Seasonal and Sexual Variations in Corticosterone and Total Triiodothyronine: A Pilot Study in Mediterranean Tortoises (Testudo hermanni)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Individuals, Location, and Management Conditions
2.2. Blood Sampling and Ethics
2.3. Hormone Quantification and Assay Validation
2.4. Statistical Analysis
3. Results
3.1. Assay Validations
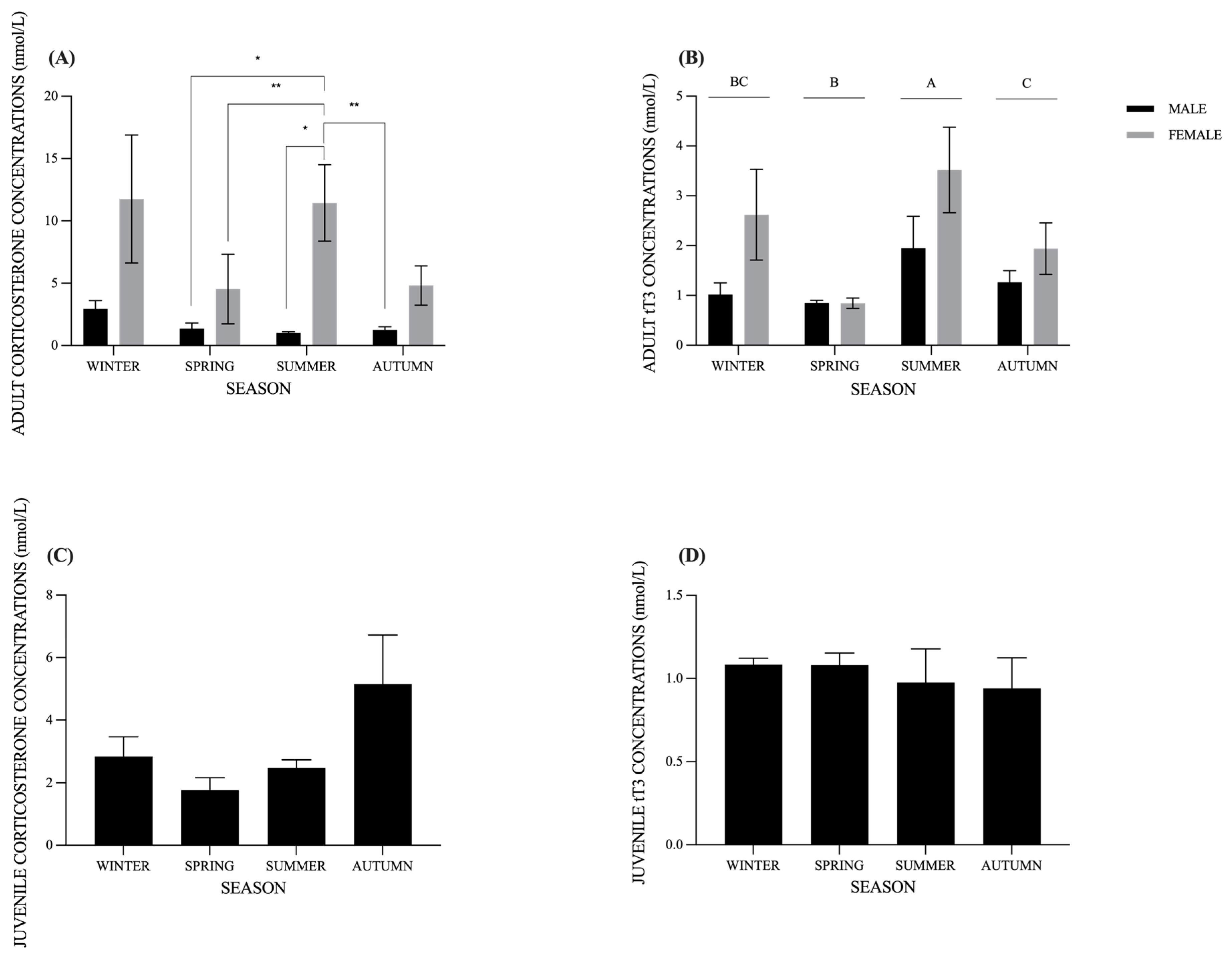
3.2. CORT and tT3 Seasonal Variations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez, M.; Livoreil, B.; Mantovani, S.; Boisselier, M.-C.; Crestanello, B.; Abdelkrim, J.; Bonillo, C.; Goutner, V.; Lambourdiere, J.; Pierpaoli, M.; et al. Genetic Variation and Population Structure in the Endangered Hermann’s Tortoise: The Roles of Geography and Human-Mediated Processes. J. Hered. 2014, 105, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zoboli, D.; Georgalis, G.L.; Arca, M.; Tuveri, C.; Carboni, S.; Lecca, L.; Pillola, G.L.; Rook, L.; Villani, M.; Chesi, F.; et al. An Overview of the Fossil Turtles from Sardinia (Italy). Hist. Biol. 2023, 35, 1484–1513. [Google Scholar] [CrossRef]
- Morales Pérez, J.V.; Serra, A.S. The Quaternary Fossil Record of the Genus Testudo in the Iberian Peninsula. Archaeological Implications and Diachronic Distribution in the Western Mediterranean. J. Archaeol. Sci. 2009, 36, 1152–1162. [Google Scholar] [CrossRef]
- Biello, R.; Zampiglia, M.; Corti, C.; Deli, G.; Biaggini, M.; Crestanello, B.; Delaugerre, M.; Di Tizio, L.; Leonetti, F.L.; Casari, S.; et al. Mapping the Geographic Origin of Captive and Confiscated Hermann’s Tortoises: A Genetic Toolkit for Conservation and Forensic Analyses. Forensic Sci. Int. Genet. 2021, 51, 102447. [Google Scholar] [CrossRef] [PubMed]
- Bech, N.; Nivelle, D.; Caron, S.; Ballouard, J.M.; Arnal, V.; Arsovski, D.; Golubović, A.; Bonnet, X.; Montgelard, C. Extent of Introgressive Hybridization in the Hermann’s Tortoise (Testudo hermanni hermanni) from the South of France. Eur. J. Wildl. Res. 2022, 68, 37. [Google Scholar] [CrossRef]
- Zenboudji, S.; Arnal, V.; Cheylan, M.; Bertolero, A.; Druelle, G.; Dubois, M.-P.; Montgelard, C. Isolation and Characterization of 32 Microsatellite Markers in Hermann’s Tortoise, Testudo hermanni (Testudinidae). Chelonian Conserv. Biol. 2018, 17, 291. [Google Scholar] [CrossRef]
- Highfield, A.C. Mediterranean Tortoises (Testudo spp.). In Companion Animal Care and Welfare: The UFAW Companion Animal Handbook; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 425–439. [Google Scholar]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring Stress in Wildlife: Techniques for Quantifying Glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Tracy, C.R.; Nussear, K.E.; Esque, T.C.; Dean-Bradley, K.; Tracy, C.R.; DeFalco, L.A.; Castle, K.T.; Zimmerman, L.C.; Espinoza, R.E.; Barber, A.M. The Importance of Physiological Ecology in Conservation Biology. Integr. Comp. Biol. 2006, 46, 1191–1205. [Google Scholar] [CrossRef]
- Moberg, G.P. Biological Response to Stress: Implications for Animal Welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI: Wallingford, UK, 2000; pp. 1–21. ISBN 9780851993591. [Google Scholar]
- Carbajal, A.; Serres-Corral, P.; Olvera-Maneu, S.; López-Béjar, M. Non-invasive Measurement of Glucocorticoids: The Reptile Perspective. J. Zool. 2024, 323, 87–96. [Google Scholar] [CrossRef]
- Martínez Silvestre, A. How to Assess Stress in Reptiles. J. Exot. Pet Med. 2014, 23, 240–243. [Google Scholar] [CrossRef]
- Rivera, S.; Lock, B. The Reptilian Thyroid and Parathyroid Glands. Vet. Clin. N. Am.-Exot. Anim. Pract. 2008, 11, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zena, L.A.; Dillon, D.; Hunt, K.E.; Navas, C.A.; Buck, C.L.; Bícego, K.C. Hormonal Correlates of the Annual Cycle of Activity and Body Temperature in the South-American Tegu Lizard (Salvator merianae). Gen. Comp. Endocrinol. 2020, 285, 113295. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.E.; Innis, C.J.; Merigo, C.; Rolland, R.M. Endocrine Responses to Diverse Stressors of Capture, Entanglement and Stranding in Leatherback Turtles (Dermochelys coriacea). Conserv. Physiol. 2016, 4, cow022. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Hazlerigg, D.G.; Ebling, F.J.P. Thyroid Hormone and Seasonal Rhythmicity. Front. Endocrinol. 2014, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Michael Romero, L. Seasonal Changes in Plasma Glucocorticoid Concentrations in Free-Living Vertebrates. Gen. Comp. Endocrinol. 2002, 128, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Leineweber, C.; Öfner, S.; Mathes, K.; Piepho, H.-P.; Marschang, R.E.; Stöhr, A.C. Thyroid Hormone Concentrations in Testudo spp. by Season and Sex. J. Herpetol. Med. Surg. 2022, 32, 84–95. [Google Scholar] [CrossRef]
- Norris, D.O. Thyroid and Reproduction in Amphibians and Reptiles. J. Exp. Zool. A Ecol. Integr. Physiol. 2023, 339, 869–877. [Google Scholar] [CrossRef]
- Licht, P.; Breitenbach, G.L.; Congdon, J.D. Seasonal Cycles in Testicular Activity, Gonadotropin, and Thyroxine in the Painted Turtle, Chrysemys picta, under Natural Conditions. Gen. Comp. Endocrinol. 1985, 59, 130–139. [Google Scholar] [CrossRef]
- Raiti, P. Endocrinology. In Mader’s Reptile and Amphibian Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 835–848.e3. ISBN 978-0-323-48253-0. [Google Scholar]
- Leineweber, C.; Öfner, S.; Stöhr, A.C.; Marschang, R.E.; Mathes, K. A Comparison of Thyroid Hormone Levels and Plasma Capillary Zone Electrophoresis in Red-Eared Sliders (Trachemys scripta elegans) and Map Turtles (Graptemys spp.) Depending on Season and Sex. Vet. Clin. Pathol. 2020, 49, 78–90. [Google Scholar] [CrossRef]
- Sengupta, A.; Ray, P.P.; Chaudhuri-Sengupta, S.; Maiti, B.R. Thyroidal Modulation Following Hypo- and Hyper-Thermia in the Soft-Shelled Turtle Lissemys punctata punctata Bonnoterre. Eur. J. Morphol. 2003, 41, 149–154. [Google Scholar]
- Boggs, A.S.P.; Hamlin, H.J.; Lowers, R.H.; Guillette, L.J. Seasonal Variation in Plasma Thyroid Hormone Concentrations in Coastal versus Inland Populations of Juvenile American Alligators (Alligator mississippiensis): Influence of Plasma Iodide Concentrations. Gen. Comp. Endocrinol. 2011, 174, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Sengupta, A.; Chaudhuri-Sengupta, S.; Maiti, B. Thyroid Responses to Altered Photoperiod in the Soft-Shelled Turtle Lissemys punctata punctata Bonnoterre. Acta Biol. Hung. 2007, 58, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hişmioğullari, Ş.E.; Kontaş Aşkar, T.; Altuğ, M.E.; Ergün, Y. Hormonal Profile of Mediterranean Green Turtles (Chelonia mydas). Turk. J. Vet. Anim. Sci. 2020, 44, 588–593. [Google Scholar] [CrossRef]
- Milnes, M.R.; Woodward, A.R.; Rooney, A.A.; Guillette, L.J. Plasma Steroid Concentrations in Relation to Size and Age in Juvenile Alligators from Two Florida Lakes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 923–930. [Google Scholar] [CrossRef]
- Currylow, A.F.T.; Rafeliarisoa, T.H.; Louis, E.E.; Stanford, C.B.; Randrianjafizanaka, S.T.; Chinn, S.M.; Crocker, D.E. Characterization of Seasonal Reproductive and Stress Steroid Hormones in Wild Radiated Tortoises, Astrochelys radiata. Gen. Comp. Endocrinol. 2017, 253, 70–78. [Google Scholar] [CrossRef]
- Kohel, K.A.; MacKenzie, D.S.; Rostal, D.C.; Grumbles, J.S.; Lance, V.A. Seasonality in Plasma Thyroxine in the Desert Tortoise, Gopherus agassizii. Gen. Comp. Endocrinol. 2001, 121, 214–222. [Google Scholar] [CrossRef]
- Sinervo, B.; Miles, D.B. Hormones and Behavior of Reptiles. In Hormones and Reproduction of Vertebrates; Elsevier: Amsterdam, The Netherlands, 2011; pp. 215–246. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://cran.r-project.org/package=emmeans (accessed on 4 February 2022).
- Bartolero, A. Tortuga Mediterránea–Testudo hermanni. Available online: http://www.vertebradosibericos.org/ (accessed on 20 August 2024).
- Ott, J.A.; Mendonça, M.T.; Guyer, C.; Michener, W.K. Seasonal Changes in Sex and Adrenal Steroid Hormones of Gopher Tortoises (Gopherus polyphemus). Gen. Comp. Endocrinol. 2000, 117, 299–312. [Google Scholar] [CrossRef]
- West, J.M.; Klukowski, M. Seasonal Changes in Baseline Corticosterone, Association with Innate Immunity, and Effects of Confinement in Free-Ranging Eastern Box Turtles, Terrapene carolina carolina. Gen. Comp. Endocrinol. 2018, 262, 71–80. [Google Scholar] [CrossRef]
- Sibeaux, A.; Michel, C.L.; Bonnet, X.; Caron, S.; Fournière, K.; Gagno, S.; Ballouard, J.M. Sex-Specific Ecophysiological Responses to Environmental Fluctuations of Free-Ranging Hermann’s Tortoises: Implication for Conservation. Conserv. Physiol. 2016, 4, cow054. [Google Scholar] [CrossRef]
- Lance, V.A.; Grumbles, J.S.; Rostal, D.C. Sex Differences in Plasma Corticosterone in Desert Tortoises, Gopherus agassizii, during the Reproductive Cycle. J. Exp. Zool. 2001, 289, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Drake, K.K.; Nussear, K.E.; Esque, T.C.; Barber, A.M.; Vittum, K.M.; Medica, P.A.; Tracy, C.R.; Hunter, K.W. Does Translocation Influence Physiological Stress in the Desert Tortoise? Anim. Conserv. 2012, 15, 560–570. [Google Scholar] [CrossRef]
- Racic, A.; Tylan, C.; Langkilde, T. Effects of Temperature on Plasma Corticosterone in a Native Lizard. Sci. Rep. 2020, 10, 16315. [Google Scholar] [CrossRef] [PubMed]
- Dupoué, A.; Brischoux, F.; Lourdais, O.; Angelier, F. Influence of Temperature on the Corticosterone Stress–Response: An Experiment in the Children’s Python (Antaresia childreni). Gen. Comp. Endocrinol. 2013, 193, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kahn, P.F.; Guyer, C.; Mendonça, M.T. Handling, Blood Sampling, and Temporary Captivity Do Not Affect Plasma Corticosterone or Movement Patterns of Gopher Tortoises (Gopherus polyphemus). Copeia 2007, 2007, 614–621. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Williams, C.A. Thyroid Function in a Lizard, a Tortoise and a Crocodile, Compared with Mammals. Comp. Biochem. Physiol. A Physiol. 1988, 90, 41–48. [Google Scholar] [CrossRef]
- Pajdak-Czaus, J.; Terech-Majewska, E.; Będzłowicz, D.; Mączyński, M.; Krystkiewicz, W.; Łabuć, S.; Platt-Samoraj, A.; Szweda, W. Applicability of Thyroxine Measurements and Ultrasound Imaging in Evaluations of Thyroid Function in Turtles. J. Vet. Res. 2019, 63, 267–273. [Google Scholar] [CrossRef]
- Virgilio, F.; Sciarrillo, R.; de Falco, M.; Laforgia, V.; Cavagnuolo, A.; Varano, L. Seasonality in Thyroid Function in Chalcides ocellatus (Reptilia, Scincidae). Ital. J. Zool. 2004, 71, 53–57. [Google Scholar] [CrossRef]
- Tyrrell, C.; Cree, A. Plasma Corticosterone Concentrations in Wild and Captive Juvenile Tuatara (Sphenodon punctatus). N. Z. J. Zool. 1994, 21, 407–416. [Google Scholar] [CrossRef]
| Winter | Spring | Summer | Autumn | |
|---|---|---|---|---|
| Female (n = 9) | 907 ± 92.5 (493–1415) | 919 ± 101 (500–1408) | 958 ± 93.4 (518–1432) | 926 ± 103 (478–1379) |
| Male (n = 7) | 557 ± 67.1 (339–830) | 622 ± 80.1 (357–851) | 606 ± 49.6 (378–807) | 588 ± 52.9 (351–791) |
| Juveniles (n = 6) | 104 ± 4.8 (84–117) | 113 ± 7.7 (91–135) | 107 ± 7.7 (83–138) | 97.8 ± 8.3 (75–127) |
| Winter | Spring | Summer | Autumn | |
|---|---|---|---|---|
| Female (n = 9) | 191 ± 18.1 (123–273) | 161.1 ± 31.7 (52–302) | 165.2 ± 63.5 (52–656) | 242.6 ± 98 (81–909) |
| Male (n = 7) | 139.3 ± 14.3 (74–184) | 82.1 ± 7.7 (52–117) | 103.3 ± 28.9 (28–308) | 262 ± 95.8 (106–797) |
| Juveniles (n = 6) | 176.5 ± 20 (122–147) | 96.5 ± 23.3 (63–211) | 59.7 ± 5.6 (41–83) | 144 ± 44.1 (88–320) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olvera-Maneu, S.; Navarro, X.; Serres-Corral, P.; Carbajal, A.; Martínez-Silvestre, A.; López-Béjar, M. Seasonal and Sexual Variations in Corticosterone and Total Triiodothyronine: A Pilot Study in Mediterranean Tortoises (Testudo hermanni). Animals 2024, 14, 2810. https://doi.org/10.3390/ani14192810
Olvera-Maneu S, Navarro X, Serres-Corral P, Carbajal A, Martínez-Silvestre A, López-Béjar M. Seasonal and Sexual Variations in Corticosterone and Total Triiodothyronine: A Pilot Study in Mediterranean Tortoises (Testudo hermanni). Animals. 2024; 14(19):2810. https://doi.org/10.3390/ani14192810
Chicago/Turabian StyleOlvera-Maneu, Sergi, Xavier Navarro, Paula Serres-Corral, Annaïs Carbajal, Albert Martínez-Silvestre, and Manel López-Béjar. 2024. "Seasonal and Sexual Variations in Corticosterone and Total Triiodothyronine: A Pilot Study in Mediterranean Tortoises (Testudo hermanni)" Animals 14, no. 19: 2810. https://doi.org/10.3390/ani14192810






