Morphophysiological Assessment of the Cervix during the Reproductive Cycle and Early Pregnancy in Does Using Computed Tomography and Oxytocin Receptor Immunohistochemistry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Computerized Tomography Scans (CT Scans)
2.3. Morphological Data
2.4. Masson Trichrome and Alcian Blue Staining
2.5. Oxytocin Receptor Immunohistochemistry
2.6. RNA Extraction, Reverse Transcription, and Real-Time qPCR
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Cervical Canal Morphometric Data, Gross Appearance, and CT Imaging
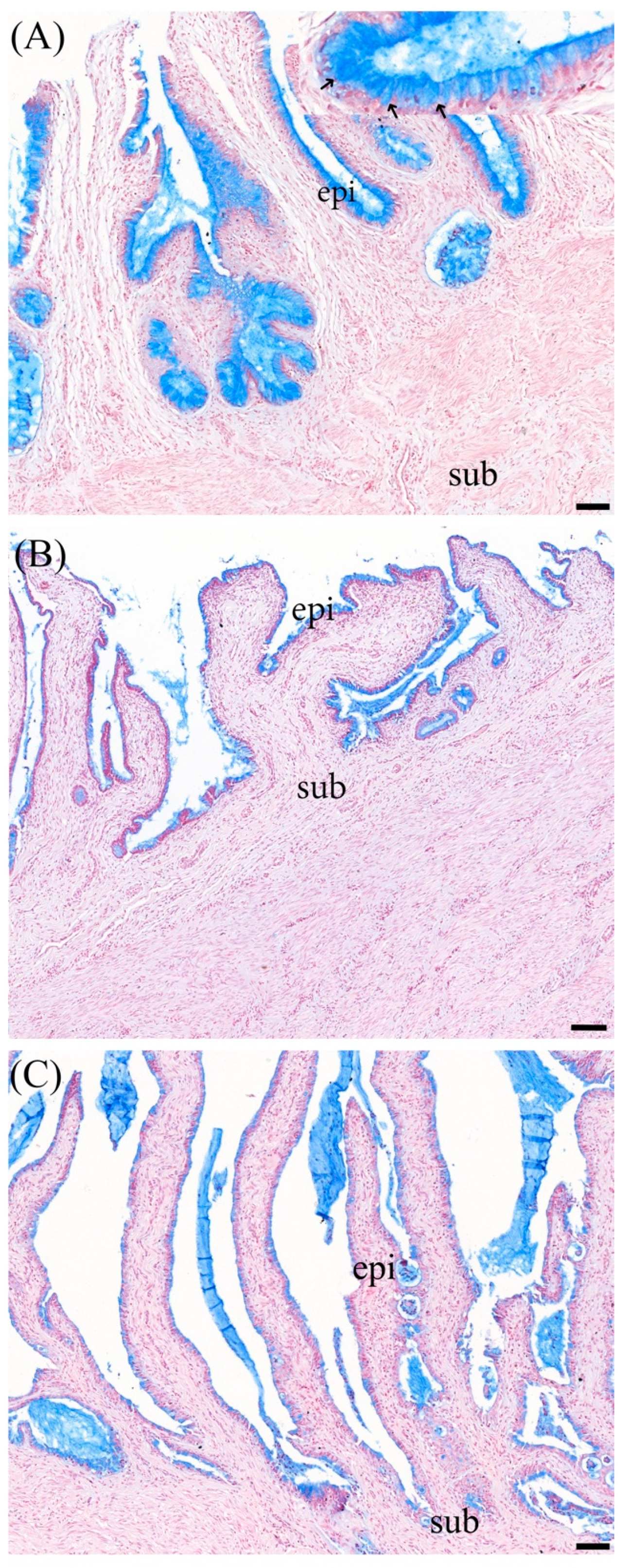
3.2. Masson Trichrome Staining
3.3. Alcian Blue Staining
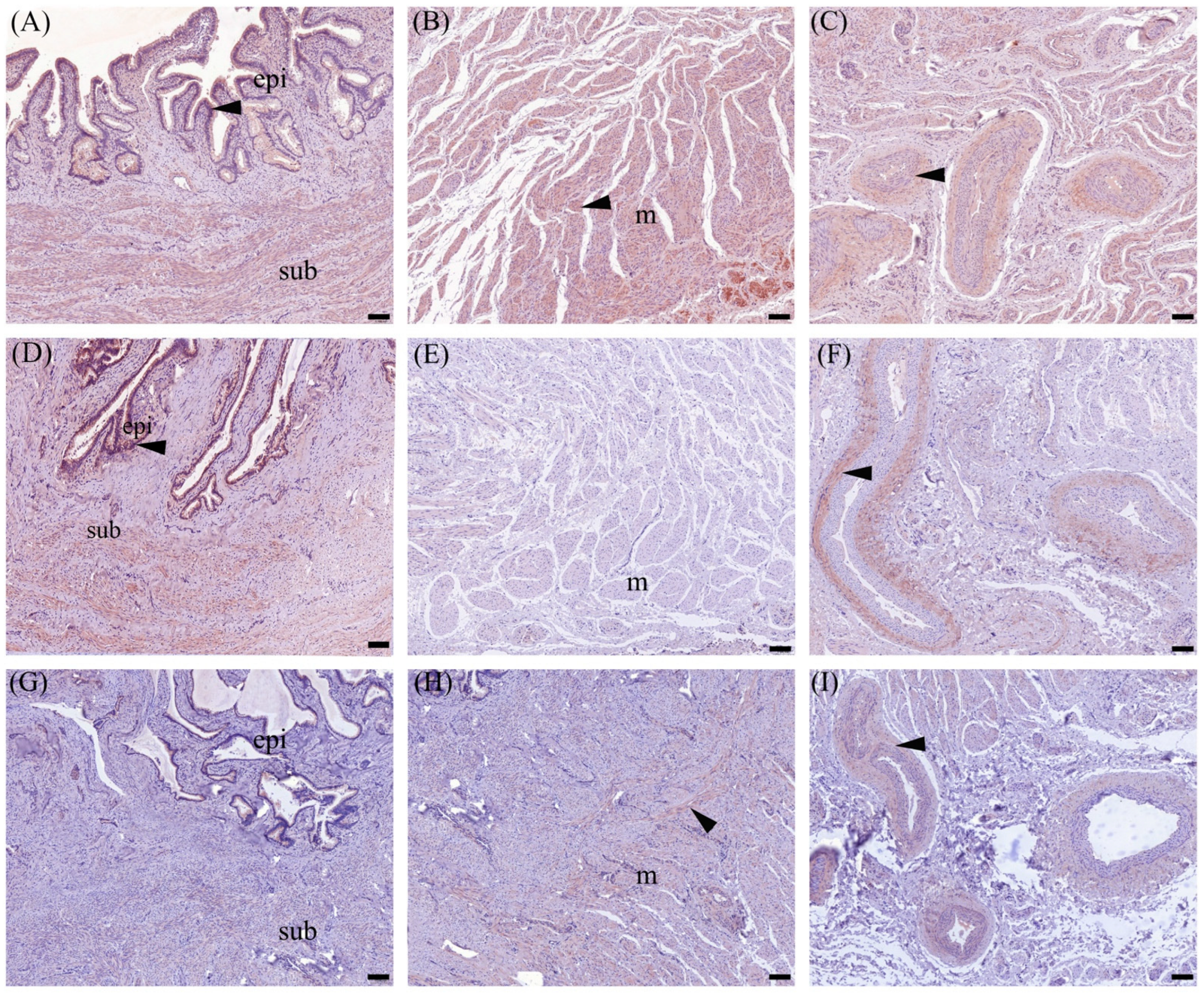
3.4. Immunohistochemistry
3.4.1. OXTR Expression in Cervix Tissue
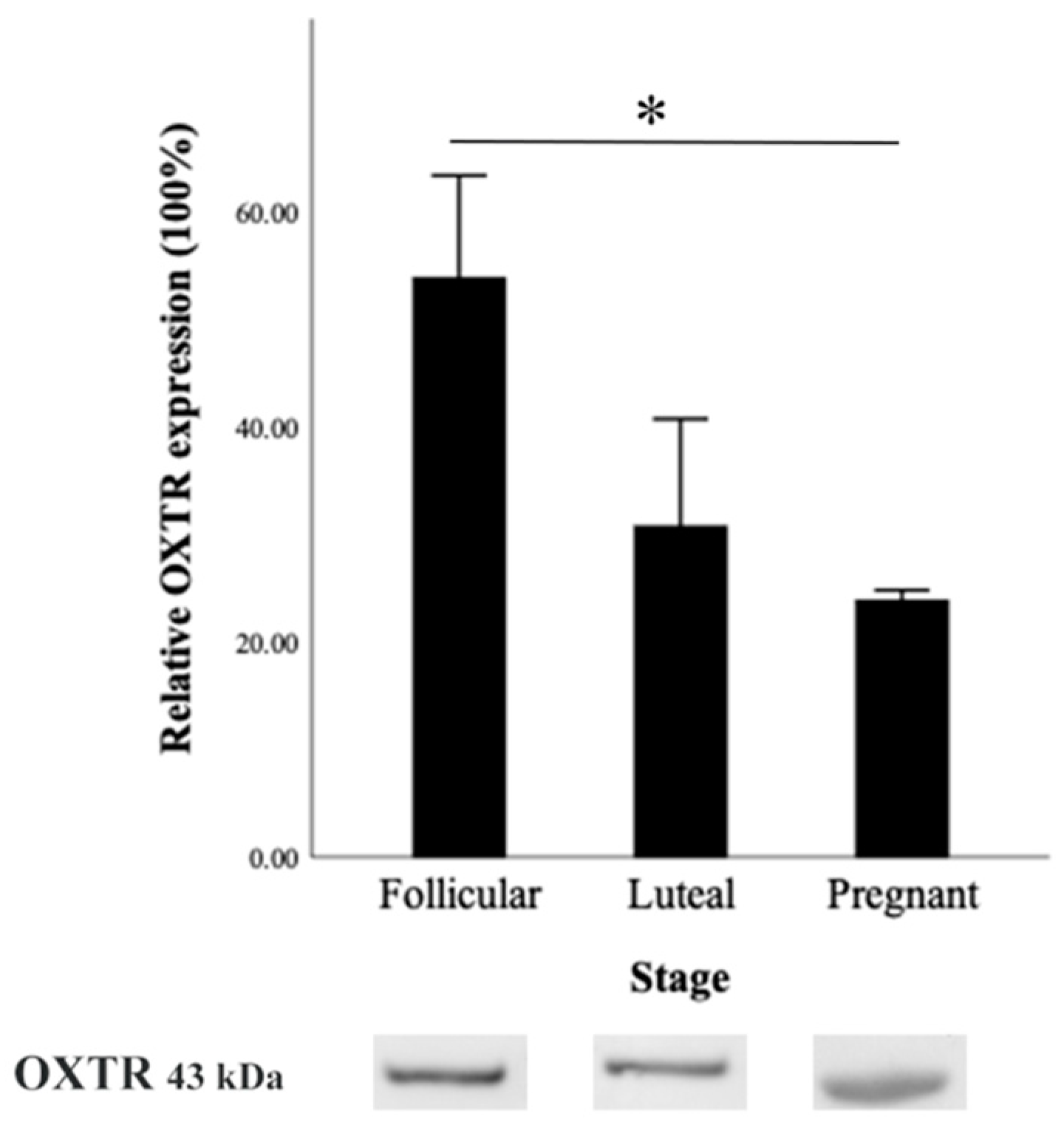
3.4.2. OXTR Protein Expression by Western Blot
3.4.3. OXTR mRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fatet, A.; Pellicer-Rubio, M.T.; Leboeuf, B. Reproductive cycle of goats. Anim. Reprod. Sci. 2011, 124, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Dayan, M.; Besoluk, K.; Eken, E.; Özkadif, S. Anatomy of the Cervical Canal in the Angora Goat (Capra hircus). Kafkas Univ. Vet. Fak. Derg. 2010, 16, 847–850. [Google Scholar]
- Kershaw, C.M.; Khalid, M.; McGowan, M.R.; Ingram, K.; Leethongdee, S.; Wax, G.; Scaramuzzi, R.J. The anatomy of the sheep cervix and its influence on the transcervical passage of an inseminating pipette into the uterine lumen. Theriogenology 2005, 64, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Piñón, M.; Tasende, C.; Casuriaga, D.; Bielli, A.; Genovese, P.; Garófalo, E.G. Collagen and matrix metalloproteinase-2 and -9 in the ewe cervix during the estrous cycle. Theriogenology 2015, 84, 818–826. [Google Scholar] [CrossRef]
- Falchi, L.; Taema, M.; La Clanche, S.; Scaramuzzi, R.J. The pattern of cervical penetration and the effect of topical treatment with prostaglandin and/or FSH and oxytocin on the depth of cervical penetration in the ewe during the peri-ovulatory period. Theriogenology 2012, 78, 376–384. [Google Scholar] [CrossRef]
- Leethongdee, S. Induction of Cervical Relaxation for Artificial Insemination in Sheep. Thai J. Vet. Med. 2011, 41, 403–407. [Google Scholar] [CrossRef]
- Russo, M.; England, G.C.W.; Catone, G.; Marino, G. Imaging of Canine Neoplastic Reproductive Disorders. Animals 2021, 11, 1213. [Google Scholar] [CrossRef]
- Kershaw-Young, C.M.; Scaramuzzi, R.J.; McGowan, M.R.; Pitsillides, A.A.; Wheeler-Jones, C.P.; Khalid, M. The effect of estradiol on COX-2, EP2, and EP4 mRNA expression and the extracellular matrix in the cervix of the hypogonadotrophic, ovariectomized ewe. Theriogenology 2010, 73, 620–628. [Google Scholar] [CrossRef]
- Gu, G.; Gao, Q.; Yuan, X.; Huang, L.; Ge, L. Immunolocalization of Adipocytes and Prostaglandin E2 and Its Four Receptor Proteins EP1, EP2, EP3, and EP4 in the Caprine Cervix During Spontaneous Term Labor1. Biol. Reprod. 2012, 86, 159. [Google Scholar] [CrossRef]
- Mahendroo, M. Cervical remodeling in term and preterm birth: Insights from an animal model. Reproduction 2012, 143, 429–438. [Google Scholar] [CrossRef]
- Vannuccini, S.; Bocchi, C.; Severi, F.M.; Challis, J.R.; Petraglia, F. Endocrinology of human parturition. Ann. D’endocrinologie 2016, 77, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C. Anatomy and physiology of cervical ripening. Clin. Obs. Gynecol. 1995, 38, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Noakes, D.E.; Parkinson, T.J.; England, G.C.W. Arthur’s Veterinary Reproduction and Obstetrics—E-Book; Elsevier Health Sciences: Edinburgh, UK, 2018. [Google Scholar]
- Karadaev, M.; Fasulkov, I.; Vassilev, N.; Petrova, Y.; Tumbev, A.; Petelov, Y. Ultrasound monitoring of the first trimester of pregnancy in local goats through visualisation and measurements of some biometric parameters. Bulg. J. Vet. Med. 2016, 19, 209–217. [Google Scholar] [CrossRef]
- Amer, H.A. Determination of first pregnancy and foetal measurements in Egyptian Baladi goats (Capra hircus). Vet. Ital. 2008, 44, 429–437. [Google Scholar] [PubMed]
- Wojtasiak, N.; Stankiewicz, T.; Udala, J. Ultrasound examination of pregnancy in the domestic goat (Capra hircus)—A review. Anim. Sci. Genet. 2020, 16, 65–78. [Google Scholar] [CrossRef]
- Amer, H.A. Ultrasonographic assessment of early pregnancy diagnosis, fetometry and sex determination in goats. Anim. Reprod. Sci. 2010, 117, 226–231. [Google Scholar] [CrossRef]
- Jantautsa, S.; Srisuwatanasagul, K.; Kongsonthana, K.; Viboonchan, P.; Srisuwatanasagul, S. Quantitative evaluation of different fixatives for Masson trichrome staining in canine intestinal tissue: An image analysis-based comparative study. Thai J. Vet. Med. 2024, 54, 55–62. [Google Scholar] [CrossRef]
- Prapaiwan, N.; Manee-In, S.; Moonarmart, W.; Srisuwatanasagul, S. The expressions in oxytocin and sex steroid receptors in the reproductive tissues of normal and unilateral cryptorchid dogs. Theriogenology 2017, 100, 59–65. [Google Scholar] [CrossRef]
- Prapaiwan, N.; Manee-In, S.; Olanratmanee, E.; Srisuwatanasagul, S. Expression of oxytocin, progesterone, and estrogen receptors in the reproductive tract of bitches with pyometra. Theriogenology 2017, 89, 131–139. [Google Scholar] [CrossRef]
- Srisuwatanasagul, K.; Panyaboriban, S.; Karapan, S.; Wittayarat, M.; Srisuwatanasagul, S. Geographic Variation in Testicular Morphometrics, Androgen Receptor Expression and Anti-Mullerian Hormone Levels in the Intermediate Roundleaf Bats across Distinct Regions in Thailand. Animals 2023, 13, 3287. [Google Scholar] [CrossRef]
- Permkam, C.; Suriyaphol, G.; Sirisawadi, S.; Tuntivanich, N. Biological Compositions of Canine Amniotic Membrane and Its Extracts and the Investigation of Corneal Wound Healing Efficacy In Vitro. Vet. Sci. 2022, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Maloy, A.; Alexander, S.; Andreas, A.; Nyunoya, T.; Chandra, D. Stain-Free total-protein normalization enhances the reproducibility of Western blot data. Anal. Biochem. 2022, 654, 114840. [Google Scholar] [CrossRef] [PubMed]
- Bergelin, I.; Valentin, L. Normal cervical changes in parous women during the second half of pregnancy–A prospective, longitudinal ultrasound study. Acta Obs. Gynecol. Scand. 2002, 81, 31–38. [Google Scholar] [CrossRef]
- Naqvi, S.M.K.; Pandey, G.K.; Gautam, K.K.; Joshi, A.; Geethalakshmi, V.; Mittal, J.P. Evaluation of gross anatomical features of cervix of tropical sheep using cervical silicone moulds. Anim. Reprod. Sci. 2005, 85, 337–344. [Google Scholar] [CrossRef]
- Kaoutar, E.L.; Allai, L.; Fatet, A.; Benmoula, A.; Hamidallah, N.; Badi, A.; El Amiri, B. Morphometry and depth of inseminating catheter penetration in prolific and non- prolific ewes at different ages: A post mortem study. Anim. Reprod. Sci. 2018, 196, 43–47. [Google Scholar] [CrossRef]
- van Engelen, E.; de Groot, M.W.; Breeveld-Dwarkasing, V.N.; Everts, M.E.; van der Weyden, G.C.; Taverne, M.A.; Rutten, V.P. Cervical ripening and parturition in cows are driven by a cascade of pro-inflammatory cytokines. Reprod. Domest. Anim. 2009, 44, 834–841. [Google Scholar] [CrossRef]
- Tantengco, O.A.G.; Menon, R. Contractile function of the cervix plays a role in normal and pathological pregnancy and parturition. Med. Hypotheses 2020, 145, 110336. [Google Scholar] [CrossRef]
- Timmons, B.; Akins, M.; Mahendroo, M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol. Metab. 2010, 21, 353–361. [Google Scholar] [CrossRef]
- Fair, S.; Meade, K.G.; Reynaud, K.; Druart, X.; de Graaf, S.P. The biological mechanisms regulating sperm selection by the ovine cervix. Reproduction 2019, 158, R1–R13. [Google Scholar] [CrossRef]
- Abril-Parreño, L.; Krogenæs, A.K.; Byrne, C.J.; Donovan, A.; Stuen, S.; Caldas, E.; Diskin, M.; Druart, X.; Fair, S. Ewe breed differences in cervical anatomy and cervicovaginal mucus properties: An international study. Theriogenology 2021, 160, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.; Hanrahan, J.P.; Tharmalingam, T.; Carrington, S.D.; Lonergan, P.; Evans, A.C.O.; Fair, S. Cervical mucus sialic acid content determines the ability of frozen-thawed ram sperm to migrate through the cervix. Reproduction 2019, 157, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Interactions of spermatozoa with the female reproductive tract: Inspiration for assisted reproduction. Reprod. Fertil. Dev. 2006, 19, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Loux, S.C.; Scoggin, K.E.; Troedsson, M.H.; Squires, E.L.; Ball, B.A. Characterization of the cervical mucus plug in mares. Reproduction 2017, 153, 197–210. [Google Scholar] [CrossRef]
- Pluta, K.; McGettigan, P.A.; Reid, C.J.; Browne, J.A.; Irwin, J.A.; Tharmalingam, T.; Corfield, A.; Baird, A.; Loftus, B.J.; Evans, A.C.; et al. Molecular aspects of mucin biosynthesis and mucus formation in the bovine cervix during the periestrous period. Physiol. Genom. 2012, 44, 1165–1178. [Google Scholar] [CrossRef]
- Leethongdee, S.; Khalid, M.; Scaramuzzi, R.J. The effect of the intracervical administration of FSH or LH on the levels of hyaluronan, COX2, and COX2 mRNA in the cervix of the nonpregnant ewe. Theriogenology 2016, 86, 2244–2253. [Google Scholar] [CrossRef]
- Xu, Q.; Zhuo, K.; Zhang, X.; Zhang, Y.; Xue, J.; Zhou, M.S. Oxytocin-induced endothelial nitric oxide dependent vasorelaxation and ERK1/2-mediated vasoconstriction in the rat aorta. Korean J. Physiol. Pharmacol. 2022, 26, 255–262. [Google Scholar] [CrossRef]
- Robinson, R.S.; Mann, G.E.; Lamming, G.E.; Wathes, D.C. Expression of oxytocin, oestrogen and progesterone receptors in uterine biopsy samples throughout the oestrous cycle and early pregnancy in cows. Reproduction 2001, 122, 965–979. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Ing, N.H. Steroid Hormones Regulate Gene Expression Posttranscriptionally by Altering the Stabilities of Messenger RNAs. Biol. Reprod. 2005, 72, 1290–1296. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Zhong, K.; Tong, A.; Jia, D. Targeted protein degradation: Mechanisms, strategies and application. Signal Transduct. Target. Ther. 2022, 7, 113. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Reproductive Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follicular Phase (n = 15) | Luteal Phase (n = 10) | Early Pregnancy (n = 15) | ||||||||
| Cervical folds (n) | 5.13 | ± | 0.26 | 5.50 | ± | 0.31 | 5.20 | ± | 0.26 | |
| Cervical width (mm) | 14.42 | ± | 0.66 a | 14.23 | ± | 0.89 a | 11.08 | ± | 0.70 b | |
| Cervical length (cm) | 4.47 | ± | 0.22 | 4.76 | ± | 0.30 | 4.47 | ± | 0.22 | |
| Cervical canal size | ||||||||||
| 1st fold | 1.82 | ± | 0.11 a | 1.37 | ± | 0.09 a,b | 0.36 | ± | 0.20 b | |
| 2nd fold | 1.81 | ± | 0.14 a | 1.21 | ± | 0.07 a,b | 0.32 | ± | 0.17 b | |
| 3rd fold | 1.71 | ± | 0.13 a | 1.02 | ± | 0.25 b | 0.11 | ± | 0.12 c | |
| Average canal size | 1.76 | ± | 0.09 a | 1.23 | ± | 0.10 b | 0.34 | ± | 0.18 c | |
| Cervical grade | ||||||||||
| Grade 1 | 6 | 5 | 7 | |||||||
| Grade 2 | 8 | 2 | 4 | |||||||
| Grade 3 | 1 | 3 | 4 | |||||||
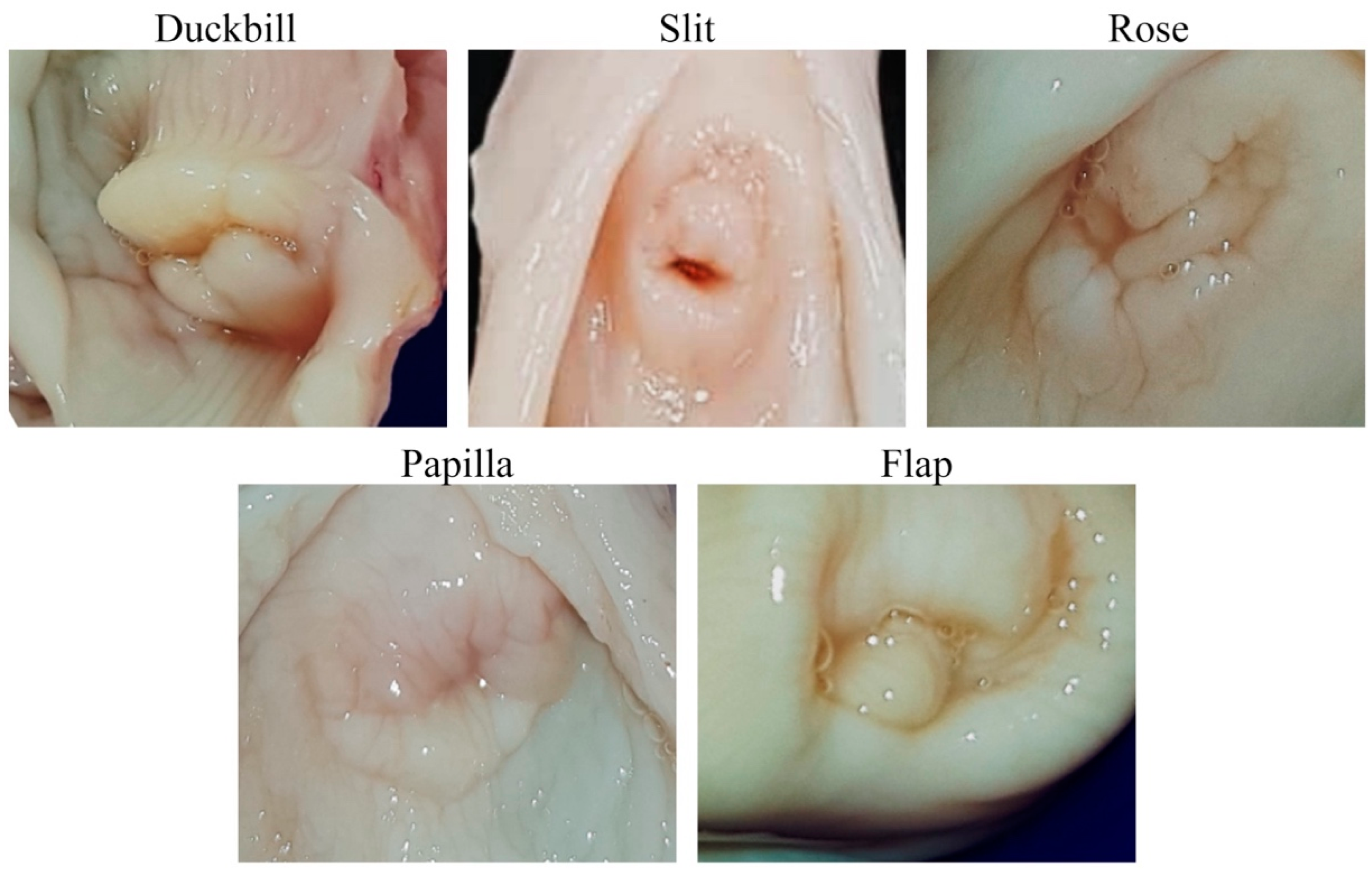
| External os type | ||||||||||
| Duckbill | 2 | 4 | 3 | |||||||
| Slit | 2 | 3 | 3 | |||||||
| Rose | 3 | 1 | 6 | |||||||
| Papilla | 2 | - | 1 | |||||||
| Flap | 6 | 2 | 2 | |||||||
| Parameter | Reproductive Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follicular Phase (n = 15) | Luteal Phase (n = 10) | Early Pregnancy (n = 15) | ||||||||
| Masson trichrome (1) Subepithelial layer | ||||||||||
| Collagen | 38.94 | ± | 1.98 | 36.52 | ± | 2.20 | 36.12 | ± | 1.87 | |
| Cervical muscle | ||||||||||
| Collagen | 15.28 | ± | 1.12 a | 13.14 | ± | 1.16 a | 22.44 | ± | 1.05 b | |
| Alcian blue stain (2) | ||||||||||
| Epithelial layer | 14.52 | ± | 0.57 | 16.93 | ± | 0.58 | 15.43 | ± | 1.11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanthawat, S.; Srisuwatanasagul, K.; Thatsanabunjong, F.; Chaivoravitsakul, N.; Panyaboriban, S.; Srisuwatanasagul, S. Morphophysiological Assessment of the Cervix during the Reproductive Cycle and Early Pregnancy in Does Using Computed Tomography and Oxytocin Receptor Immunohistochemistry. Animals 2024, 14, 2793. https://doi.org/10.3390/ani14192793
Kanthawat S, Srisuwatanasagul K, Thatsanabunjong F, Chaivoravitsakul N, Panyaboriban S, Srisuwatanasagul S. Morphophysiological Assessment of the Cervix during the Reproductive Cycle and Early Pregnancy in Does Using Computed Tomography and Oxytocin Receptor Immunohistochemistry. Animals. 2024; 14(19):2793. https://doi.org/10.3390/ani14192793
Chicago/Turabian StyleKanthawat, Supapit, Kongkiat Srisuwatanasagul, Fueangrat Thatsanabunjong, Nardtiwa Chaivoravitsakul, Saritvich Panyaboriban, and Sayamon Srisuwatanasagul. 2024. "Morphophysiological Assessment of the Cervix during the Reproductive Cycle and Early Pregnancy in Does Using Computed Tomography and Oxytocin Receptor Immunohistochemistry" Animals 14, no. 19: 2793. https://doi.org/10.3390/ani14192793






