mcdonald, I. A radiographic study of skeletal growth and development in the pig. Temporal pattern of growth. J. Agric. Sci. 1973, 80, 123–133. [Google Scholar] [CrossRef]
- Boyed, J.S. Radiographic appearance of the centers of ossification of the limb bones in the feline fetus. Br. Vet. J. 1968, 124, 365–372. [Google Scholar] [CrossRef]
- Bagnall, K.M.; Harris, P.F.; Jones, P.R.M. A radiographic study of the longitudinal growth of primary ossification centers in limb long bones of the human fetus. Anat. Rec. 1982, 203, 293–299. [Google Scholar] [CrossRef]
- Delle Donne, H., Jr.; Faúndes, A.; Tristão, E.G.; Helena de Sousa, M.; Antonio Urbanetz, A. Sonographic identification and measurement of the epiphyseal ossification centers as markers of fetal gestational age. J. Clin. Ultrasound 2005, 33, 394–400. [Google Scholar] [CrossRef]
- Moradi, B.; Ghanbari, A.; Rahmani, M.; Kazemi, M.A.; Tahmasebpour, A.R.; Shakiba, M. Evaluation of bi-iliac distance and timing of ossification of sacrum by sonography in the second trimester of pregnancy. Iran. J. Radiol. 2019, 16, e79940. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.S.; Majeed, Z.Z. The embryonic development of the sternum in sheep and goats. J. Vet. Med. Sci. 2008, 7, 50–57. [Google Scholar]
- Lindsay, F.E.F. Observations on the loci of ossification in the prenatal and neonatal bovine skeleton. I. The appendicular skeleton. Br. Vet. J. 1969, 125, 101–111. [Google Scholar] [CrossRef]
- Soana, S.; Bertoni, G.; Gnudi, G.; Botti, P. Osteogenesis of the fetal bovine skull. Anat. Histol. Embryol. 1996, 25, 167–173. [Google Scholar] [CrossRef]
- Rosignoli, L.; Tonni, G.; Centini, G. Cranial development in the first trimester: The use of 3D in the study of complex structures. Imaging Med. 2010, 2, 251–257. [Google Scholar] [CrossRef]
- Silva, G.P.; Monteiro, F.O.B.; Pereira, T.H.D.S.; de Matos, S.E.R.; dos Santos de Andrade, R.; El Bizri, H.R.; Coutinho, L.N.; Valsecchi, J.; López-Plana, C.; Mayor, P.; et al. Fetal bone development in the lowland paca (Cuniculus paca, Rodentia, Cuniculidae) determined using ultrasonography. J. Anat. 2020, 237, 105–118. [Google Scholar] [CrossRef]
- Souza Pereira, T.H.; Barros Monteiro, F.O.; Pereira da Silva, G.; Rodrigues de Matos, S.E.; Rocha El Bizri, H.; Valsecchi, J.; Bodmer, R.E.; Perez Pena, P.; Coutinho, L.N.; Lopez Plana, C.; et al. Ultrasound evaluation of fetal bone development in the collared (Pecari tajacu) and white-lipped peccary (Tayassu pecari). J. Anat. 2022, 241, 741–755. [Google Scholar] [CrossRef]
- Hautier, L.; Stansfield, F.J.; Allen, W.R.T.; Asher, R.J. Skeletal development in the African elephant and ossification timing in placental mammals. Proc. R. Soc. B Biol. Sci. 2012, 279, 2188–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, B.M. Sequential pattern of prenatal ossification in the fore limb bones in white New Zealand rabbits by double stained techniques and computed tomography. PSM Vet. Res. 2019, 4, 24–35. [Google Scholar]
- Patton, J.T.; Kaufman, M.H. The timing of mineralization of the limb bones, and growth rates of various long bones of the fore and hind limbs of the prenatal and early postnatal laboratory mouse. J. Anat. 1995, 186, 175–185. [Google Scholar] [PubMed]
- Phillips, I.R. Skeletal development in the foetal and neonatal marmoset (Callithrix jacchus). Lab. Anim. 1976, 10, 317–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starck, J.M.; Ricklefs, R.E. Patterns of development: The altricial-precocial spectrum. In Avian Growth and Development. Evolution within the Altricial Precocial Spectrum; JM Stark & RE Ricklefs, Oxford University Press: New York, NY, USA, 1998; pp. 3–30. [Google Scholar]
- Connolly, S.A.; Jaramillo, D.; Hong, J.K.; Shapiro, F. Skeletal development in fetal pig specimens: MR imaging of femur with histological comparison. Radiology 2004, 233, 505–514. [Google Scholar] [CrossRef]
- Modina, S.C.; Veronesi, M.C.; Moioli, M.; Meloni, T.; Lodi, G.; Bronzo, V.; Di Giancamillo, M. Small-sized newborn dogs skeletal development: Radiologic, morphometric, and histological findings obtained from spontaneously dead animals. BMC Vet. Res. 2017, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of bone tissue: Structures, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, C.M. Maternal behavior and lamb survival: From neuroendocrinology to practical application. Animal 2014, 8, 102–112. [Google Scholar] [CrossRef] [Green Version]





| CRL (cm) | BPD (cm) | Day of Pregnancy (% of Gestational Period) | Dynamic of Ossification |
|---|---|---|---|
| 2.01 ± 0.045– 2.54 ± 0.022 | 0.49 ± 0.002– 0.66 ± 0.007 | 30th–35th (20–23) | Skeleton cartilaginous |
| 3.34 ± 0.088– 4.05 ± 0.058 | 1.03 ± 0.01– 1.12 ± 0.026 | 40th–45th (26.6–30) | Beginning of jaw ossification and humerus and radius diaphysis |
| 7.06 ± 0.037– 8.15 ± 1.32 | 1.38 ± 0.026– 1.63 ± 0.055 | 50th–55th (33.3–36.6) | Beginning of ossification of rachis, ossification of ribs, diaphysis of femur, and tibia. Beginning of intra-membranous ossification of the skull. |
| 8.65 ± 0.058– 9.2 ± 0.153 | 2.41 ± 0.024– 2.52 ± 0.03 | 65th–70th (43.3–46.6) | Almost all skeleton is ossified, except ends of the limbs, cervical vertebra and chondrocostal junctions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Succu, S.; Contu, E.; Bebbere, D.; Gadau, S.D.; Falchi, L.; Nieddu, S.M.; Ledda, S. Fetal Growth and Osteogenesis Dynamics during Early Development in the Ovine Species. Animals 2023, 13, 773. https://doi.org/10.3390/ani13050773
Succu S, Contu E, Bebbere D, Gadau SD, Falchi L, Nieddu SM, Ledda S. Fetal Growth and Osteogenesis Dynamics during Early Development in the Ovine Species. Animals. 2023; 13(5):773. https://doi.org/10.3390/ani13050773
Chicago/Turabian StyleSuccu, Sara, Efisiangelo Contu, Daniela Bebbere, Sergio Domenico Gadau, Laura Falchi, Stefano Mario Nieddu, and Sergio Ledda. 2023. "Fetal Growth and Osteogenesis Dynamics during Early Development in the Ovine Species" Animals 13, no. 5: 773. https://doi.org/10.3390/ani13050773






