Electrolytically Ionized Abrasive-Free CMP (EAF-CMP) for Copper
Abstract
:1. Introduction
- (1)
- Effortless maintenance of particle dispersion stability;
- (2)
- Continuous mixing in a slurry tank and filtration are not required;
- (3)
- Maintenance of the flow velocity of the slurry to prevent particle agglomeration is not necessary.
2. Experimental Setup
2.1. Electrolytic Ionization of CMP Solution and CMP Machine
2.2. Experimental Conditions
3. Results and Discussion
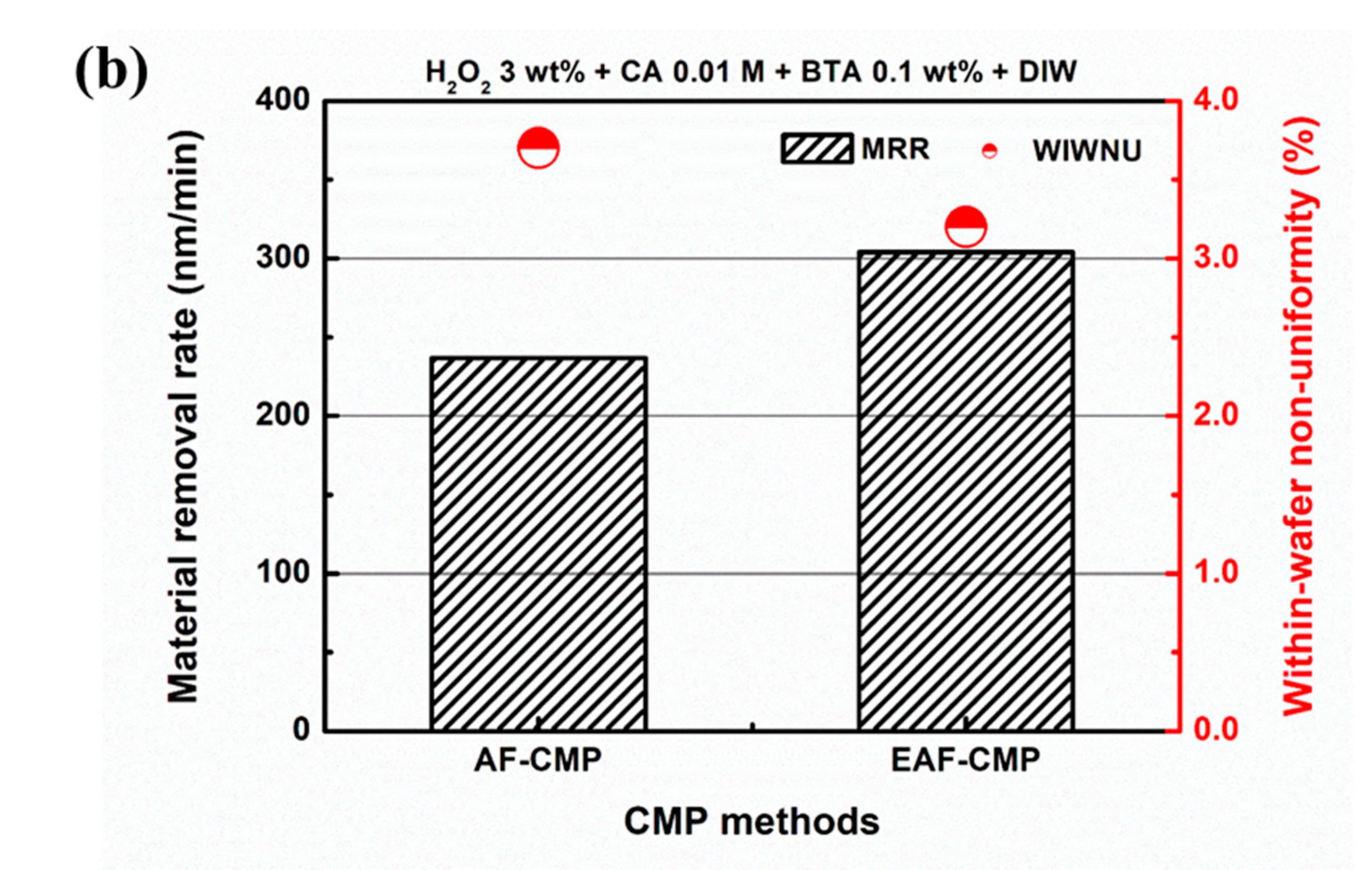
3.1. Comparison of AF-CMP and EAF-CMP
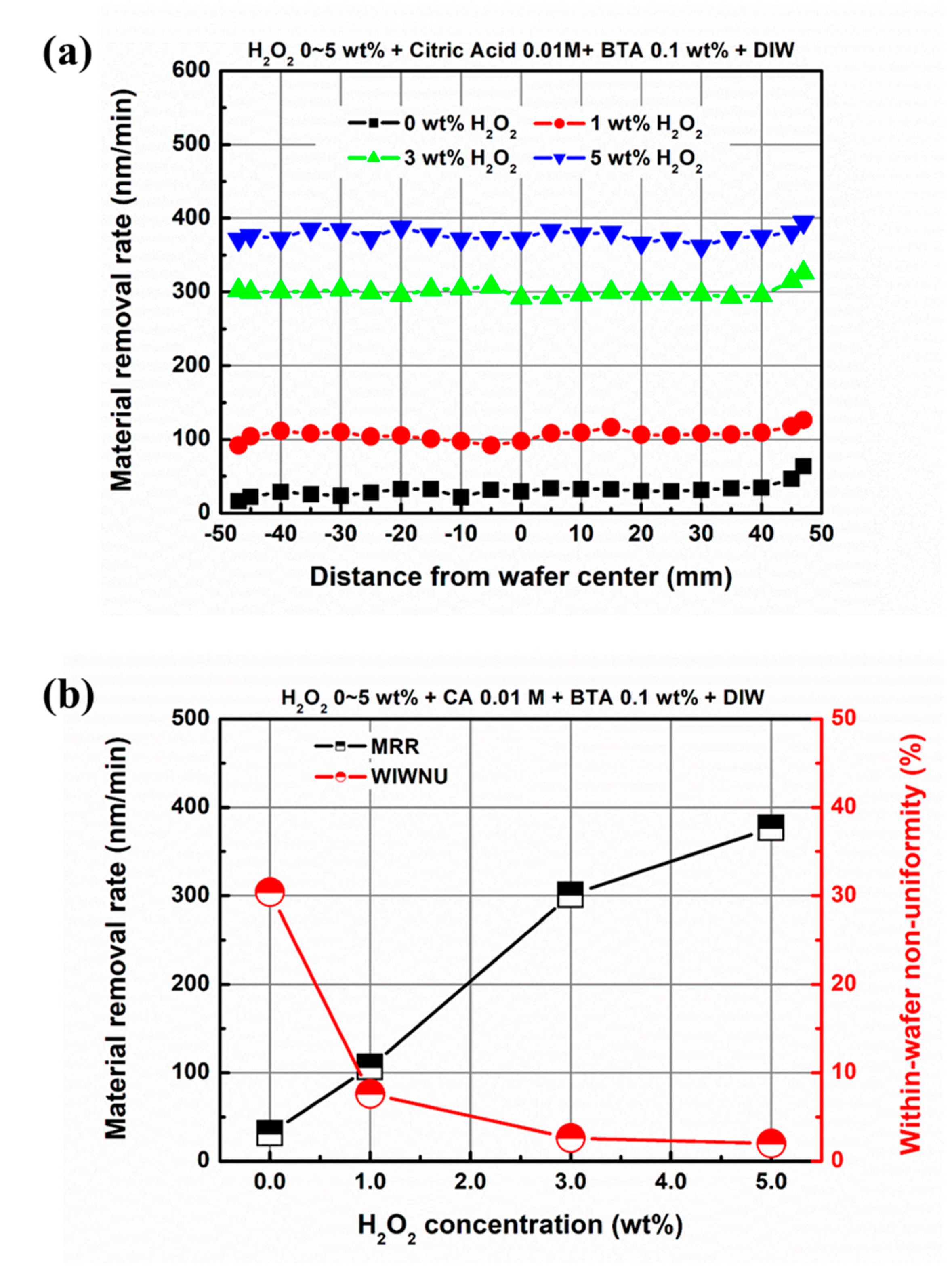
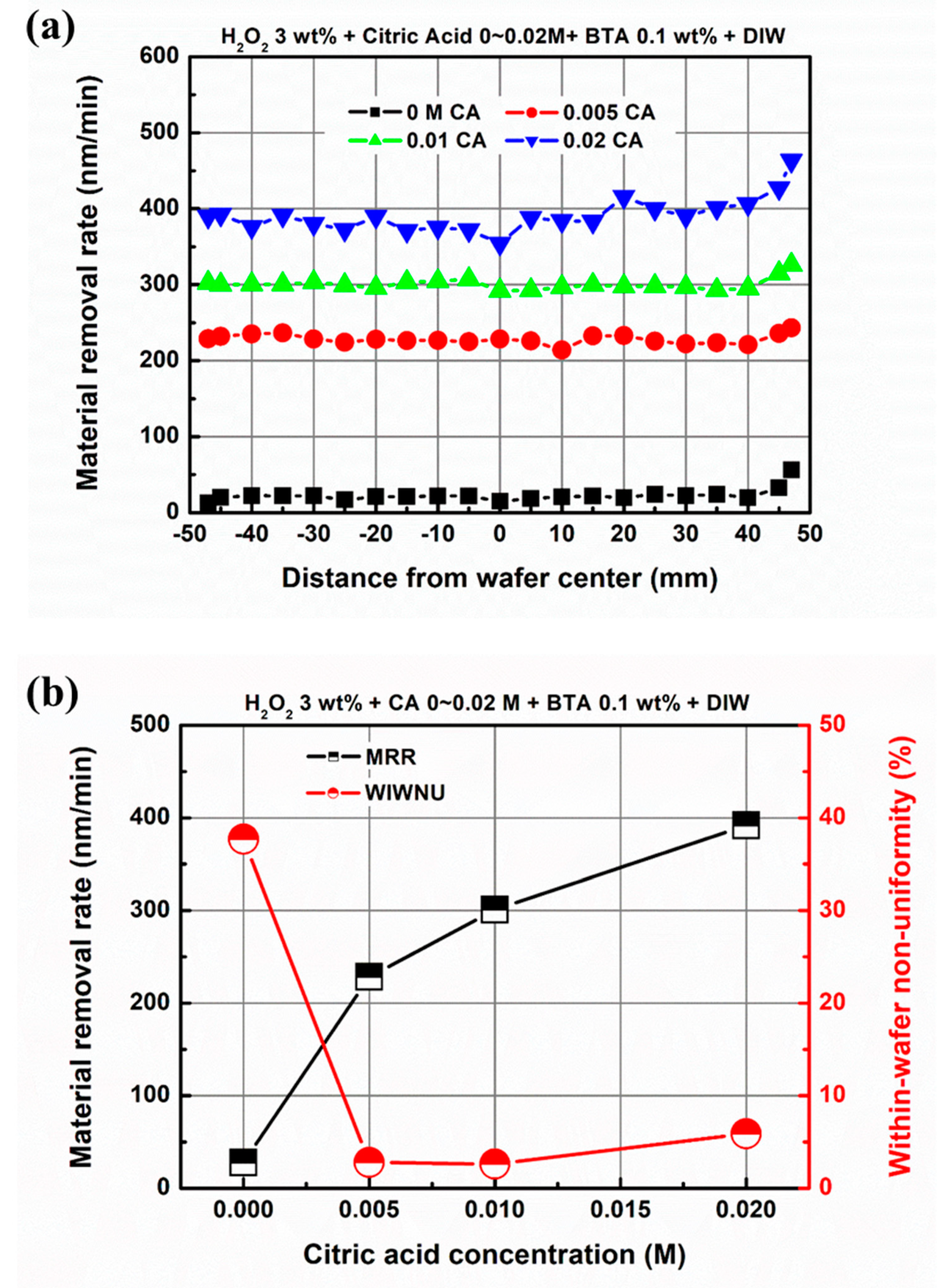
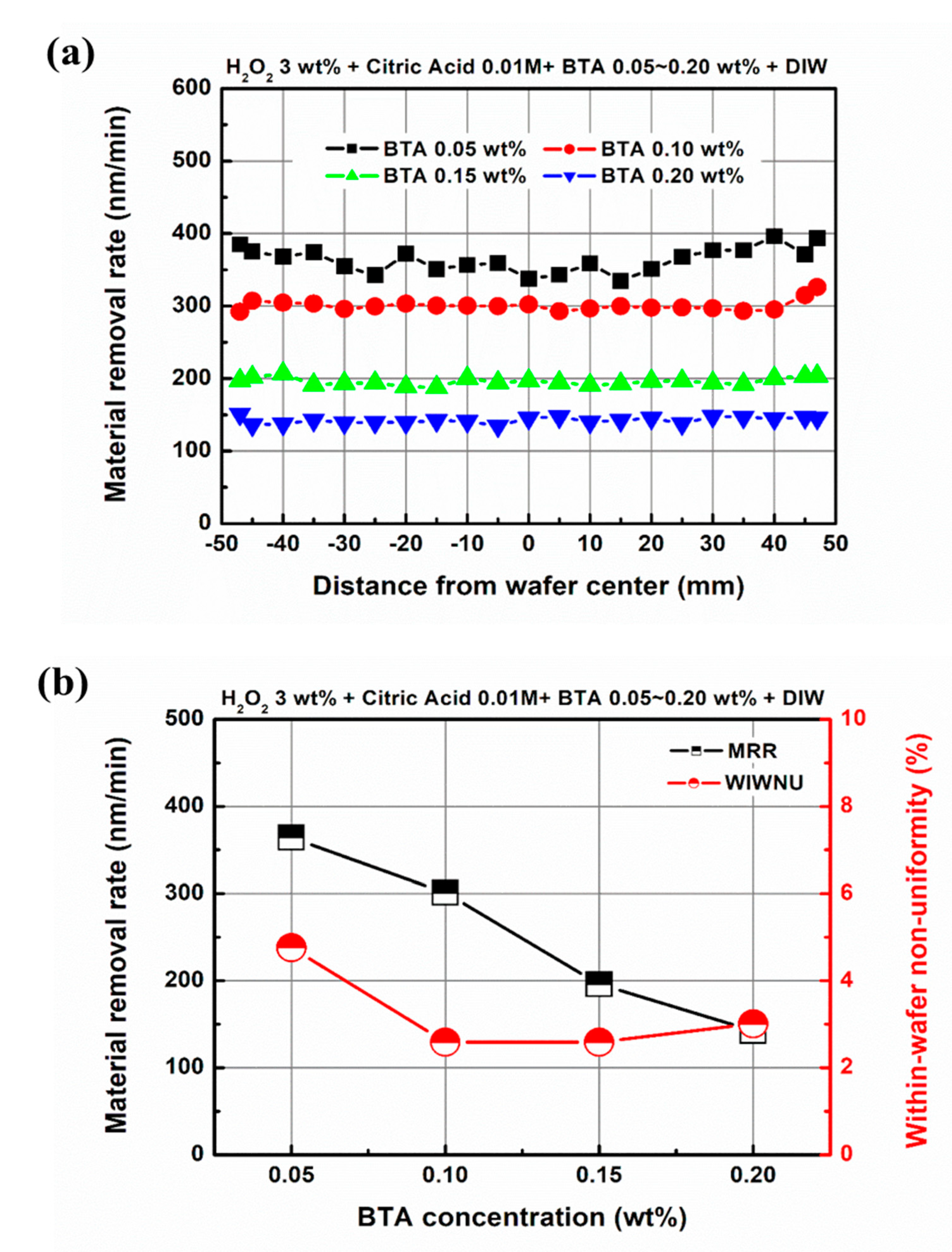
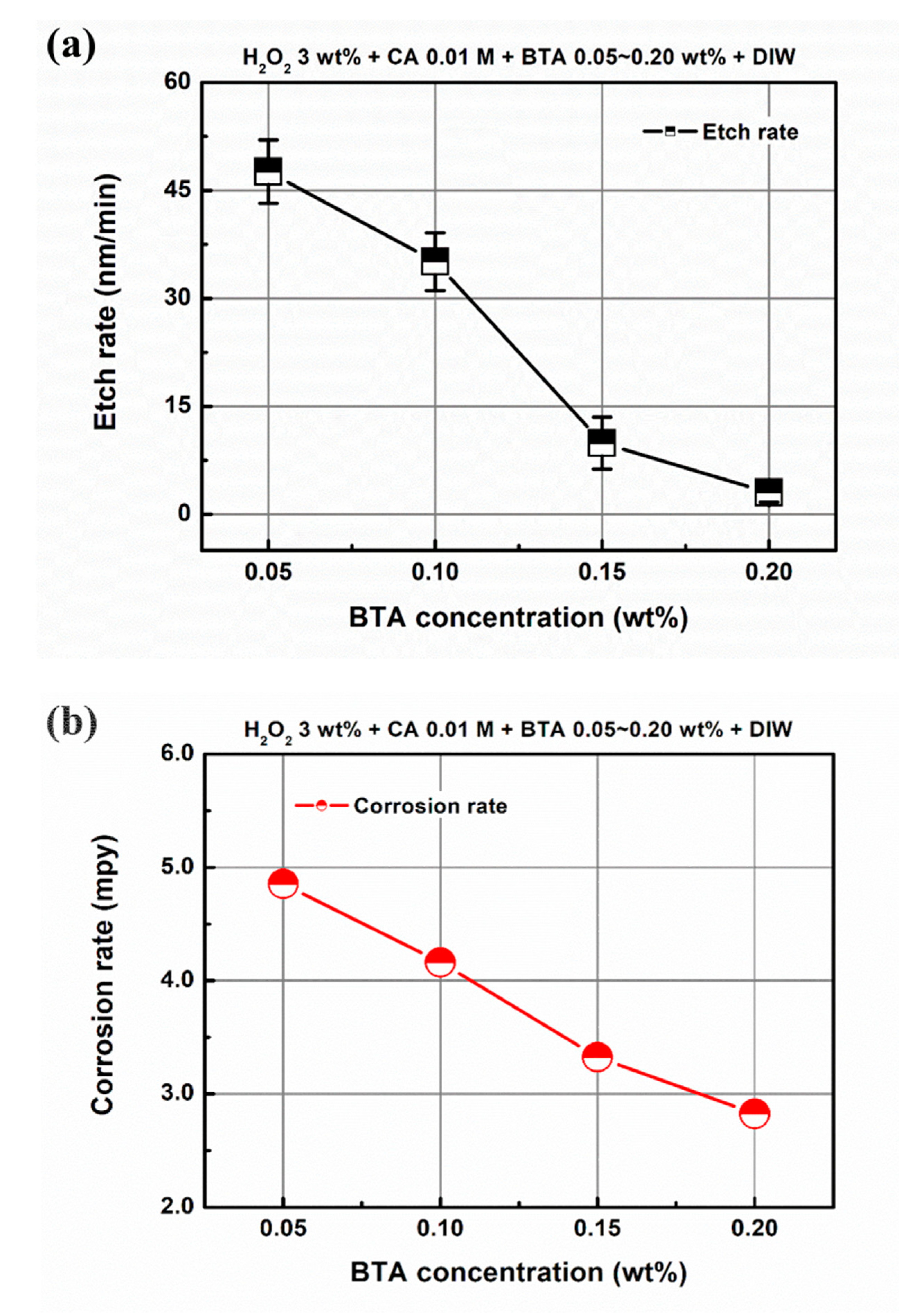
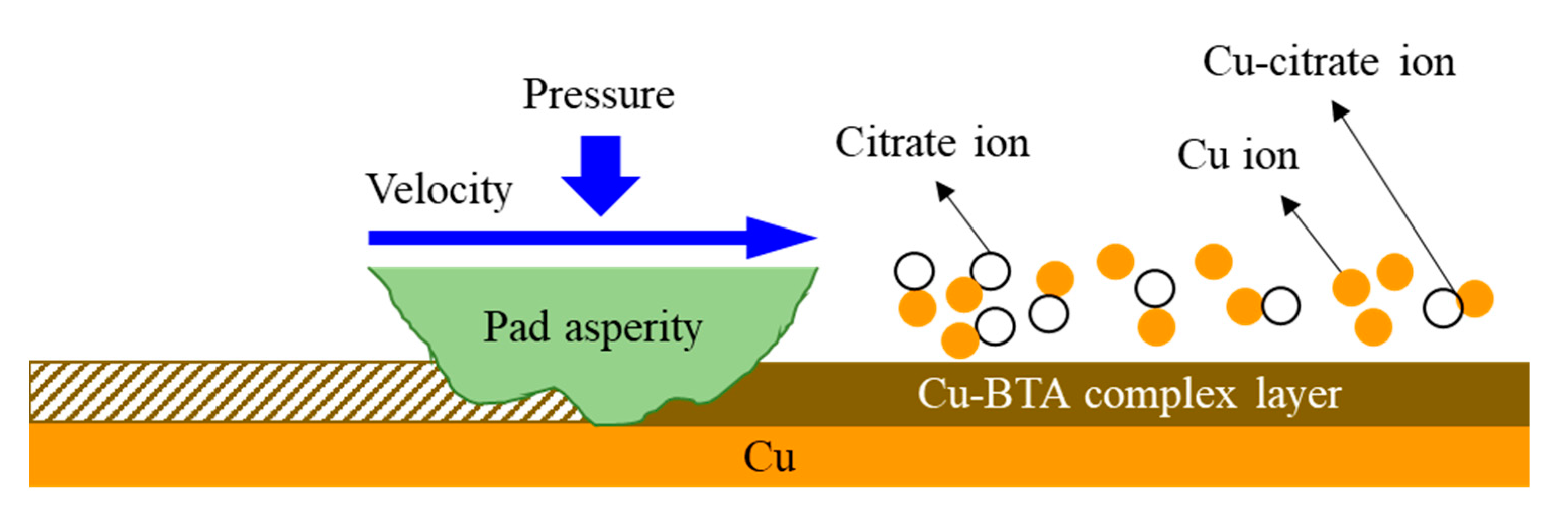
3.2. Effect of Chemical Component Concentration on Material Removal in EAF-CMP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Lee, D.; Jeong, H. Mechanical aspects of the chemical mechanical polishing process: A review. Int. J. Precis. Eng. Manuf. 2016, 17, 525–536. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S. Investigation of pad wear in CMP with swing-arm conditioning and uniformity of material removal. Precis. Eng. 2017, 49, 85–91. [Google Scholar] [CrossRef]
- Zhao, G.; Wei, Z.; Wang, W.; Feng, D.; Xu, A.; Liu, W.; Song, Z. Review on modeling and application of chemical mechanical polishing. Nanotechnol. Rev. 2020, 9, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Hara, T.; Balakumar, S. Chemical mechanical polishing of copper layer employing MnO2 slurry. Thin Solid Films 2004, 462–463, 186–191. [Google Scholar] [CrossRef]
- Hu, C.-K.; Luther, B.; Kaufman, F.B.; Hummel, J.; Uzoh, C.; Pearson, D.J. Copper interconnect integration and reliability. Thin Solid Films 1995, 262, 84–92. [Google Scholar] [CrossRef]
- Wrschka, P.; Hernandez, J.; Oehrlein, g.S.; King, J. Chemical mechanical planarization of copper damascene structures. J. Electrochem. Soc. 2000, 147, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Jeong, H. A wafer-scale material removal rate profile model for copper chemical mechanical planarization. Int. J. Mach. Tools Manuf. 2011, 51, 395–403. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, H.D. Chemical and mechanical balance in polishing of electronic materials for defect-free surfaces. CIRP Ann. Manuf. Technol. 2009, 58, 485–490. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.; Jeong, H. Slurry Components in Metal Chemical Mechanical Planarization (CMP) Process: A Review. Int. J. Precis. Eng. Manuf. 2016, 17, 1751–1762. [Google Scholar] [CrossRef]
- Lee, H. Environmental Impact of Concentration of Slurry Components in thick Copper CMP. Int. J. Precis. Eng. Manuf.-Green Tech. 2017, 4, 13–18. [Google Scholar] [CrossRef]
- Rui, X.; Yongsheng, W.; Yipu, W.; Haixu, L.; Jianxiu, S. Study on Oxidant in Chemical Mechanical Polishing of Copper. Trans. Electr. Electron. Mater. 2020, 21, 580–586. [Google Scholar] [CrossRef]
- Du, T.; Tamboli, D.; Desai, V. Electrochemical characterization of copper chemical mechanical polishing. Microelectron. Eng. 2003, 69, 1–9. [Google Scholar] [CrossRef]
- Gorantla, V.R.K.; Matijević, E.; Babu, S.V. Amino Acids as Complexing Agents in Chemical−Mechanical Planarization of Copper. Chem. Mater. 2005, 17, 2076–2080. [Google Scholar] [CrossRef]
- Jang, S.; Jeong, H.; Yuh, M.; Park, I.; Park, J. Effect of glycine on copper CMP. Int. J. Precis. Eng. Manuf.-Green Tech. 2016, 3, 155–159. [Google Scholar] [CrossRef]
- Kim, T.; Lee, H. Preliminary Study on fluidized Bed Chemical Mechanical Polishing (FB-CMP) Process for Stainless Steel 304 (SS304). Micromachines 2020, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-Y.; Cho, B.-J.; Yerriboina, N.P.; Lee, C.-H.; Hwang, J.-K. Selection and Optimization of Corrosion Inhibitors for Improved Cu CMP and Post-Cu CMP Cleaning. ECS J. Solid State Sci. Technol. 2019, 8, P3058. [Google Scholar] [CrossRef]
- Deshpande, D.; Kuiry, S.C.; Klimov, M.; Obeng, Y.; Seal, S. Chemical mechanical planarization of copper: Role of oxidants and inhibitors. J. Electrochem. Soc. 2004, 151, G788–G800. [Google Scholar] [CrossRef]
- Du, T.; Luo, Y.; Desai, V. The combinatorial effect of complexing agent and inhibitor on chemical-mechanical planarization of copper. Microelectron. Eng. 2004, 71, 90–97. [Google Scholar] [CrossRef]
- Eom, D.-H.; Kim, I.-K.; Han, J.-H.; Park, J.-G. The Effect of Hydrogen Peroxide in a Citric Acid Based Copper Slurry on Cu Polishing. J. Electrochem. Soc. 2007, 154, D38–D44. [Google Scholar] [CrossRef]
- Gorantla, V.R.K.; Assiongbon, K.A.; Babu, S.V.; Roy, D. Citric Acid as a Complexing Agent in CMP of Copper: Investigation of Surface Reactions Using Impedance Spectroscopy. J. Electrochem. Soc. 2005, 152, G404–G410. [Google Scholar] [CrossRef]
- Jung, W.; Park, B.; Lee, H.; Jeong, H. Effects of Citric Acid as a Complexing Agent on Material Removal in Cu CMP. J. Korean Inst. Electr. Electron. Mater. Eng. 2006, 19, 889–893. [Google Scholar]
- Krishnan, M.; Nalaskowski, J.W.; Cook, L.M. Chemical Mechanical Planarization: Slurry Chemistry, Materials, and Mechanisms. Chem. Rev. 2010, 110, 178–205. [Google Scholar] [CrossRef]
- Cho, B.-J.; Shima, S.; Hamada, S.; Park, J.-G. Investigation of cu-BTA complex formation during Cu chemical mechanical planarization process. Appl. Surf. Sci. 2016, 384, 505–510. [Google Scholar] [CrossRef]
- Lee, H.; Joo, S.; Jeong, H. Mechanical Effect of colloidal silica in copper chemical mechanical planarization. J. Mater. Proc. Technol. 2009, 209, 6134–6139. [Google Scholar] [CrossRef]
- Lee, H.; Park, B.; Jeong, H. Mechanical effect of process condition and abrasive concentration on material removal rate profile in copper chemical mechanical planarization. J. Mater. Proc. Technol. 2009, 209, 1729–1735. [Google Scholar] [CrossRef]
- Wei, K.-H.; Wang, Y.-S.; Liu, C.-P.; Chen, K.-W.; Wang, Y.-L.; Cheng, Y.-L. The influence of abrasive particle size in copper chemical mechanical planarization. Surf. Coat. Technol. 2013, 231, 543–545. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Han, J.-H.; Song, J.-H.; Park, J.-G. Frictional behavior and particle adhesion of abrasive particles during Cu CMP. Mater. Res. Soc. Symp. Proc. 2005, 867, W6.2.1–W6.2.6. [Google Scholar] [CrossRef]
- Kwon, T.-Y.; Ramachandran, M.; Park, J.-G. Scratch formation and its mechanism in chemical mechanical planarization (CMP). Friction 2013, 1, 279–305. [Google Scholar] [CrossRef] [Green Version]
- Pandija, S.; Roy, D.; Babu, S.V. Chemical mechanical planarization of copper using abrasive-free solutions of oxalic acid and hydrogen peroxide. Mater. Chem. Phys. 2007, 102, 144–151. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Janjam, S.V.S.B.; Patri, U.B.; Roy, D.; Babu, S.V. Comparison of dicarboxylic acids as complexing agents for abrasive-free chemical mechanical planarization of copper. Microelectron. Eng. 2007, 84, 80–86. [Google Scholar] [CrossRef]
- Denardis, D.; Sorooshian, J.; Habiro, M.; Rogers, C.; Philipossian, A. Tribology and Removal Rate Characteristics of Abrasive-Free Slurries for Copper CMP Applications. Jpn. J. Appl. Phys. 2003, 42, 6809–6814. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Sun, L.; Tsai, S.; Liu, F.Q.; Chen, L. A Low Cost and Residue-Free Abrasive-Free Copper CMP Process with Low Dishing, Erosion and Oxide Loss. In Proceedings of the IEEE 2001 International Interconnect Technology Conference (Cat. No.01EX461), Burlingame, CA, USA, 6 June 2001; pp. 137–139. [Google Scholar]
- Hanazono, M.; Amanokura, J.; Kamigata, Y. Development and Application of an Abrasive-Free Polishing Solution for Copper. MRS Bull. 2002, 27, 772–775. [Google Scholar] [CrossRef]
- Penta, N.K. 9-Abrsive-free and ultra-low abrasive chemical mechanical polishing (CMP) processes. In Advances in Chemical Mechanical Planarization (CMP), 1st ed.; Babu, S., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 213–227. [Google Scholar]
- Lee, D.; Lee, H.; Jeong, S.; Yuh, M.; Jeong, H. Surface Activation by Electrolytically Ionized Slurry during Cu CMP. ECS J. Solid State Sci. Technol. 2019, 8, P3053–P3057. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel Slopes and Corrosion Rates Obtained in the Pre-Tafel Region of Polarization Curves. Corrosio Sci. 2005, 47, 3178–3186. [Google Scholar] [CrossRef]
- Lu, J.; Garland, J.E.; Pettit, C.M.; Babu, S.V.; Roy, D. Relative Roles of H2O2 and Glycine in CMP of Copper Studied with Impedance Spectroscopy. J. Electrochem. Soc. 2004, 151, G717–G722. [Google Scholar] [CrossRef]
- Nolan, L.M.; Cadien, K.C. Chemically enhanced synergistic wear: Acopper chemical mechanical polishing case study. Wear 2013, 307, 155–163. [Google Scholar] [CrossRef]

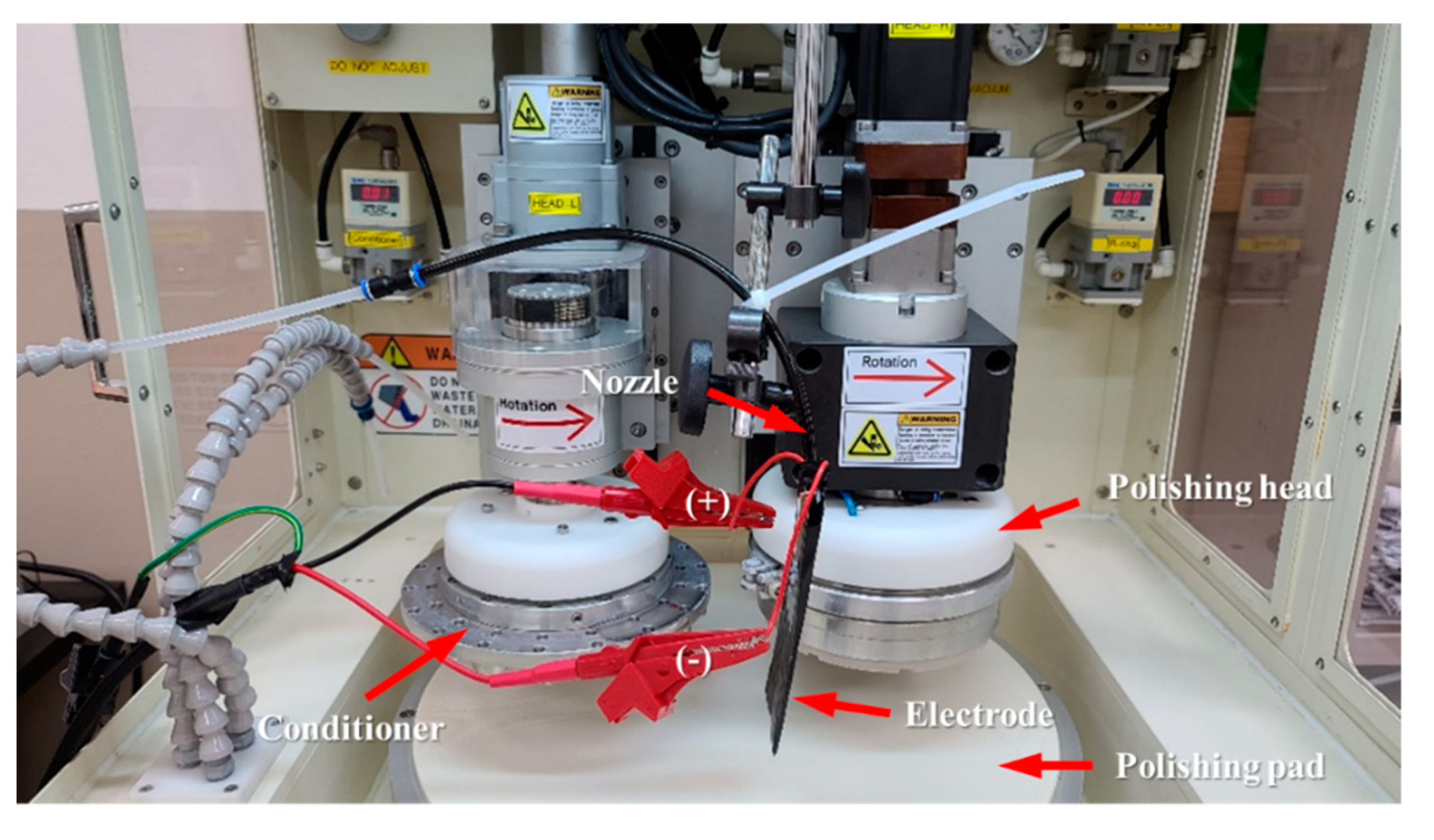
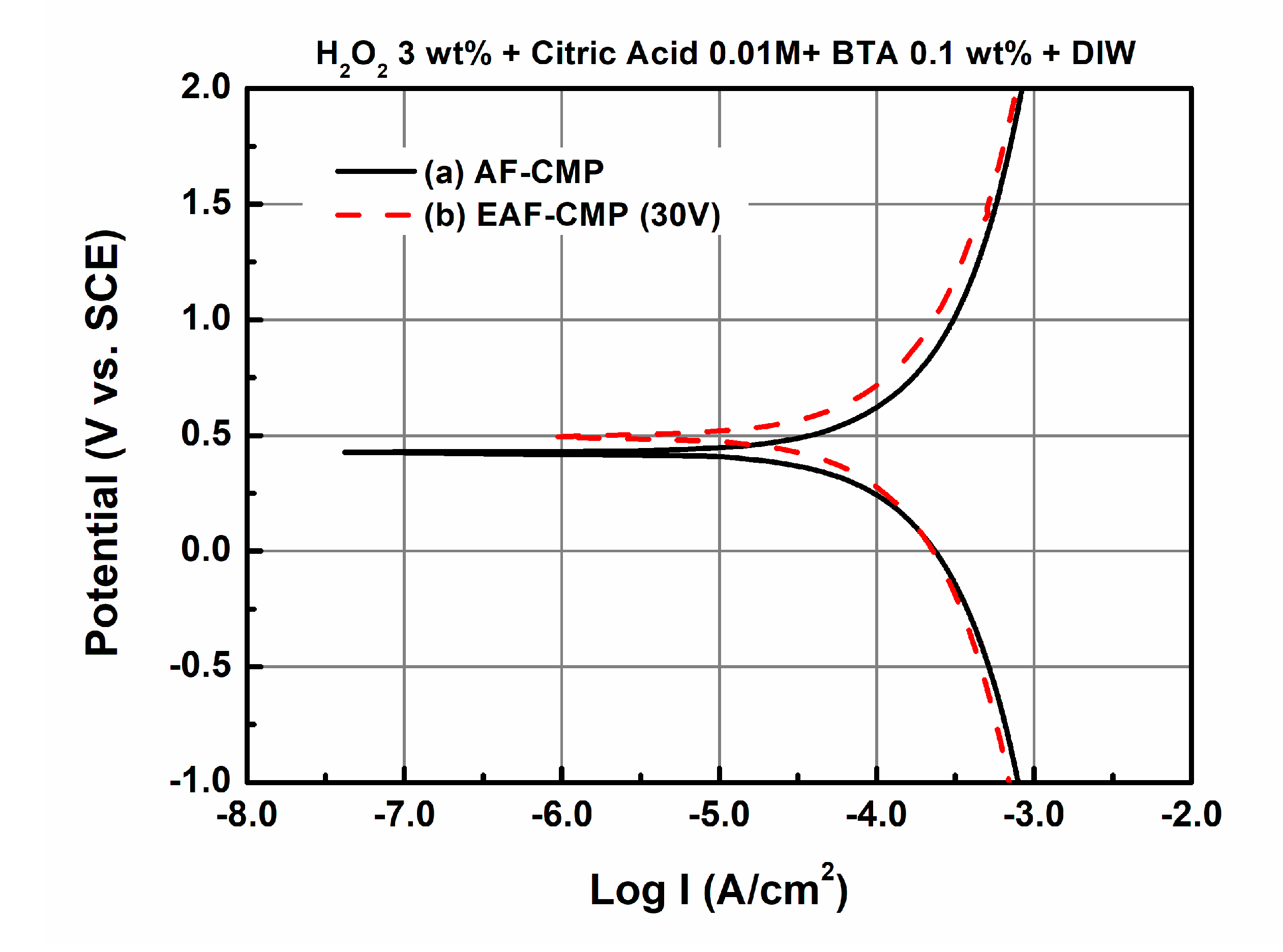
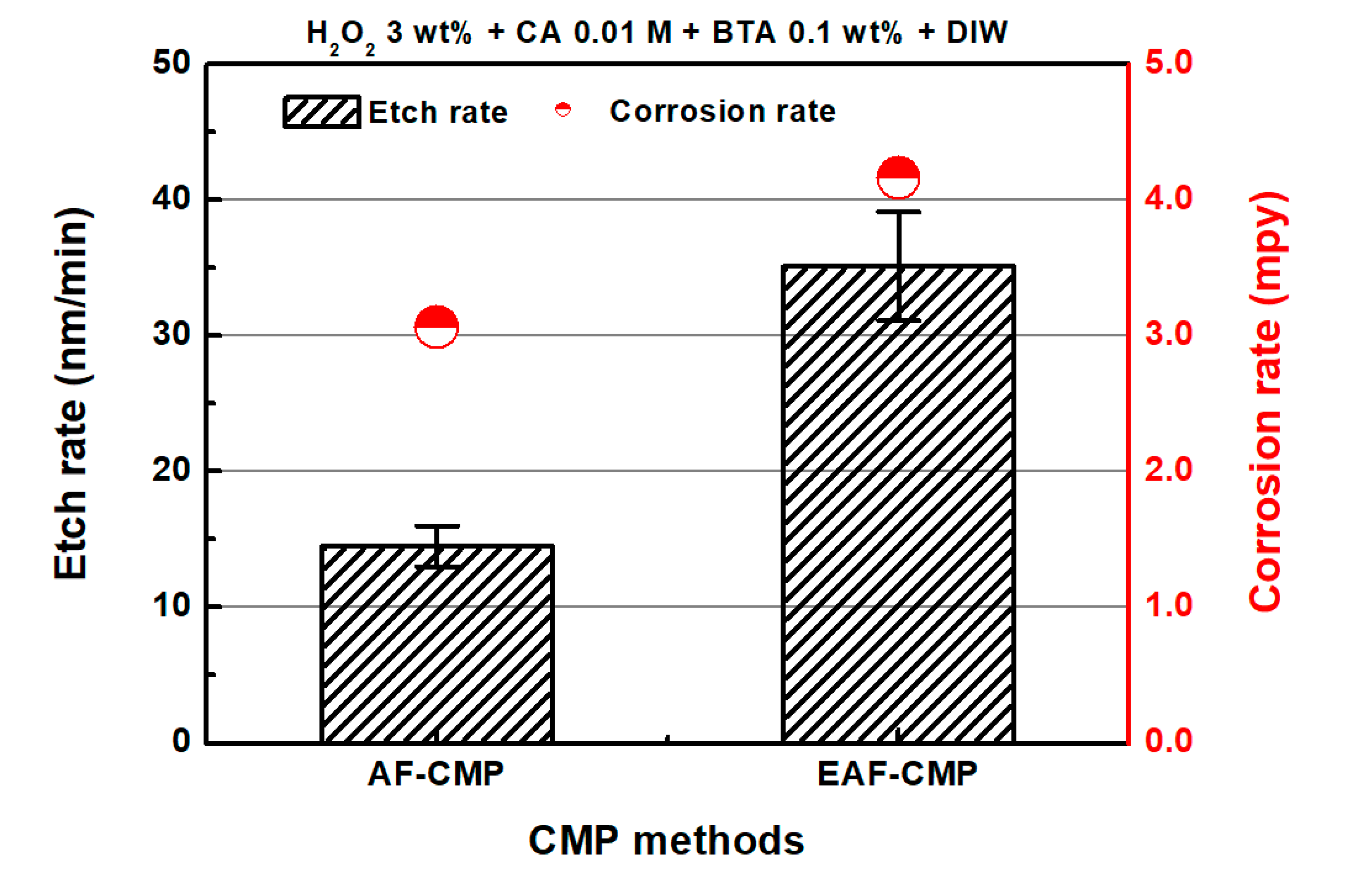
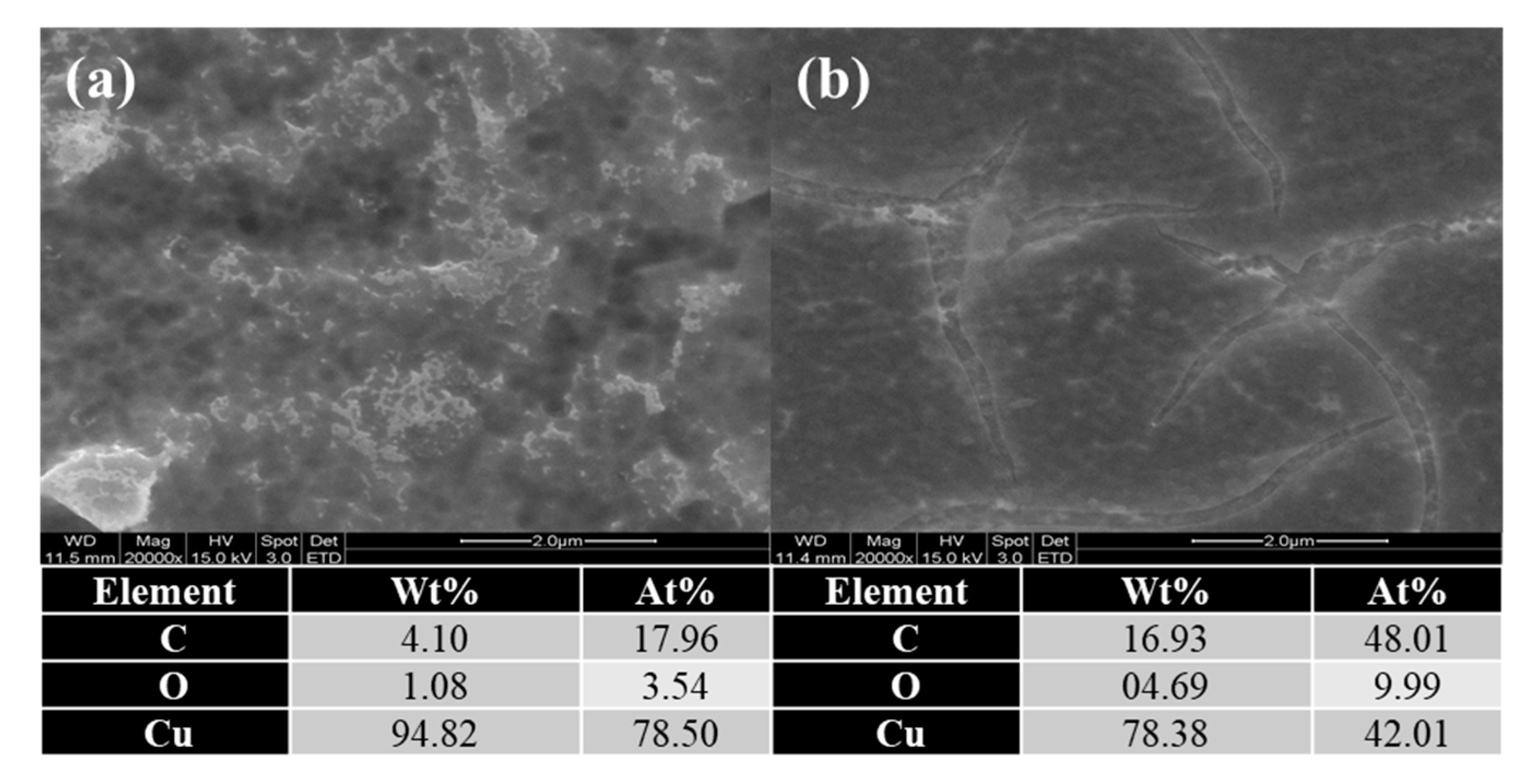




| Parameters | Values |
|---|---|
| Rotational speed of platen (rpm) | 80 |
| Rotational speed of head (rpm) | 80 |
| Wafer pressure (kPa) | 29.4 |
| Retaining ring pressure (kPa) | 39.2 |
| Slurry flow rate (mL/min) | 150 |
| Polishing time (s) | 60 |
| Wafer | 100 mm-diameter, 1.5 μm-thick Cu |
| Abrasive-free slurry | H2O2 + Citric acid + BTA + Deionized water |
| Voltage (V) | 30 |
| Parameters | AF–CMP | EAF–CMP |
|---|---|---|
| Abrasive | No | No |
| Electrolytic ionization | No | Yes |
| Average friction force (kgf) | 5.509 | 7.946 |
| MRR (nm/min) | 236.9 | 303.9 |
| WIWNU (%) | 3.7 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, H. Electrolytically Ionized Abrasive-Free CMP (EAF-CMP) for Copper. Appl. Sci. 2021, 11, 7232. https://doi.org/10.3390/app11167232
Park S, Lee H. Electrolytically Ionized Abrasive-Free CMP (EAF-CMP) for Copper. Applied Sciences. 2021; 11(16):7232. https://doi.org/10.3390/app11167232
Chicago/Turabian StylePark, Seonghyun, and Hyunseop Lee. 2021. "Electrolytically Ionized Abrasive-Free CMP (EAF-CMP) for Copper" Applied Sciences 11, no. 16: 7232. https://doi.org/10.3390/app11167232






