Heat-Killed Lactobacillus acidophilus Promotes Growth by Modulating the Gut Microbiota Composition and Fecal Metabolites of Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Preparation of Heat-Killed Lactobacillus acidophilus IFFI 6005
2.3. Animal and Experimental Design
2.4. Animal Administrations
2.5. Test Indicator Determination
2.6. Fecal Sample Collection and Index Determination
2.6.1. Fecal Sample Collection
2.6.2. Illumina Sequencing
2.6.3. Determination and Analysis of the Metabolome
2.7. Integration Analysis between Microbiota and Metabolome
2.8. Statistical Analysis
3. Results
3.1. Preparation of Heat-Killed Lactobacillus acidophilus IFFI 6005
3.2. Growth Performance and Diarrhea Rate
3.3. Analysis of the Intestinal Microbiota
3.3.1. Analysis of Alpha Diversity in Intestinal Microorganisms
3.3.2. Identification of Target Microorganisms by Differential Analysis
3.4. Analysis of Fecal Metabolites
3.5. Association Analysis of Differential Microorganisms and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, N.; Chen, S.; Chao, L.; Sun, L.N.; Zheng, D.M.; Liu, Q.; Zhang, Q. Research of antibiotics pollution in soil environments and theirs biological degradation. Appl. Mech. Mater. 2014, 3308, 800–803. [Google Scholar] [CrossRef]
- Huang, C.; Feng, S.Y.; Huo, F.J.; Liu, H.L. Effects of four antibiotics on the diversity of the intestinal microbiota. Microbiol. Spectr. 2022, 10, e0190421. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document. the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastro. Hepat. 2017, 11, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lokhande, A.; Kim, E.K.; Kwon, I.K.; Kim, Y.H.; Chae, B.J. Effects of dietary supplementation with Bacillus subtilis LS 1-2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim. Feed. Sci. Tech. 2014, 188, 102–110. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, J.R.; Lin, J.D. Probiotics in aquaculture: Challenges and outlook. Aquaculture 2008, 281, 1–4. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B. Enhancing probiotic stability in industrial processes. Microb. Ecol. Health. Dis. 2012, 23, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Goderska, K.; Johnson, E.M.; Das, B.; Indira, D.; Yadav, R.; Ojha, S.; Jayabalan, R.; Moumita, S. Evaluation of the viability of free and encapsulated lactic acid bacteria using in-vitro gastro intestinal model and survivability studies of synbiotic microcapsules in dry food matrix during storage. LWT 2017, 77, 460–467. [Google Scholar]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends. Food. Sci. Tech. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes. Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef]
- Tartrakoon, W.; Charoensook, R.; Incharoen, T.; Numthuam, S.; Pechrkong, T.; Onoda, S.; Shoji, G.; Brenig, B. Effects of heat-Killed Lactobacillus plantarum L-137 supplementation on growth performance, blood profiles, intestinal morphology, and immune gene expression in pigs. Vet. Sci. 2023, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, J.J.; Cho, J.H.; Choe, J.; Kyoung, H.; Kim, S.H.; Kim, H.B.; Song, M. Effects of dietary inactivated probiotics on growth performance and immune responses of weaned pigs. J. Anim. Sci. Technol. 2021, 63, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kyoung, H.; Park, K.I.; Oh, S.; Song, M.; Kim, Y. Postbiotic heat-killed lactobacilli modulates on body weight associated with gut microbiota in a pig model. AMB Express 2022, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xing, S.; He, L.; Li, C.; Wang, X.; Zeng, X.; Dai, Y. Characterization, high-density fermentation, and the production of a directed vat set starter of Lactobacilli used in the food industry: A review. Foods 2022, 11, 3063. [Google Scholar] [CrossRef]
- Papizadeh, M.; Rohani, M.; Nahrevanian, H.; Hosseini, S.N.; Shojaosadati, S.A.; Pourshafifie, M.R. Using various approaches of design of experiments for high cell density production of the functionally probiotic Lactobacillus plantarum strain RPR42 in a cane molasses-based medium. Curr. Microbiol. 2020, 77, 1756–1766. [Google Scholar] [CrossRef]
- Heatley, N.G. A method for the assay of penicillin. Biochem. J. 1944, 38, 61–65. [Google Scholar] [CrossRef]
- Phillips, M.; Kailasapathy, K.; Tran, L. Viability of commercial probiotic cultures (L. acidophilus, Bifidobacterium sp., L. casei, L. paracasei and L. rhamnosus) in cheddar cheese. Int. J. Food. Microbiol. 2006, 108, 276–280. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Marquardt, R.R.; Jin, L.Z.; Kim, J.W.; Fang, L.; Frohlich, A.A.; Baidoo, S.K. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS. Immunol. Med. Microbiol. 1999, 23, 283–288. [Google Scholar] [CrossRef]
- Sun, T.H.; Miao, H.B.; Zhang, C.B.; Wang, Y.S.; Liu, S.; Jiao, P.; Li, W.; Li, Y.; Huang, Z.X. Effect of dietary Bacillus coagulans on the performance and intestinal microbiota of weaned piglets. Animal 2022, 16, 100561. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platformindependent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metabolomics. 2013, 1, 92–107. [Google Scholar] [PubMed]
- Westerhuis, J.A.; van Velzen, E.J.J.; Hoefsloot, H.C.J.; Smilde, A.K. Multivariate paired data analysis: Multilevel PLSDA versus OPLSDA. Metabolomics 2010, 6, 119–128. [Google Scholar] [CrossRef]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–371. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic. Acids. Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MSEA: A web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic. Acids. Res. 2010, 38, 71–77. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, 316–324. [Google Scholar]
- Lim, S.M. Cultural conditions and nutritional components affecting the growth and bacteriocin production of Lactobacillus plantarum KC21. Food. Sci. Biotechnol. 2010, 19, 793–802. [Google Scholar] [CrossRef]
- Lv, X.P.; Liu, G.F.; Sun, X.M.; Chen, H.Y.; Sun, J.H.; Feng, Z. Short communication: Nutrient consumption patterns of Lactobacillus acidophilus KLDS 1.0738 in controlled pH batch fermentations. J. Dairy. Sci. 2017, 100, 5188–5194. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, K.Y.; Tran, H.N.; Kim, I.H. Effect of a protected blend of organic acids and medium-chain fatty acids on growth performance, nutrient digestibility, blood profiles, meat quality, faecal microflora, and faecal gas emission in finishing pigs. Can. J. Anim. Sci. 2016, 99, 448–455. [Google Scholar] [CrossRef]
- Shin, S.; Yasuhiro, I.; Kaoruko, Y.; Rao, S.; Oka, K.; Arakawa, F.; Fujimura, T.; Murakami, H.; Kurazono, H.; Takahashi, M.; et al. Effects of oral administration of heat-killed Enterococcus faecium strain NHRD IHARA in post-weaning piglets. Anim. Sci. J. 2014, 85, 454–460. [Google Scholar]
- Zumbado, M.E.; Scheele, C.W.; Kwakernaak, C. Chemical composition, digestibility, and metabolizable energy content of different fat and oil by-products. J. App. Poult. Res. 1999, 8, 263–271. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.W.; Ci, W.J.; Zheng, Y.Y.; Han, X.Y.; Huang, J.P.; Zhu, J.J. Effects of dietary supplementation with Lactobacillus acidophilus and Bacillus subtilis on mucosal immunity and intestinal barrier are associated with its modulation of gut metabolites and microbiota in late-phase laying hens. Probiotics. Antimicro. 2022, 15, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Amerah, A.M.; Quiles, A.; Medel, P.; Sánchez, J.; Lehtinen, M.J.; Gracia, M.I. Effect of pelleting temperature and probiotic supplementation on growth performance and immune function of broilers fed maize/soy-based diets. Anim. Feed. Sci. Tech. 2013, 180, 55–63. [Google Scholar] [CrossRef]
- Yang, J.W.C.; Yih, S.C.; Lung, T.L.T. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar]
- Konstantinov, S.R.; Awati, A.; Williams, B.A.; Miller, B.G.; Jones, P.; Stokes, C.R.; Akkermans, A.D.L.; Smidt, H.; de Vos, W.M. Post-natal development of the porcine microbiota composition and activities. Environ. Microbiol. 2006, 8, 1191–1199. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.M.; Jiang, L.L.; Wang, W.; Li, T.T.; Zuo, B.; Li, Z.; Wang, J.J. Differences in the gut microbiota establishment and metabolome characteristics between lowand normal-birth-weight piglets during early-life. Front. Microbiol. 2018, 9, 1798. [Google Scholar]
- Sato, Y.; Kuroki, Y.; Oka, K.; Takahashi, M.; Rao, S.; Sukegawa, S.; Fujimura, T. Effects of dietary supplementation with Enterococcus faecium and Clostridium butyricum, either alone or in combination, on growth and fecal microbiota composition of post-weaning pigs at a commercial farm. Front. Vet. Sci. 2019, 6, 26. [Google Scholar] [CrossRef]
- Lan, W.; Yang, C.L. Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total. Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and carbon sources oxidation. Indian, J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Shi, X.M.; Yang, J.; Zhao, Y.Y.; Xue, L.X.; Xu, L.; Cai, J. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein. Cell 2021, 12, 346–359. [Google Scholar] [CrossRef]
- Cheng, S.S.; Ma, X.; Geng, S.J.; Jiang, X.M.; Li, Y.; Hu, L.S.; Li, J.R.; Wang, Y.Z.; Han, X.Y. Fecal microbiota transplantation beneficially regulates intestinal mucosal autophagy and alleviates gut barrier injury. Msystems 2018, 3, e00137-18. [Google Scholar] [CrossRef]
- Huang, I.F.; Lin, I.C.; Liu, P.F.; Cheng, M.F.; Liu, Y.C.; Hsieh, Y.D.; Chen, J.J.; Chen, C.L.; Chang, H.W.; Shu, C.W. Lactobacillus acidophilus attenuates Salmonella-induced intestinal inflammation via TGF-β signaling. BMC. Microbiol. 2015, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Kutsukake, E.; Fukui, T.; Sato, I.; Shirai, T.; Kurihara, T.; Okada, N.; Danbara, H.; Toba, M.; Kohda, N.; et al. Oral administration of heat-killed Lactobacillus plantarum strain b240 protected mice against Salmonella enterica Serovar Typhimurium. Biosci. Biotechnol. Biochem. 2010, 74, 1338–1342. [Google Scholar] [CrossRef]
- Lin, W.H.; Yu, B.; Lin, C.K.; Hwang, W.Z.; Tsen, H.Y. Immune effect of heat-killed multistrain of Lactobacillus acidophilus against Salmonella typhimurium invasion to mice. J. Appl. Microbiol. 2007, 102, 22–31. [Google Scholar] [CrossRef]
- Boeckman, J.X.; Sprayberry, S.; Korn, A.M.; Suchodolski, J.S.; Paulk, C.; Genovese, K.; Rech, R.R.; Giaretta, P.R.; Blick, A.K.; Callaway, T.; et al. Effect of chronic and acute enterotoxigenic E. coli challenge on growth performance, intestinal infammation, microbiome, and metabolome of weaned piglets. Sci. Rep. 2022, 12, 5024. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, D.W.; Mao, X.B.; He, J.; Yu, J.; Zheng, P.; Luo, J.Q.; Gao, J.; Htoo, J.K.; Yu, B. Evaluation of standardized ileal digestible lysine requirement for 8-20 kg pigs fed low crude protein diets. Anim. Sci. J. 2019, 90, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Che, D.S.; Adams, S.; Zhao, B.; Qin, G.X.; Jiang, H.L. Effects of dietary L-arginine supplementation from conception to post-weaning in piglets. Curr. Protein. Pept. Sci. 2019, 20, 736–749. [Google Scholar] [CrossRef]
- Lee, W.J.; Ryu, S.; Kang, A.N.; Song, M.; Shin, M.; Oh, S.; Kim, Y. Molecular characterization of gut microbiome in weaning pigs supplemented with multi-strain probiotics using metagenomic, culturomic, and metabolomic approaches. Anim. Microb. 2022, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Gy, W.; Flynn, N.E.; Knabe, D.A. Polyamine metabolism in the intestine of piglets is altered by weaning and proline supplementation. J. Anim. Sci. 2016, 94, 423–428. [Google Scholar]
- Clouard, C.; Lannuzel, C.; Bourgo, C.L.; Gerrits, W.J.J. Lactose and digestible maltodextrin in milk replacers differently affect energy metabolism and substrate oxidation: A calorimetric study in piglets. J. Nutr. 2020, 150, 3114–3122. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, L.; Meng, Q.S.; Wu, W.D.; Lee, Y.K.; Xie, J.J.; Zhang, H.F. Effects of dietary pectin on the profile and transport of intestinal bile acids in young pigs. J. Anim. Sci. 2018, 96, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Dilkin, P.; Direito, G.; Simas, M.M.S.; Mallmann, C.A.; Corrêa, B. Toxicokinetics and toxicological effects of single oral dose of fumonisin B1 containing Fusarium verticillioides culture material in weaned piglets. Chem. Biol. Interact. 2010, 185, 157–162. [Google Scholar] [CrossRef]
- Zhen, R.; Liu, C.Y.; Wei, C.W.; Luo, Y.Y.; Hu, X.X.; Liu, G.Y.; Yi, H.B.; Huang, Y.N. Effect of different dosage of sodium butyrate and niacin on growth, fecal microbiota and vitamin B metabolism in weaned piglets. J. Appl. Microbiol. 2022, 132, 4466–4475. [Google Scholar] [CrossRef]
- He, Q.; Zou, T.D.; Chen, J.; He, J.; Jian, L.; Xie, F.; You, J.M.; Wang, Z.R. Methyl-donor micronutrient for gestating sows: Effects on gut microbiota and metabolome in offspring piglets. Front. Nutr. 2021, 8, 675640. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.Y.; Ma, Y.H.; Cai, S.; Yang, L.J.; Fan, Y.X.; Zeng, X.F.; Qiao, S.Y. Lactobacillus reuteri improves the development and maturation of fecal microbiota in piglets through mother-to-infant microbe and metabolite vertical transmission. Microbiome 2022, 10, 211. [Google Scholar] [CrossRef]
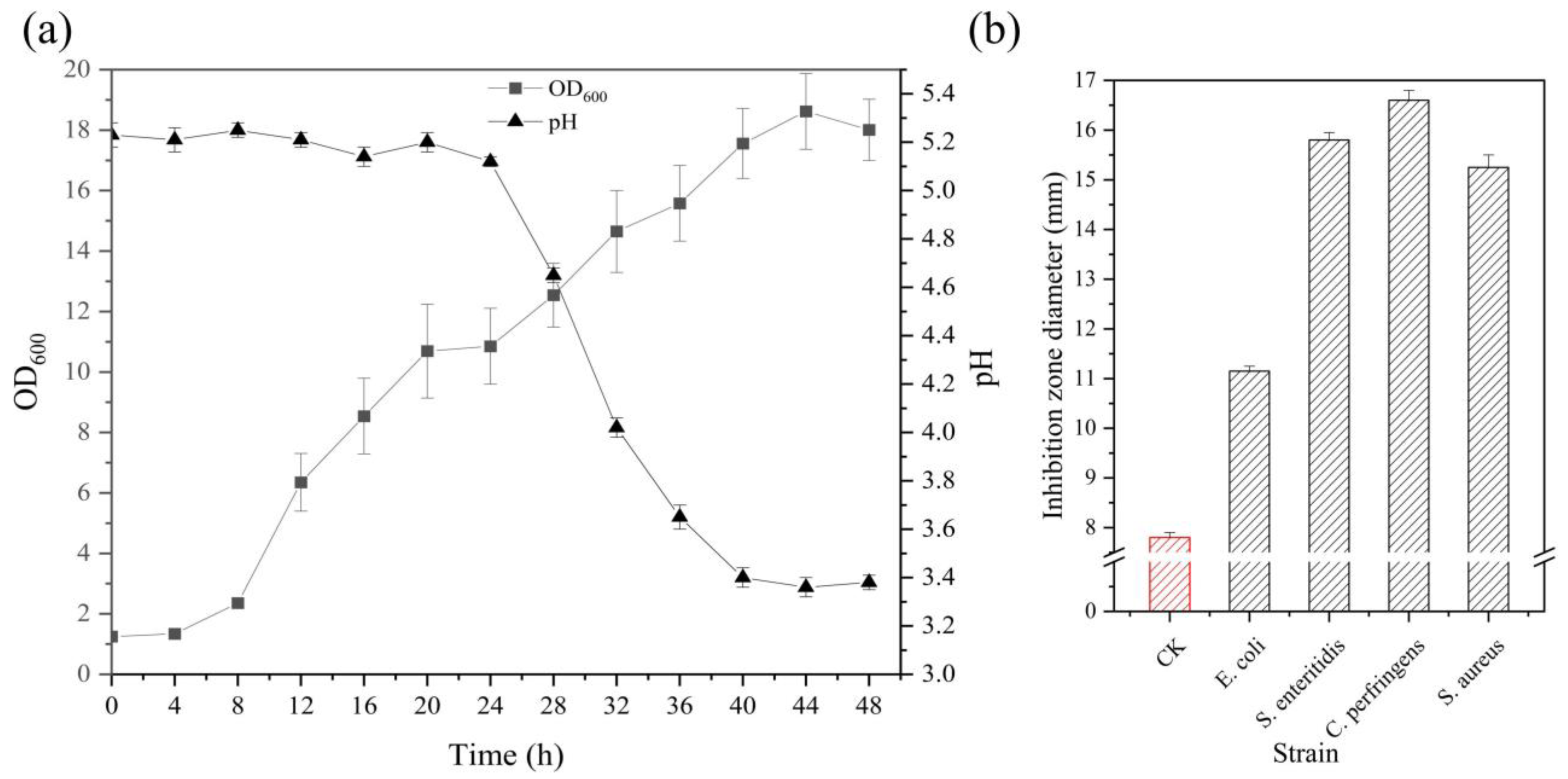

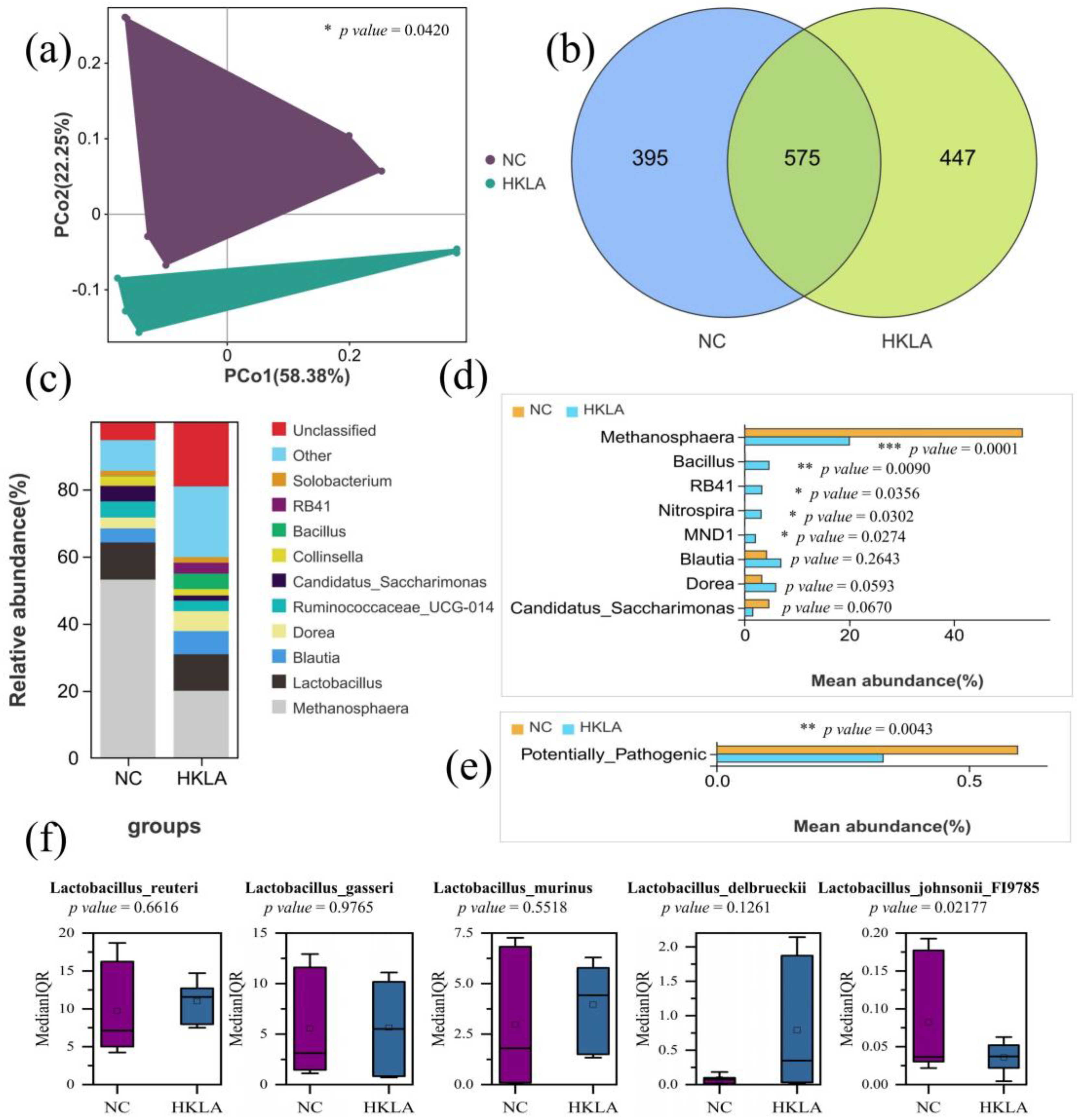
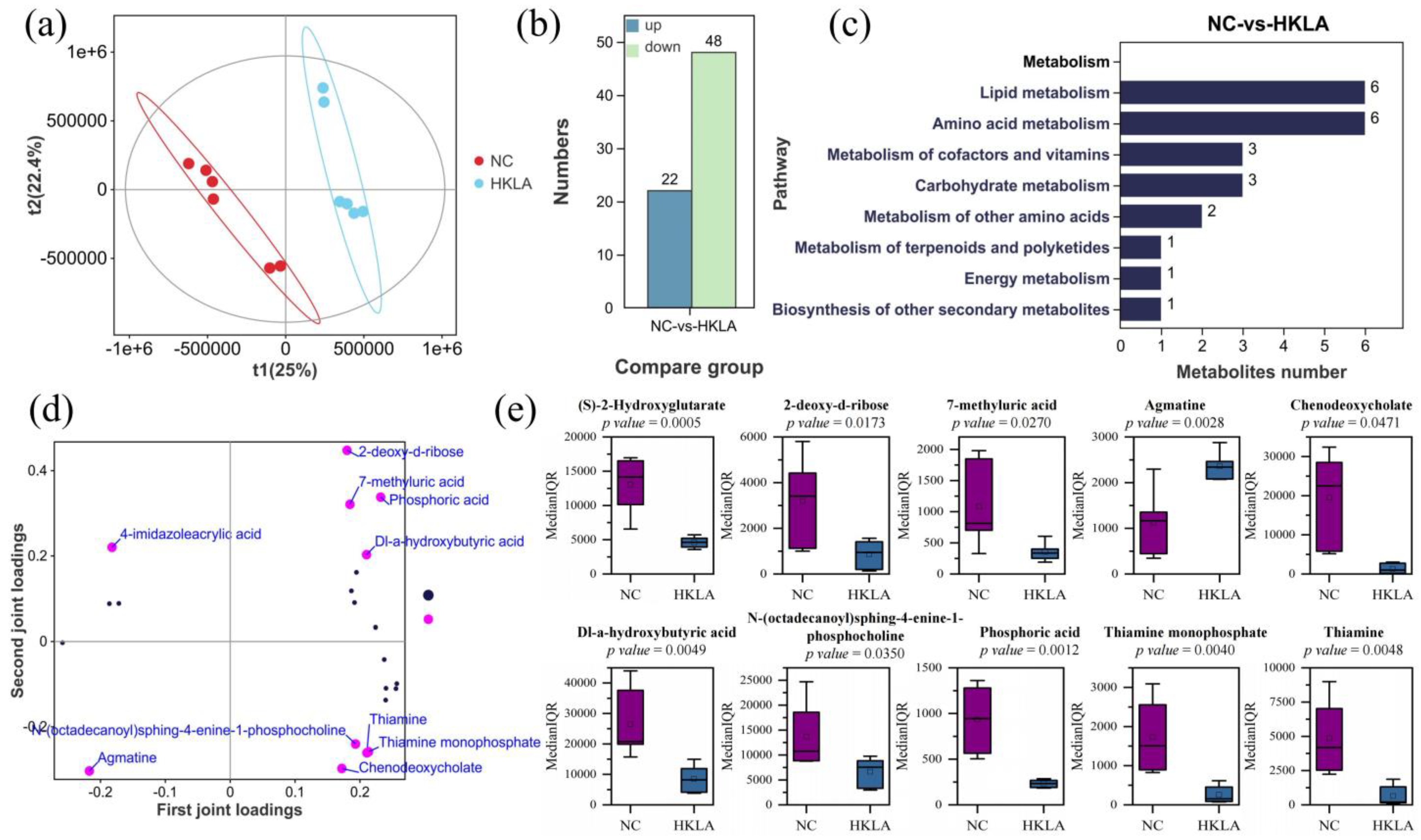
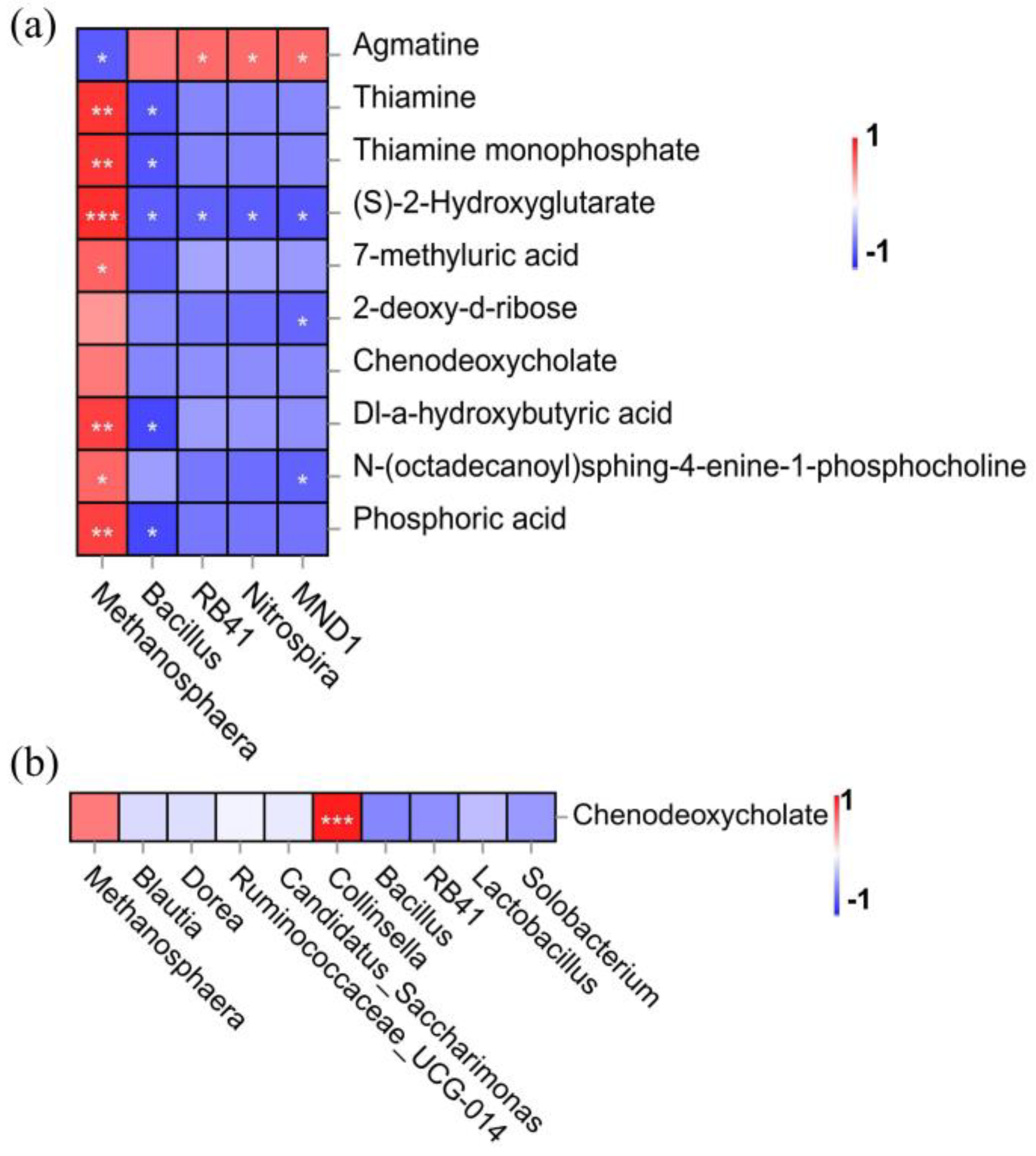
| Items | NC | PC | LA | HKLA | SEM | p Value |
|---|---|---|---|---|---|---|
| BW1 (kg) | 14.28 | 14.36 | 14.63 | 14.25 | 0.37 | 0.843 |
| BW30 (kg) | 35.75 a | 37.55 b | 36.25 a | 38.74 b | 0.69 | 0.045 |
| ADG (g) | 715.67 a | 773.00 bc | 720.67 a | 816.33 c | 13.36 | 0.025 |
| ADFI (g) | 1090.76 a | 1051.28 a | 1095.42 a | 1142.86 b | 18.64 | 0.0659 |
| F:G | 1.52 a | 1.36 b | 1.52 a | 1.40 b | 0.02 | 0.017 |
| Diarrhoea rate (%) | 5.83 a | 3.33 b | 5.14 a | 2.64 b | 0.15 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, H.; Liang, J.; Lan, G.; Wu, Q.; Huang, Z. Heat-Killed Lactobacillus acidophilus Promotes Growth by Modulating the Gut Microbiota Composition and Fecal Metabolites of Piglets. Animals 2024, 14, 2528. https://doi.org/10.3390/ani14172528
Miao H, Liang J, Lan G, Wu Q, Huang Z. Heat-Killed Lactobacillus acidophilus Promotes Growth by Modulating the Gut Microbiota Composition and Fecal Metabolites of Piglets. Animals. 2024; 14(17):2528. https://doi.org/10.3390/ani14172528
Chicago/Turabian StyleMiao, Huabiao, Jing Liang, Ganqiu Lan, Qian Wu, and Zunxi Huang. 2024. "Heat-Killed Lactobacillus acidophilus Promotes Growth by Modulating the Gut Microbiota Composition and Fecal Metabolites of Piglets" Animals 14, no. 17: 2528. https://doi.org/10.3390/ani14172528





