A Review of Swine Breeding Herd Biosecurity in the United States to Prevent Virus Entry Using Porcine Reproductive and Respiratory Syndrome Virus as a Model Pathogen
Abstract
:Simple Summary
Abstract
1. Introduction
2. A Review of the Concepts of Biosecurity
2.1. Bio-Exclusion
2.2. Biocontainment
2.3. Bio-Management
3. What Are the Components of a Successful Biosecurity Program?
- (1)
- science-based,
- (2)
- a way of life,
- (3)
- continuously validated,
- (4)
- cost-effective,
- (5)
- benchmarked over time.
3.1. Biosecurity Must Be Science-Based
3.2. Biosecurity Must Be a Way of Life
3.3. Biosecurity Must Be Continuously Validated
3.4. Biosecurity Must Be Cost-Effective
3.5. Biosecurity Must Be Benchmarked over Time
4. Transmission of Porcine Reproductive and Respiratory Syndrome Virus and the Biosecurity Protocols Designed to Prevent Infection of the Breeding Herd
4.1. Pigs
4.2. Semen
4.3. Needles
4.4. Fomites
4.5. Personnel
4.6. Transport
4.7. Avian Species
4.8. Rodents
4.9. Feral/Domestic Animals
4.10. Insects
4.11. Swine Slurry and Lagoon Effluent
4.12. Pig Meat
4.13. Aerosols
4.14. Feed
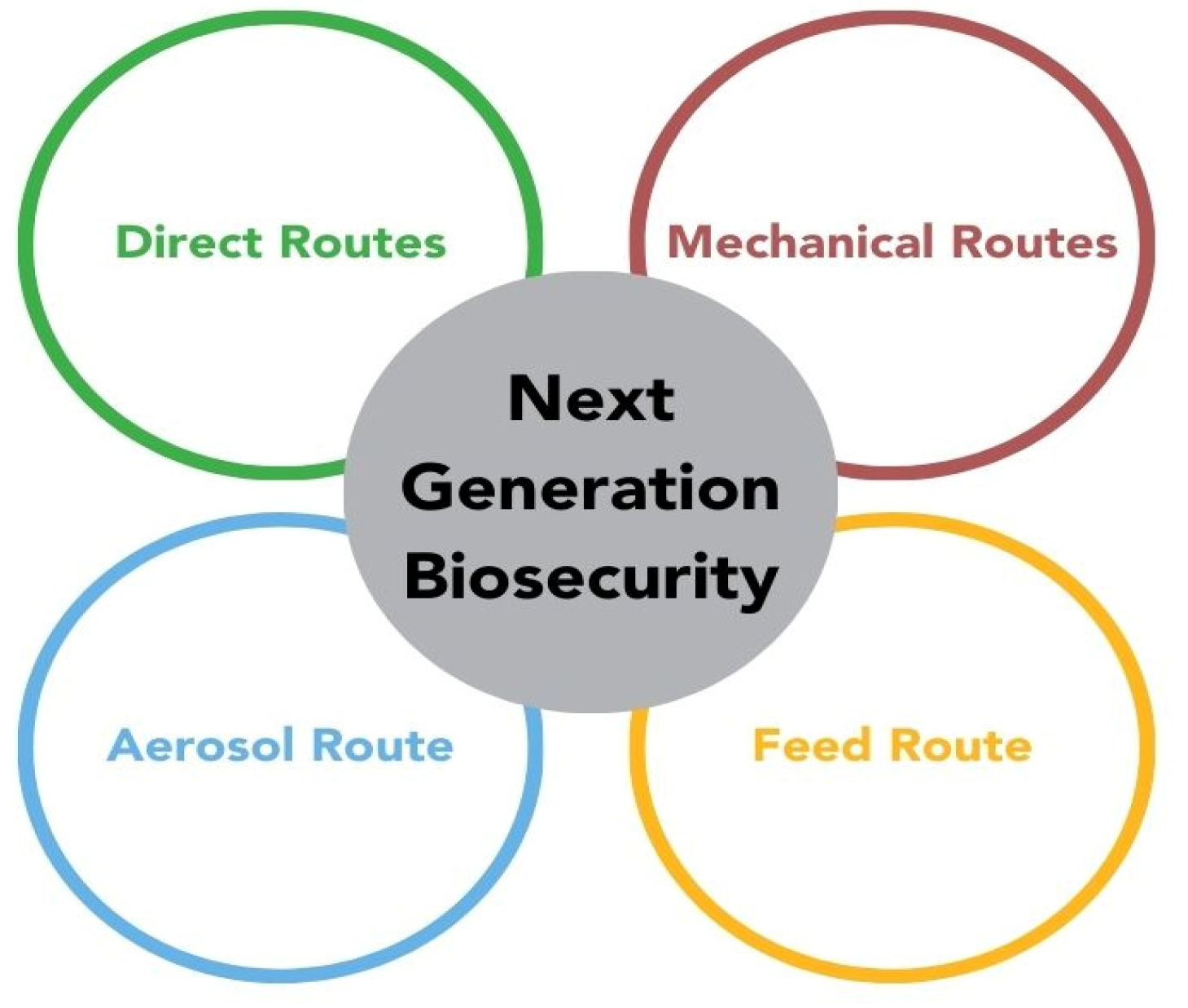
5. Next Generation Biosecurity: A Model for the Swine Industry
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holtkamp, D.; Kliebenstein, J.; Neumann, E.; Zimmerman, J.; Rotto, H.; Yoder, T.; Wang, C.; Yeske, P.; Mowrer, C.; Haley, C. Assessment of the economical impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. 2013, 21, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Studdert, V.P.; Gay, C.C.; Hinchcliff, K.W. Saunders Comprehensive Veterinary Dictionary, 5th ed.; Elsevier: St. Luis, MO, USA, 2021; p. 130. [Google Scholar]
- Cieslak, T.J.; Kortepeter, M.G. A brief history of biocontainment. Curr. Treat. Options Infect. Dis. 2016, 8, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, D.J.; Lin, H.; Wang, C.; O’connor, A.M. Identifying questions in the American Association of Swine Veterinarian’s PRRS risk assessment survey that are important for retrospective classifying swine herds according to whether they reported clinical PRRS outbreaks in the previous 3 years. Prev. Veter Med. 2012, 106, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Furutani, A.; Furuichi, T.; Hayakawa, Y.; Ishizeki, S.; Kano, R.; Koike, F.; Miyashita, M.; Mizukami, Y.; Watanabe, Y.; et al. Development of a biosecurity assessment tool and assessment of biosecurity levels by this tool on Japanese commercial swine farms. Prev. Veter. Med. 2020, 175, 104848. [Google Scholar] [CrossRef]
- Ritter, C.; Jansen, J.; Roche, S.; Kelton, D.F.; Adams, C.L.; Orsel, K.; Erskine, R.J.; Benedictus, G.; Lam, T.J.; Barkema, H.W. Invited review: Determinants of farmers’ adoption of management-based strategies for infectious disease prevention and control. J. Dairy Sci. 2017, 100, 3329–3347. [Google Scholar] [CrossRef]
- Vilalta, C.; Arruda, A.G.; Tousignant, S.J.P.; Valdes-Donoso, P.; Muellner, P.; Muellner, U.; Alkhamis, M.A.; Morrison, R.B.; Perez, A.M. A Review of the quantitative tools used to assess the epidemiology of porcine reproductive and respiratory syndrome virus in U.S. swine farms using Dr. Morrison’s Swine Health Monitoring Program Data. Front. Veter. Sci. 2017, 4, 94. [Google Scholar] [CrossRef]
- Wensvoort, G.; Terpstra, C.; Pol, J.M.A.; Ter Laak, E.A.; Bloemraad, M.; De Kluyver, E.P.; Kragten, C.; Van Buiten, L.; den Besten, A.; Wagenaar, F.; et al. Mystery swine diseases in the Netherlands: The isolation of Lelystad virus. Veter. Q. 1991, 13, 121–130. [Google Scholar] [CrossRef]
- Meulenberg, J.J. PRRSV, the virus. Vet. Res. 2000, 31, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, C.J.; Murtaugh, M.P.; Faaberg, K.S. Porcine reproductive and respiratory virus comparison: Divergent evolution on two continents. J. Virol. 1999, 73, 270–280. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yoon, K.-J.; Zimmerman, J.J.; Harmon, K.M.; Dixon, P.M.; Dvorak, C.M.T.; Murtaugh, M.P. Evolution of porcine reproductive and respiratory syndrome virus during sequential passages in pigs. J. Virol. 2002, 76, 4750–4763. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Lee, K.; Liu, X.; Gauger, P.C.; Zhang, J.; Martínez-López, B. Molecular evolution of porcine reproductive and respiratory syndrome virus field strains from two swine production systems in the Midwestern United States from 2001 to 2020. Microbiol. Spectr. 2022, 10, e0263421. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Li, G.; Moura, C.A.A.; Coleman, K.; Thomas, P.; Zhang, J.; Gauger, P.; Zeller, M.; Linhares, D. Complete coding genome sequence of a novel porcine reproductive and respiratory syndrome virus 2 restriction fragment length polymorphism 1-4-4 lineage 1C variant identified in Iowa, USA. Microbiol. Resour. Announc. 2021, 10, e0044821. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Deen, J.; A Dee, S. Influence of isolate pathogenicity on the aerosol transmission of Porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 2007, 71, 23–27. [Google Scholar] [PubMed]
- Brar, M.S.; Murtaugh, M.P.; Shi, M.; Leung, F.C.-C. Evolutionary diversification of type 2 porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2015, 96 Pt 7, 1570–1580. [Google Scholar] [CrossRef]
- Wills, R.; Zimmerman, J.; Yoon, K.-J.; Swenson, S.; McGinley, M.; Hill, H.; Platt, K.; Christopher-Hennings, J.; Nelson, E. Porcine reproductive respiratory syndrome virus: A persist infection. Veter. Microbiol. 1997, 55, 231–240. [Google Scholar] [CrossRef]
- Bierk, M.D.; Dee, S.A.; Rossow, K.D.; Otake, S.; Collins, J.E.; Molitor, T.W. Transmission of porcine reproductive and syndrome virus from persistently infected sows to contact controls. Can. J. Vet. Res. 2001, 65, 261–266. [Google Scholar]
- Christopher-Hennings, J.; Nelson, E.A.; Hines, R.J.; Nelson, J.K.; Swenson, S.L.; Zimmerman, J.J.; Chase, C.C.L.; Yaeger, M.J.; Benfield, D.A. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J. Veter. Diagn. Investig. 1995, 7, 456–464. [Google Scholar] [CrossRef]
- Swenson, S.L.; Hill, H.T.; Zimmerman, J.J.; Evans, L.E.; Landgraf, J.G.; Wills, R.W.; Sanderson, T.P.; McGinley, M.J.; Brevik, A.K.; Ciszewski, D.K.; et al. Excretion of porcine reproductive and respiratory syndrome virus in semen after experimentally induced infection in boars. J. Am. Veter. Med. Assoc. 1994, 204, 1943–1948. [Google Scholar] [CrossRef]
- Christopher-Hennings, J.; Nelson, E.A.; Hines, R.J.; Swenson, S.L.; Howard, T.H. Detection and duration of porcine reproductive and respiratory syndrome virus in semen by PCR. J. Clin. Microbiol. 1995, 33, 1730–1734. [Google Scholar] [CrossRef]
- Gradil, C.; Dubuc, C.; Eaglesome, M.D. Porcine reproductive and respiratory syndrome virus: Seminal transmission. Veter. Rec. 1996, 138, 521–522. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.A.; Rossow, K.D.; Joo, H.S.; Deen, J.; Molitor, T.W.; Pijoan, C. Transmission of porcine reproductive and respiratory syndrome virus by needles. Veter. Rec. 2002, 150, 114–115. [Google Scholar] [CrossRef]
- Madapong, A.; Saeng-Chuto, K.; Tantituvanont, A.; Nilubol, D. Safety of PRRSV-2 MLV vaccines administrated via the intramuscular or intradermal route and evaluation of PRRSV transmission upon needle-free and needle delivery. Sci. Rep. 2021, 11, 23107. [Google Scholar] [CrossRef]
- Pirtle, E.C.; Beran, G.W. Stability of porcine reproductive and respiratory syndrome virus in the presence of fomites commonly found on farms. J. Am. Veter. Med. Assoc. 1996, 208, 390–392. [Google Scholar] [CrossRef]
- Dee, S.; Deen, J.; Rossow, K.; Wiese, C.; Otake, S.; Joo, H.S.; Pijoan, C. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during cold weather. Can. J. Vet. Res. 2002, 66, 232–239. [Google Scholar] [PubMed]
- Dee, S.; Deen, J.; Rossow, K.; Weise, C.; Eliason, R.; Otake, S.; Joo, H.S.; Pijoan, C. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during warm weather. Can. J. Vet. Res. 2003, 67, 12–19. [Google Scholar] [PubMed]
- Mesa, V.L.; Munoz, A.Q.; Sobhy, N.M.; Corzo, C.A.; Goyal, S.M. Survival of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in the Environment. Veter. Sci. 2024, 11, 22. [Google Scholar] [CrossRef]
- Dee, S.; Deen, J.; Pijoan, C. Evaluation of 4 intervention strategies to prevent the mechanical transmission of porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 2004, 68, 19–26. [Google Scholar]
- Otake, S.; Dee, S.A.; Rossow, K.D.; Deen, J. Transmission of porcine reproductive and respiratory syndrome virus by coveralls, boots, and personnel. J. Swine Health Prod. 2002, 10, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, A.; Deen, J.; Dee, S. Further assessment of fomites and personnel as vehicles for the mechanical transport and transmission of porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 2009, 73, 298–302. [Google Scholar]
- Pitkin, A.; Otake, S.; Dee, S. A one-night downtime period prevents the spread of porcine reproductive and respiratory virus and Mycoplasma hyopneumoniae by personnel and fomites (boots and coveralls). J. Swine Health Prod. 2011, 19, 345–348. [Google Scholar] [CrossRef]
- Dee, S.A.; Deen, J.; Otake, S.; Pijoan, C. An experimental model to evaluate the role of transport vehicles as a source of transmission of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can. J. Vet. Res. 2004, 68, 128–133. [Google Scholar] [PubMed]
- Galvis, J.A.; Corzo, C.A.; Machado, G. Modelling and assessing additional transmission routes for porcine reproductive and respiratory syndrome virus: Vehicle movements and feed ingredients. Transbound. Emerg. Dis. 2022, 69, e1549–e1560. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.; Deen, J.; Burns, D.; Douthit, G.; Pijoan, C. An evaluation of disinfectants for the sanitation of porcine reproductive and respiratory syndrome virus-contaminated transport vehicles at cold temperatures. Can. J. Vet. Res. 2005, 69, 64–70. [Google Scholar]
- Dee, S.A.; Torremorell, M.; Thompson, B.; Deen, J.; Pijoan, C. An evaluation of thermos-assisted drying and decontamination for the elimination of PRRSV from contaminated livestock transport vehicles. Can. J. Vet. Res. 2005, 69, 58–63. [Google Scholar]
- Holtkamp, D.J.; Myers, J.; Thomas, P.R.; A Karriker, L.; Ramirez, A.; Zhang, J.; Wang, C. Efficacy of an accelerated hydrogen peroxide disinfectant to inactivate porcine epidemic diarrhea virus in swine feces on metal surfaces. Can. J. Vet. Res. 2017, 81, 100–107. [Google Scholar]
- Zimmerman, J.; Yoon, K.-J.; Pirtle, E.; Wills, R.; Sanderson, T.; McGinley, M. Studies of porcine reproductive and respiratory syndrome (PRRS) virus infection in avian species. Veter. Microbiol. 1997, 55, 329–336. [Google Scholar] [CrossRef]
- Trincado, C.; Dee, S.A.; Jacobson, L.; Rossow, K.D.; Otake, S.; Halvorson, D.; Pijoan, C. Studies about porcine reproductive and respiratory syndrome virus (PRRS) transmission by aerosol and non-porcine vectors under field conditions. In Proceedings of the Annual Meeting of American Association of Swine Veterinarians, Orlando, FL, USA, September 2002; pp. 503–506. [Google Scholar]
- Dee, S.A. Biosecurity: A critical review of today’s practices. In Proceedings of the 2nd International Swine Disease Eradication Symposium, St. Paul, MN, USA, 14 September 2002; pp. 25–32. [Google Scholar]
- Hooper, C.C.; Van Alstine, W.G.; Stevenson, G.W.; Kanitz, C.L. Mice and rats (laboratory and feral) are not a reservoir of PRRS virus. J. Veter. Diagn. Investig. 1994, 6, 13–15. [Google Scholar] [CrossRef]
- Wills, R.W.; Osorio, F.A.; Doster, A.R. Susceptibility of selected non-swine species to infection with PRRS virus. In Proceedings of the31st Annual Meeting of American Association of Swine Practitioners, Indianapolis, IN, USA, 11–14 March 2020; pp. 411–413. [Google Scholar]
- Reiner, G.; Fresen, C.; Bronnert, S.; Willems, H. Porcine reproductive and respiratory syndrome virus (PRRSV) infection in wild boars. Veter. Microbiol. 2009, 136, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Lee, C.H.; Hyun, B.H.; Kim, J.J.; Lim, S.L.; Song, J.Y.; Shin, Y.K. A survey of porcine reproductive and respiratory syndrome virus among wild boar population in Korea. J. Vet. Sci. 2012, 13, 377–383. [Google Scholar] [CrossRef]
- Kitamura, Y.; Saito, T.; Tanaka, E.; Takashima, Y. A serological survey of porcine reproductive and respiratory syndrome virus in wild boar in Gifu prefecture, Japan. J. Veter. Med Sci. 2022, 84, 1406–1409. [Google Scholar] [CrossRef]
- Mellor, P.; Wilkinson, P. Experimental transmission of African swine fever virus by Ornithodoros savignyi (Audouin). Res. Veter. Sci. 1985, 39, 353–361. [Google Scholar] [CrossRef]
- Clements, A. The Biology of MOSQUITOES Volume 2: Sensory Reception and Behaviour; CABI Publishing: New York, NY, USA, 1999; pp. 319–332. [Google Scholar]
- Moon, R.D. Muscid flies, Muscidae. In Medical and Veterinary Entomology; Academic Press: New York, NY, USA, 2002; pp. 279–301. [Google Scholar]
- Otake, S.; Dee, S.A.; Rossow, K.D.; Moon, R.D.; Trincado, C.; Pijoan, C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica). Vet. Rec. 2003, 152, 73–76. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.A.; Moon, R.D.; Rossow, K.D.; Trincado, C.; Pijoan, C. Survival of porcine and reproductive and respiratory syndrome virus in houseflies (Musca domestica). Can. J. Vet. Res. 2003, 67, 198–203. [Google Scholar] [PubMed]
- Otake, S.; Dee, S.A.; Moon, R.D.; Rossow, K.D.; Trincado, C.; Pijoan, C. Studies on the carriage and transmission of porcine reproductive and respiratory virus by individual houseflies (Musca domestica). Veter. Rec. 2004, 154, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Schurrer, J.A.; Dee, S.A.; Moon, R.D.; Rossow, K.D.; Mahlum, C.; Mondaca, E.; Otake, S.; Fano, E.; Collins, J.E.; Pijoan, C. Spatial dispersal of porcine reproductive and respiratory syndrome virus-contaminated flies after contact with experimentally infected pigs. Am. J. Veter. Res. 2004, 65, 1284–1292. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.A.; Rossow, K.D.; Moon, R.D.; Pijoan, C. Mechanical transmission of porcine reproductive and respiratory syndrome virus by mosquitoes (Aedes vexans). Can. J. Vet. Res. 2002, 66, 191–195. [Google Scholar]
- Otake, S.; Dee, S.; Moon, R.; Rossow, K.; Trincado, C.; Pijoan, C. Evaluation of mosquitoes (Aedes vexans) as biological vectors of porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 2003, 67, 265–270. [Google Scholar]
- Dee, S.A.; Martinez, B.C.; Clanton, C. Survival and infectivity of porcine reproductive and respiratory syndrome virus in swine lagoon effluent. Veter. Rec. 2005, 156, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Linhares, D.; Torremorell, M.; Joo, H.S.; Morrison, R. Infectivity of porcine reproductive and respiratory virus in pig manure at different temperature. Vet. Microbiol. 2012, 160, 23–28. [Google Scholar] [CrossRef]
- Guarino, H.; Moura, J.; Cox, R.; Goyal, S.; Patnayak, D. Survival of porcine reproductive and respiratory syndrome virus in fresh pork. Acta Veter. Hung. 2014, 62, 257–263. [Google Scholar] [CrossRef]
- Martin, R.G.; Steverink, P.J.G.M. Oral transmission of PRRS virus via the feeding of infected muscle to pigs. In Proceedings of the 17th International Pig Veterinarian Society Congress, Ames, IA, USA, 2–5 June 2002; p. 215. [Google Scholar]
- Bloemraad, M.; De-Kluisver, E.P.; Peterson, A.; Burkhardt, G.B.; Wensvoot, G. Porcine reproductive and respiratory syndrome: Temperature and pH stability of Lelystad virus and its survival in tissue specimens from viremic pigs. Vet. Microbiol. 1994, 42, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Mager, R.; Robinson, Y.; Dubuc, C.; Larochelle, R. Evaluation of the persistence of porcine reproductive and respiratory syndrome virus in pig carcasses. Vet. Rec. 1995, 137, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Cano, J.P.; Murtaugh, M.P.; Dee, S.A. Evaluation of the survival of porcine reproductive and respiratory syndrome virus in non-processed pig meat. Veter. Rec. 2007, 160, 907–908. [Google Scholar] [CrossRef]
- Alexander, T. A winter of windborne spread. Br. Veter. J. 1993, 149, 507–510. [Google Scholar] [CrossRef]
- Torremorell, M.; Pijoan, C.; Janni, K.; Walker, R.; Joo, H.S. Airborne transmission of Actinobacillus pleuropneumoniae and porcine reproductive and respiratory syndrome virus in nursery pigs. Am. J. Veter. Res. 1997, 58, 828–832. [Google Scholar] [CrossRef]
- Dee, S.A.; Deen, J.; Jacobson, L.; Rossow, K.D.; Mahlum, C.; Pijoan, C. Laboratory model to evaluate the role of aerosols in the transport of porcine reproductive and respiratory syndrome virus. Veter. Rec. 2005, 156, 501–504. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.; Corzo, C.; Oliveira, S.; Deen, J. Long-distance airborne transport of infectious PRRSV and Mycoplasma hyopneumoniae from a swine population infected with multiple viral variants. Veter. Microbiol. 2010, 145, 198–208. [Google Scholar] [CrossRef]
- Dee, S.; Otake, S.; Deen, J. Use of a production region model to assess the efficacy of various air filtration systems for preventing airborne transmission of porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae: Results from a 2-year study. Virus Res. 2010, 154, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.; Brands, L.; Nerem, J.; Schelkopf, A.; Spronk, G.; Kikuti, M.; Corzo, C.; Havas, K. Improvements in swine herd biosecurity reduce the incidence risk of porcine reproductive and respiratory syndrome virus in breeding herds in the Midwestern United States. J. Am. Veter. Med. Assoc. 2023, 262, 520–525. [Google Scholar] [CrossRef]
- Dee, S.; Cano, J.P.; Spronk, G.; Reicks, D.; Ruen, P.; Pitkin, A.; Polson, D. Evaluation of the long-term effect of air filtration on the occurrence of new PRRSV infections in large breeding herds in swine-dense regions. Viruses 2012, 4, 654–662. [Google Scholar] [CrossRef]
- Dee, S.A.; Clement, T.; Schelkopf, A.; Nerem, J.; Knudsen, D.; Christopher-Hennings, J.; Nelson, E. An evaluation of contaminated complete feed as a vehicle for porcine epidemic diarrhea virus infection of naïve pigs following consumption via natural feeding behavior: Proof of concept. BMC Vet. Res. Vol. 2014, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.; Shah, A.; Cochrane, R.; Clement, T.; Singrey, A.; Edler, R.; Spronk, G.; Niederwerder, M.; Nelson, E. Use of a demonstration project to evaluate viral survival in feed: Proof of concept. Transbound. Emerg. Dis. 2021, 68, 248–252. [Google Scholar] [CrossRef]
- Jones, C.K.; Woodworth, J.; Dritz, S.S.; Paulk, C.B. Reviewing the risk of feed as a vehicle for swine pathogen transmission. Veter. Med. Sci. 2020, 6, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.A.; Bauermann, F.V.; Niederwerder, M.C.; Singrey, A.; Clement, T.; Lima, M.D.; Long, C.; Patterson, G.; Sheahan, M.A.; Stoian, A.M.M.; et al. Survival of viral pathogens in animal feed ingredients under transboundary shipping models. PLoS ONE 2018, 13, e0194509. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.A.; Niederwerder, M.C.; Edler, R.; Hanson, D.; Singrey, A.; Cochrane, R.; Spronk, G.; Nelson, E. An evaluation of additives for mitigating the risk of virus-contaminated feed using an ice-block challenge model. Transbound. Emerg. Dis. 2020, 68, 833–845. [Google Scholar] [CrossRef]
- Dee, S.; Clement, T.; Nelson, E. Transmission of porcine reproductive and respiratory syndrome virus in domestic pigs via oral ingestion of feed material. J. Am. Veter. Med. Assoc. 2023, 262, 1–4. [Google Scholar] [CrossRef]
- Spronk, G.; Havas, K.; Patterson, G.; Dee, S.A. Will swine veterinarians lead by meeting the next generation needs of our industry? J. Am. Vet. Med. Assoc. 2022, 261, 424–429. [Google Scholar] [CrossRef]
- Torremorell, M.; Baker, R. Eradication of PRRS virus by changing the pig flow and introduction of negative replacements into positive sow farms. Proc. Allen D. Leman Swine Conf. 2000, 27, 59–62. [Google Scholar]
- Dee, S.A.; Molitor, T.W. Elimination of PRRS virus using a test and removal process. Vet. Rec. 1998, 143, 474–476. [Google Scholar] [CrossRef]
- Dee, S.A.; Bierk, M.D.; Deen, J.; Molitor, T.W. An evaluation of test and removal for elimination of porcine reproductive and respiratory syndrome virus from 5 swine farms. Can. J. Vet. Res. 2001, 65, 22–27. [Google Scholar]
- Corzo, C.A.; Mondaca, E.; Wayne, S.; Torremorell, M.; Dee, S.; Davies, P.; Morrison, R.B. Control and elimination of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Havas, K.A.; Brands, L.; Cochrane, R.; Spronk, G.D.; Nerem, J.; Dee, S.A. An assessment of enhanced biosecurity interventions and their impact on porcine reproductive and respiratory syndrome virus outbreaks within a managed group of farrow-to-wean farms, 2020–2021. Front. Veter. Sci. 2023, 9, 952383. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.-È.; Poljak, Z.; Arsenault, J.; D’Allaire, S. Epidemiological investigations in regards to porcine reproductive and syndrome (PRRS) in Quebec, Canada. Part 1: Biosecurity practices and their geographical distribution in two areas of different swine density. Prev. Veter. Med. 2012, 104, 74–83. [Google Scholar] [CrossRef] [PubMed]
| Years | Milestone |
|---|---|
| 2000 | PRRSV-naïve breeding stock and semen become commercially available. |
| 2001 | Area Spread of PRRSV is described. |
| 2002–2004 | Mechanical risks (contaminated transport, clothing/footwear, and incoming supplies) for the spread of PRRSV are identified. |
| 2005–2007 | Protocols of transport sanitation, personnel entry, and supply entry are validated. |
| 2007–2010 | Aerosol transmission and long-distance airborne transport of PRRSV are identified. |
| 2009–2012 | Air filtration for PRRSV prevention is validated. |
| 2014–2023 | The risk of contaminated feed for the transmission of viruses of veterinary significance is discovered. |
| 2014–2022 | Feed biosecurity protocols (additives, storage) are developed. |
| 2021–2024 | Next Generation Biosecurity concept developed and tested. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otake, S.; Yoshida, M.; Dee, S. A Review of Swine Breeding Herd Biosecurity in the United States to Prevent Virus Entry Using Porcine Reproductive and Respiratory Syndrome Virus as a Model Pathogen. Animals 2024, 14, 2694. https://doi.org/10.3390/ani14182694
Otake S, Yoshida M, Dee S. A Review of Swine Breeding Herd Biosecurity in the United States to Prevent Virus Entry Using Porcine Reproductive and Respiratory Syndrome Virus as a Model Pathogen. Animals. 2024; 14(18):2694. https://doi.org/10.3390/ani14182694
Chicago/Turabian StyleOtake, Satoshi, Mio Yoshida, and Scott Dee. 2024. "A Review of Swine Breeding Herd Biosecurity in the United States to Prevent Virus Entry Using Porcine Reproductive and Respiratory Syndrome Virus as a Model Pathogen" Animals 14, no. 18: 2694. https://doi.org/10.3390/ani14182694






