LaFe1-xNix as a Robust Catalytic Oxygen Carrier for Chemical Looping Conversion of Toluene
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Preparation
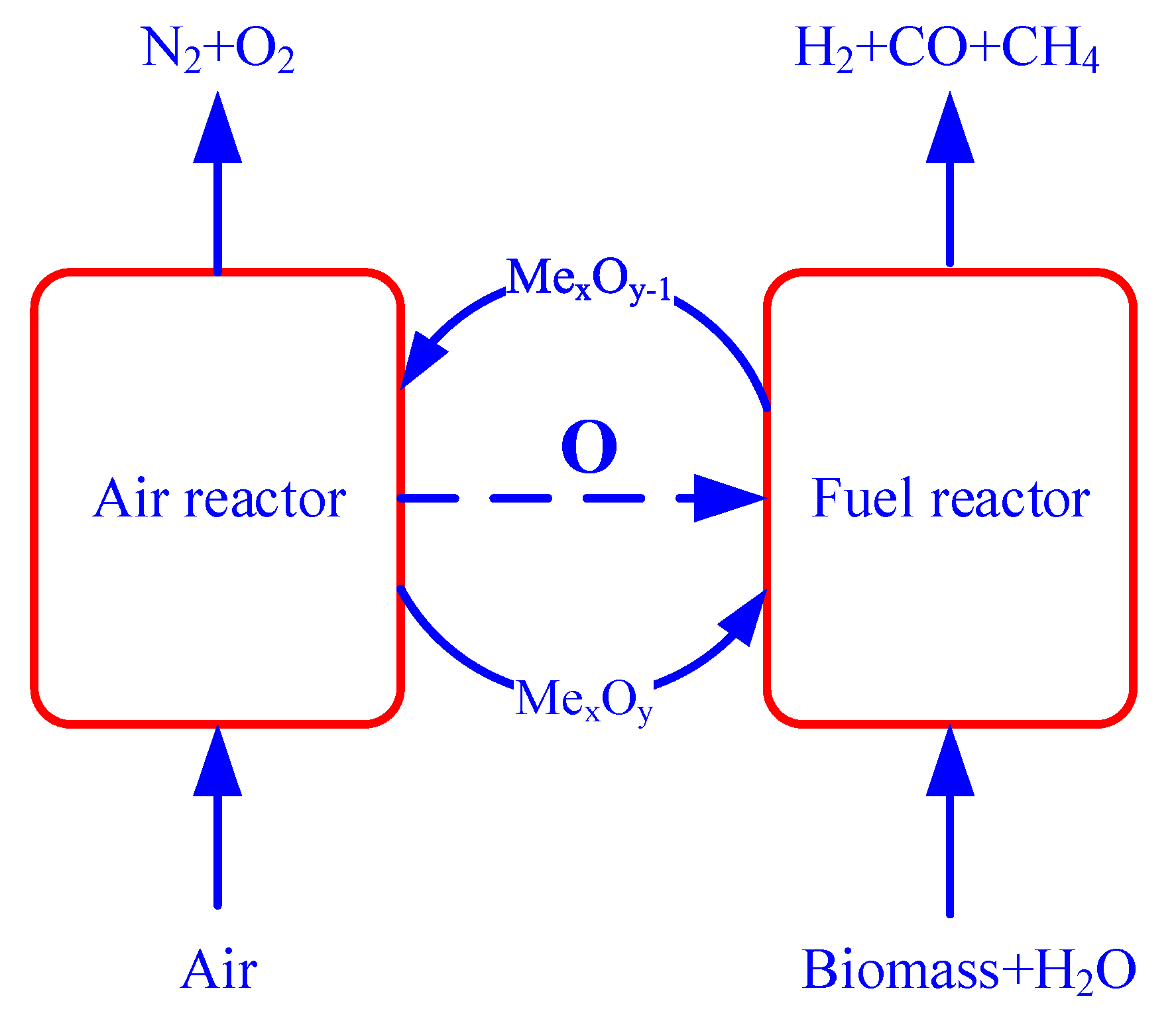
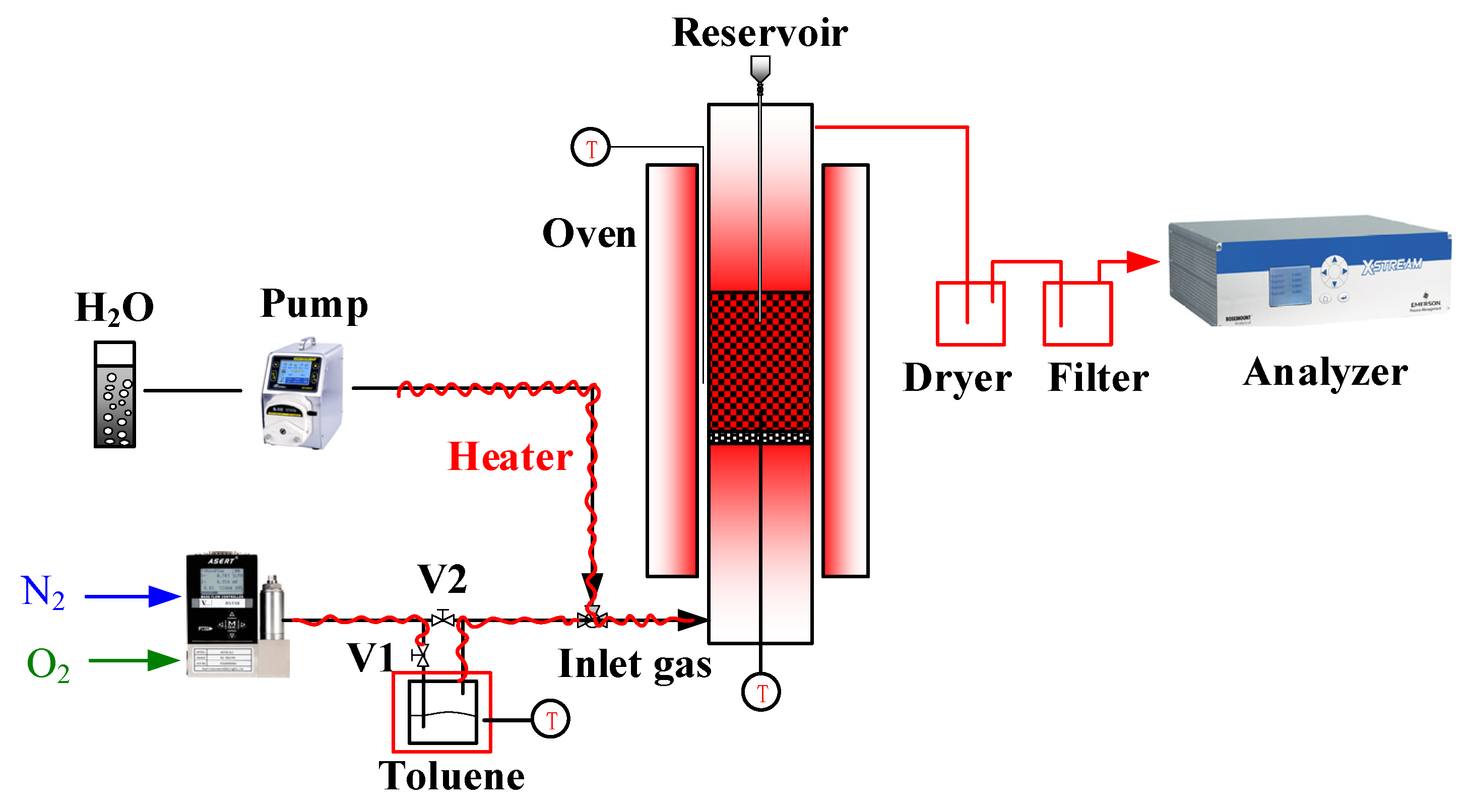
2.2. Experimental Setup and Procedure
2.3. Material Characterization
2.4. Data Process
3. Results and Discussions
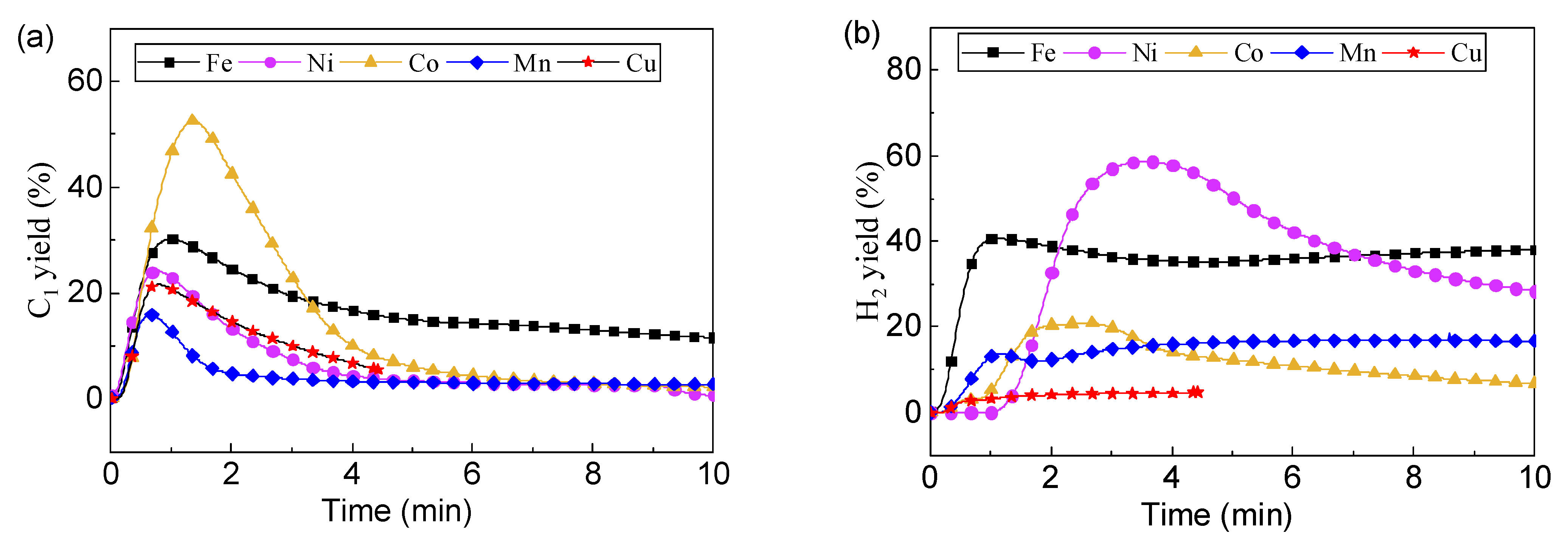
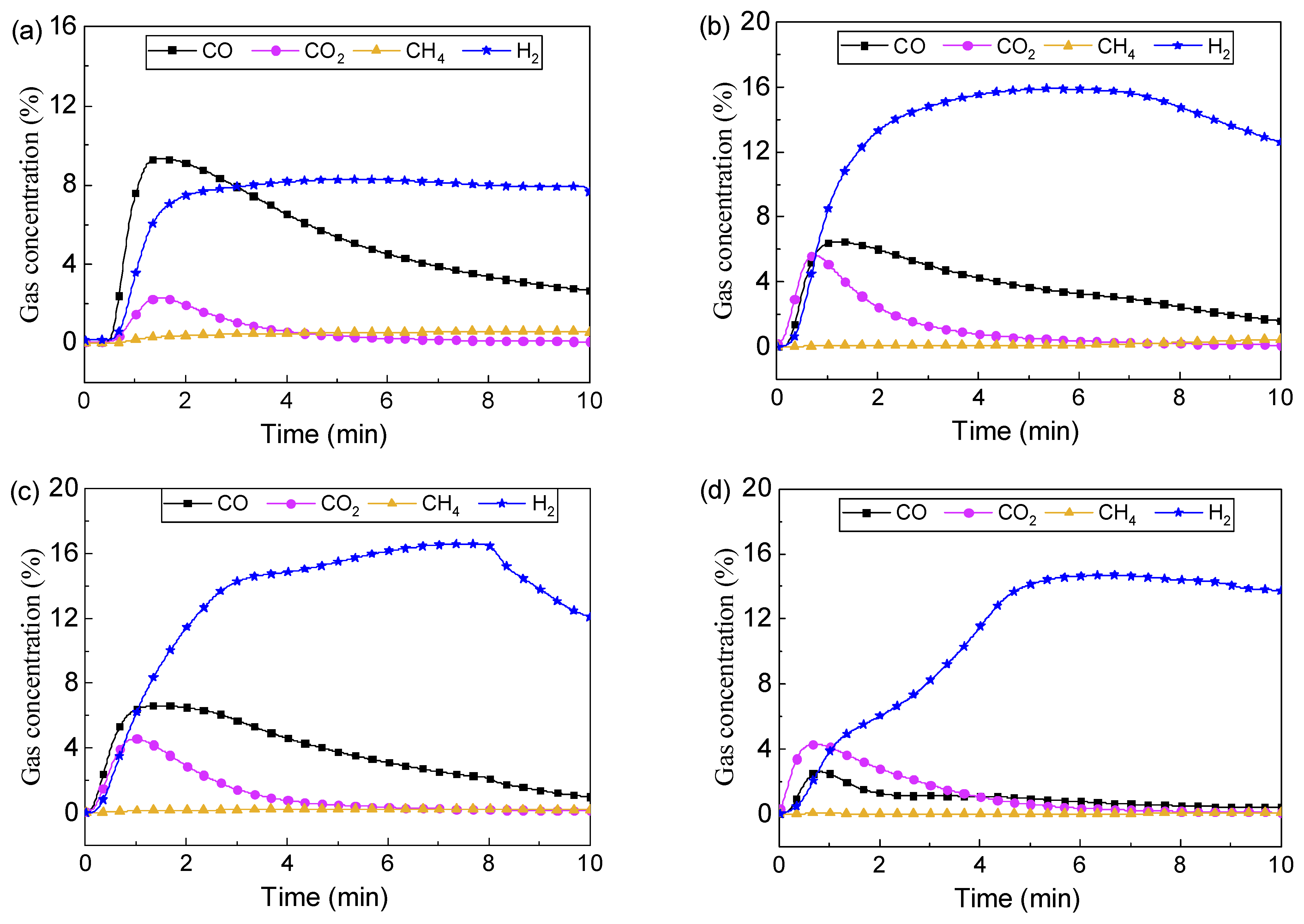
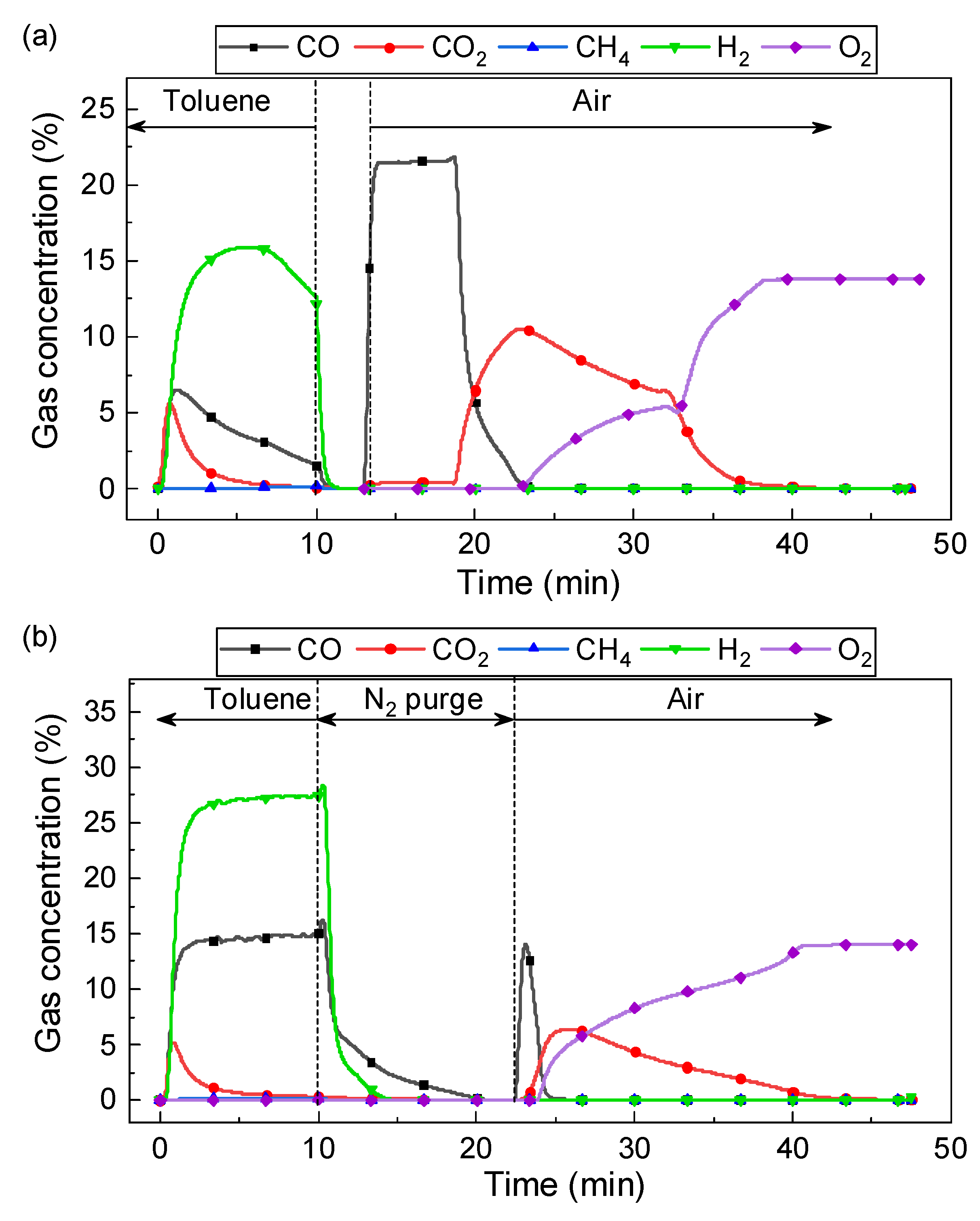
3.1. Enhanced Fuel Conversion by Catalytic Oxygen Carrier
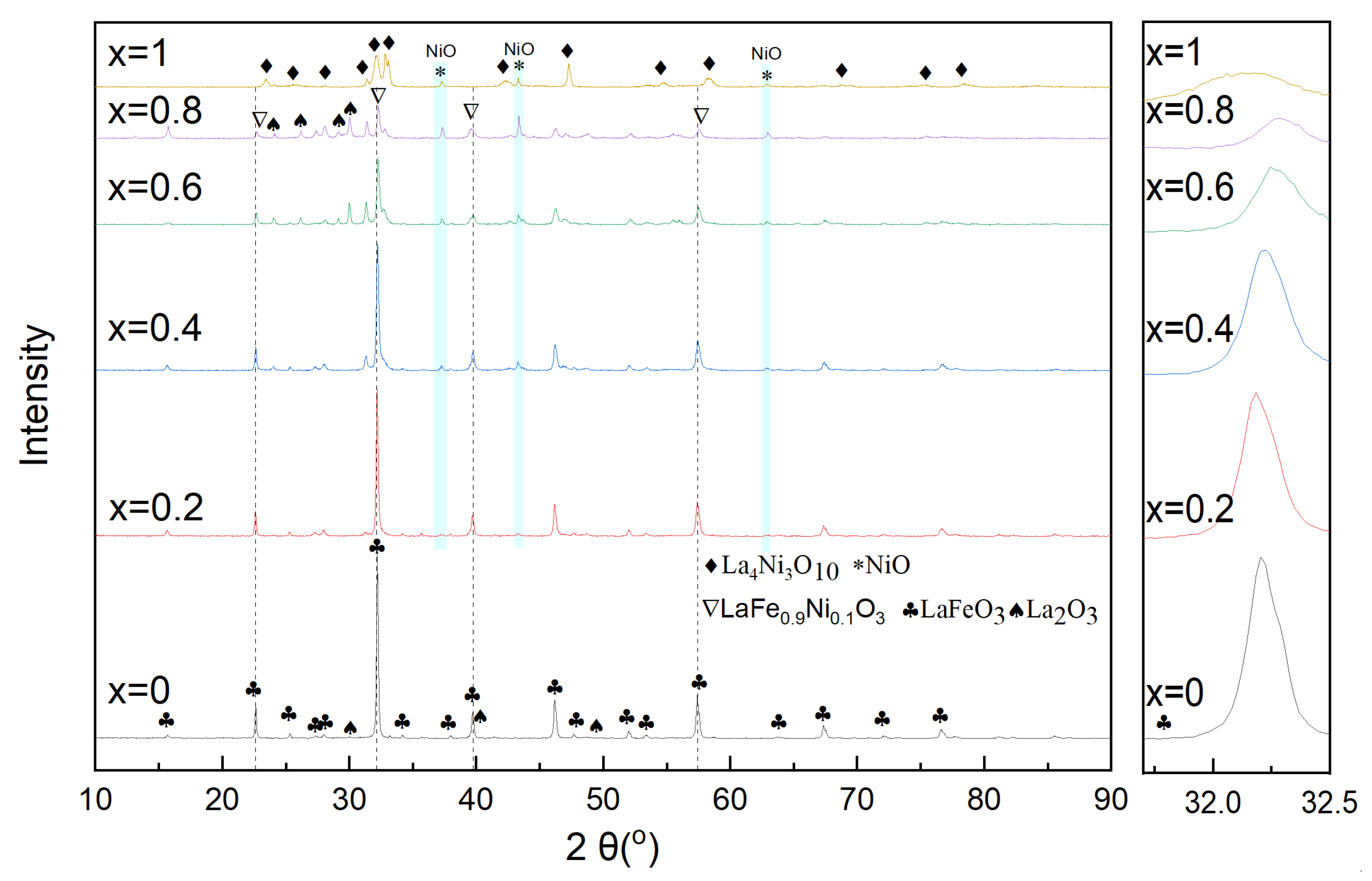
3.2. LaFe1-xNix Oxygen Carrier
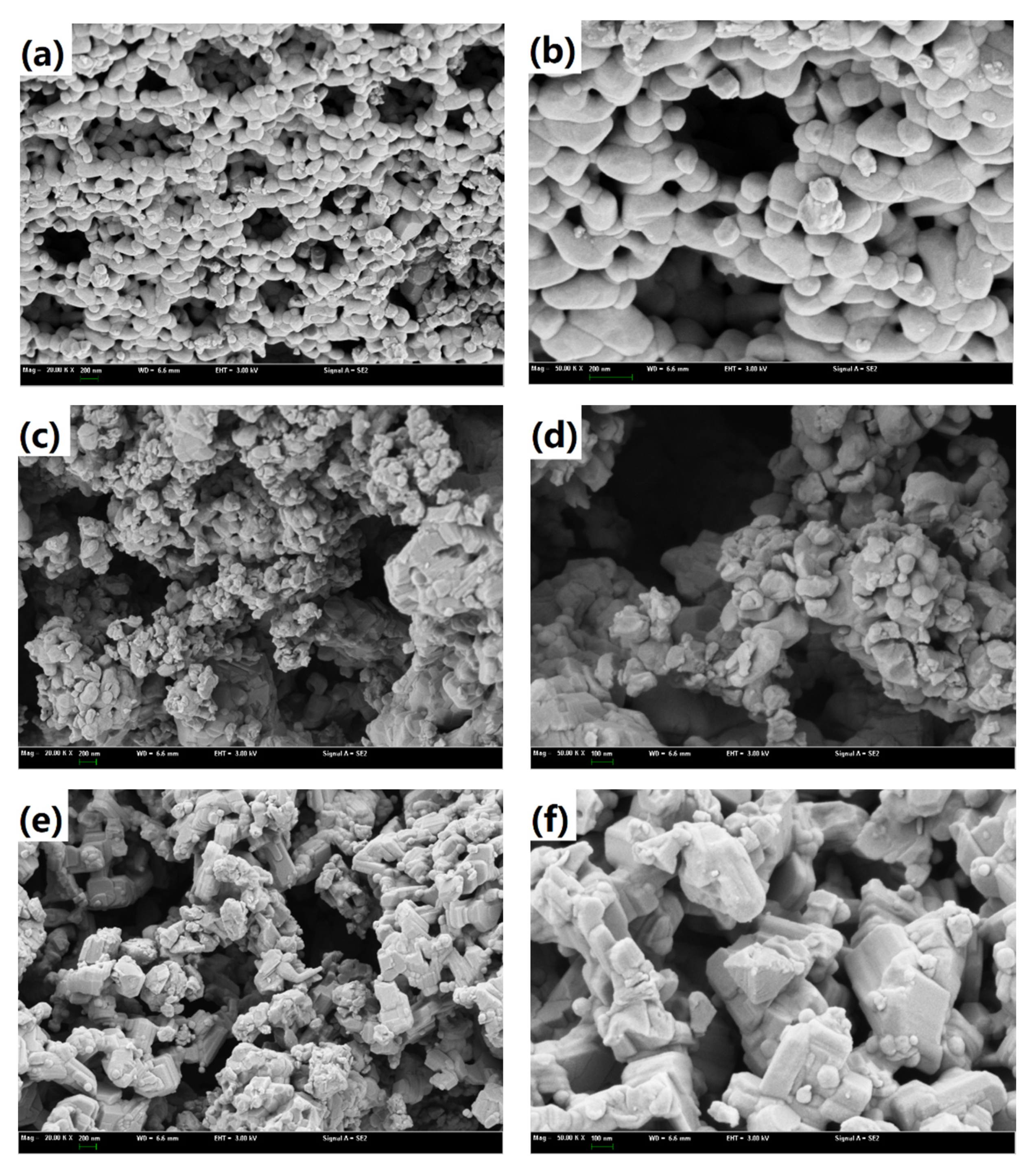
3.2.1. Characterization of Oxygen Carrier
3.2.2. Effect of Ni Substitution on Toluene Conversion
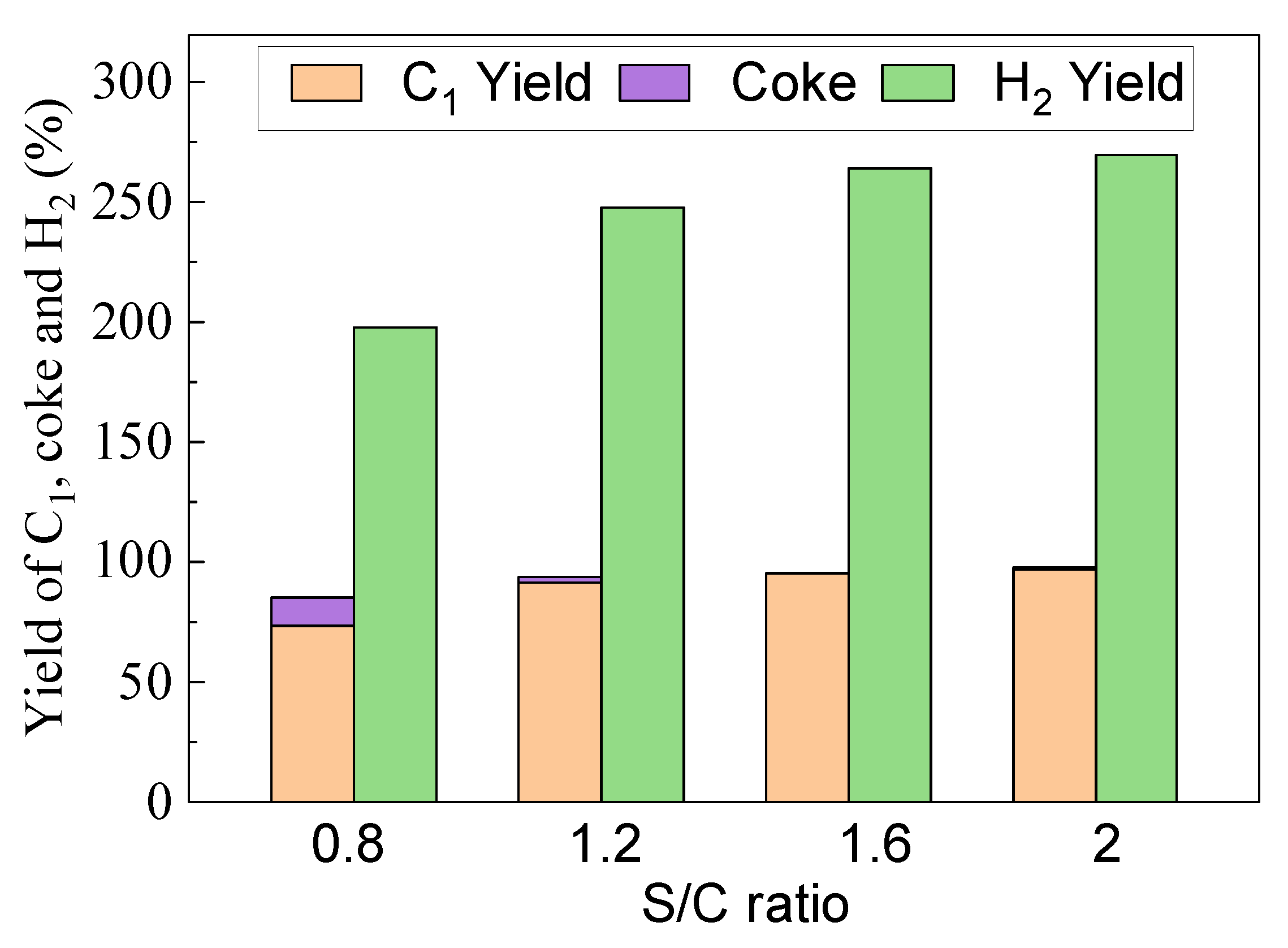
3.2.3. Steam Enhanced Syngas Production
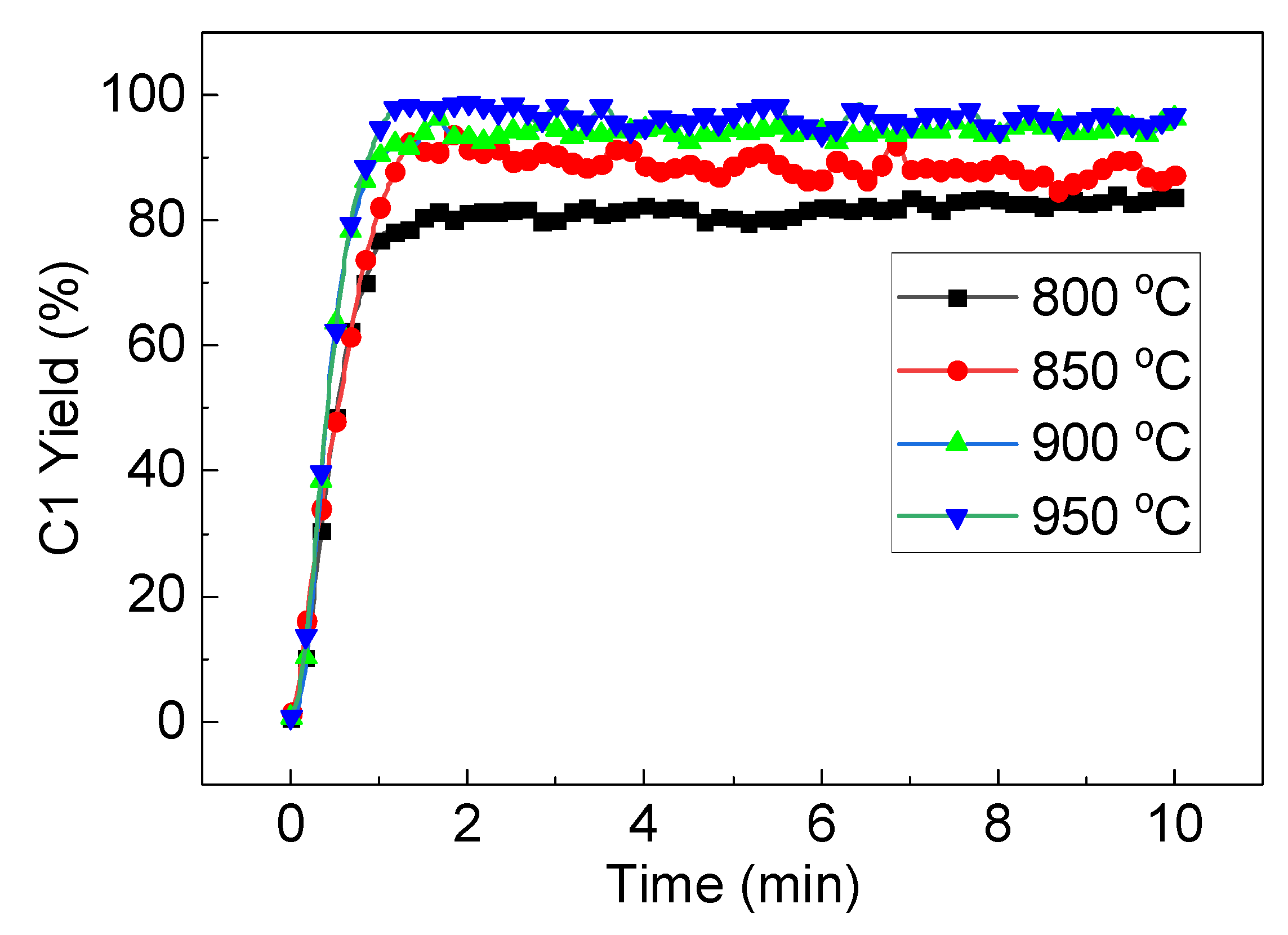
3.3. Reactivity Stability of LaFe0.6Ni0.4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carbon Dioxide. Available online: https://climate.nasa.gov/vital-signs/carbon-dioxide/ (accessed on 30 November 2021).
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Nordgreen, T.; Liliedahl, T.; Sjostrom, K. Metallic iron as a tar breakdown catalyst related to atmospheric, fluidised bed gasification of biomass. Fuel 2006, 85, 689–694. [Google Scholar] [CrossRef]
- Li, C.S.; Suzuki, K. Tar property, analysis, reforming mechanism and model for biomass gasification-An overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- Chen, G.; Andries, J.; Luo, Z.; Spliethoff, H. Biomass pyrolysis/gasification for product gas production: The overall investigation of parametric effects. Energy Convers. Manag. 2003, 44, 1875–1884. [Google Scholar] [CrossRef]
- Shanmuganandam, K.; Thanikaikarasan, S.; Anichai, J. Refining of producer gas using sand filters: An experimental study. Mater. Today Proc. 2020, 33, 4000–4002. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, D.; He, X.; He, J.; Hong, L. Scrubbing of Syngas from MSW Pyrolysis–Volatile Re-forming Process with the Co-produced Oil to Remove Tar and Particulates. Energy Fuels 2020, 34, 14312–14320. [Google Scholar] [CrossRef]
- Gomez-Rueda, Y.; Zaini, I.N.; Yang, W.; Helsen, L. Thermal tar cracking enhanced by cold plasma—A study of naphthalene as tar surrogate. Energy Convers. Manag. 2020, 208, 112540. [Google Scholar] [CrossRef]
- Bin, X.U.; Jia-Qing, L.I.; Xie, J.J.; Huang, Y.-Q.; Yin, X.-L.; Wu, C.-Z. Performance study on simultaneous tar removal and bio-syngas methanation by combining catalysis with nonthermal plasma. J. Fuel Chem. Technol. 2021, 49, 967–977. [Google Scholar] [CrossRef]
- Guo, S.; Wei, X.; Li, J.; Che, D.; Liu, H.; Sun, B.; Wang, Q. Experimental Study on Product Gas and Tar Removal in Air–Steam Gasification of Corn Straw in a Bench-Scale Internally Circulating Fluidized Bed. Energy Fuels 2020, 34, 1908–1917. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kim, J.W.; Seo, M.W.; Mun, T.-Y.; Kim, J.-S. Characteristics of two-stage air gasification of polystyrene with active carbon as a tar removal agent. Energy 2020, 219, 119681. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Chistyakov, A.V.; Konstantinov, G.I.; Borisov, R.S.; Bondarenko, G.N.; Arapova, O.V. Microwave-Assisted Plasma Catalytic Conversion of Tar to Hydrocarbon Products. Pet. Chem. 2021, 61, 721–728. [Google Scholar] [CrossRef]
- Ramadhani, B.; Kivevele, T.; Kihedu, J.H.; Jande, Y.A.C. Catalytic tar conversion and the prospective use of iron-based catalyst in the future development of biomass gasification: A review. Biomass Convers. Biorefinery 2020, 6, 1–24. [Google Scholar] [CrossRef]
- David, E.; Kopa, J. Efficient removal of tar from gas fraction resulting from thermo-chemical conversion of biomass using coal fly ash–based catalysts. Renew. Energy 2021, 171, 1290–1302. [Google Scholar] [CrossRef]
- Islam, M.W. A review of dolomite catalyst for biomass gasification tar removal. Fuel 2020, 267, 117095. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, X.; Gu, J.; Shi, J. Co-gasification of coal and biomass blends using dolomite and olivine as catalysts. Renew. Energy 2019, 132, 509–514. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wei, J.; Bai, Y.-H.; Song, X.-D.; Su, W.-G.; Yu, G.-S. Effect of alkali metal occurrence on the pyrolysis behavior of rice straw. J. Fuel Chem. Technol. 2021, 49, 752–758. [Google Scholar] [CrossRef]
- Zhao, M.; Memon, M.; Ji, G.; Yang, X.; Vuppaladadiyam, A.K.; Song, Y.; Raheem, A.; Li, J.; Wang, W.; Zhou, H. Alkali metal bifunctional catalyst-sorbents enabled biomass pyrolysis for enhanced hydrogen production. Renew. Energy 2020, 148, 168–175. [Google Scholar] [CrossRef]
- Mohamed, D.K.B.; Veksha, A.; Lim, T.; Lisak, G. Highly active and poison-tolerant nickel catalysts for tar reforming synthesized through controlled hydrothermal synthesis. Appl. Catal. A Gen. 2020, 607, 117779. [Google Scholar] [CrossRef]
- Gao, N.; Salisu, J.; Quan, C.; Williams, P. Modified nickel-based catalysts for improved steam reforming of biomass tar: A critical review. Renew. Sustain. Energy Rev. 2021, 145, 111023. [Google Scholar] [CrossRef]
- Wang, S.; Shan, R.; Gu, J.; Zhang, J.; Yuan, H.; Chen, Y. Pyrolysis municipal sludge char supported Fe/Ni catalysts for catalytic reforming of tar model compound. Fuel 2020, 279, 118494. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Su, Y.; Zhu, S. Construction of Fe embedded graphene nanoshell/carbon nanofibers catalyst for catalytic cracking of biomass tar: Effect of CO2 etching. Fuel 2021, 305, 121552. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Guo, T.; Liu, C. Effects of Cu and Fe Additives on Low-Temperature Catalytic Steam Reforming of Toluene Over Ni/AC Catalysts. Catal. Surv. Asia 2019, 23, 54–63. [Google Scholar] [CrossRef]
- Zeng, X.; Ueki, Y.; Yoshiie, R.; Naruse, I.; Wang, F.; Han, Z.; Xu, G. Recent progress in tar removal by char and the applications: A comprehensive analysis. Carbon Resour. Convers. 2020, 3, 1–18. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Zheng, A. Synthesis gas production from biomass gasification using steam coupling with natural hematite as oxygen carrier. Energy 2013, 53, 244–251. [Google Scholar] [CrossRef]
- Samprón, I.; Diego, L.; García-Labiano, F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Biomass chemical looping gasification of pine wood using a synthetic Fe2O3/Al2O3 oxygen carrier in a continuous unit. Bioresour. Technol. 2020, 316, 123908. [Google Scholar] [CrossRef] [PubMed]
- Condori, O.; de Diego, L.F.; Garcia-Labiano, F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Syngas production in a 1.5 kWth biomass chemical looping gasification unit using Fe and Mn ores as the oxygen carrier. Energy Fuels 2021, 35, 17182–17196. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, C.; Guo, Q.; Dang, J.; Zhang, Q.; Lee, D.-J.; Yang, Y. Syngas production by chemical-looping gasification of wheat straw with Fe-based oxygen carrier. Bioresour. Technol. 2018, 263, 273–279. [Google Scholar] [CrossRef]
- Sun, R.; Yan, J.; Shen, L.; Bai, H. Performance and mechanism study of LaFeO3 for biomass chemical looping gasification. J. Mater. Sci. 2020, 55, 11151–11166. [Google Scholar] [CrossRef]
- Yan, J.; Liu, W.; Sun, R.; Jiang, S.; Wang, S.; Shen, L. Chemical looping catalytic gasification of biomass over active LaNixFe1-xO3 perovskites as functional oxygen carriers. Chin. J. Chem. Eng. 2021, 36, 146–156. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, T.; Zhang, Q.; An, X.; Zhao, S.; Dang, J.; Wang, W. Application of calcium oxide/ferric oxide composite oxygen carrier for corn straw chemical looping gasification. Bioresour. Technol. 2021, 330, 125011. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, L.; Tian, H.; Li, X.; Gong, J. Enhanced lattice oxygen reactivity over Ni modified WO3-based redox catalysts for chemical looping partial oxidation of methane. ACS Catal. 2017, 7, 3548–3559. [Google Scholar] [CrossRef]
- Gao, Y.; Neal, L.M.; Li, F. Li-promoted LaxSr2–xFeO4-d core-shell redox catalysts for oxidative dehydrogenation of ethane under a cyclic redox scheme. ACS Catal. 2016, 6, 7293–7302. [Google Scholar] [CrossRef]
- Oehlschlaeger, M.A.; Davidson, D.F.; Hanson, R.K. Thermal decomposition of toluene: Overall rate and branching ratio. Proc. Combust. Inst. 2007, 31, 211–219. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, Y.; Haribal, V.P.; Qi, H.; Liu, X.; Hong, H.; Jin, H.; Li, F. Mixed conductive composites for ‘low-temperature’ thermo-chemical CO2 splitting and syngas generation. J. Mater. Chem. A 2020, 8, 13173–13182. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, A.; Deng, Z.; Wei, G.; Zhao, K.; Chen, D.; He, F.; Zhao, Z.; Li, H.; Li, F. In-situ removal of toluene as a biomass tar model compound using NiFe2O4 for application in chemical looping gasification oxygen carrier. Energy 2020, 190, 116360. [Google Scholar] [CrossRef]
- Iftikhar, S.; Jiang, Q.; Gao, Y.; Liu, J.; Gu, H.; Neal, L.; Li, F. LaNixFe1-xO3-δas a Robust Redox Catalyst for CO2 Splitting and Methane Partial Oxidation. Energy Fuels 2021, 35, 13921–13929. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, K.; Wang, H.; Zhu, X.; Wei, Y.; Zheng, M.; Luo, Y. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane. Appl. Catal. B Environ. 2017, 202, 51–63. [Google Scholar] [CrossRef]
- Zeng, D.; Kang, F.; Qiu, Y.; Cui, D.; Li, M.; Ma, L.; Zhang, S.; Xiao, R. Iron oxides with gadolinium-doped cerium oxides as active supports for chemical looping hydrogen production. Chem. Eng. J. 2020, 396, 125153. [Google Scholar] [CrossRef]


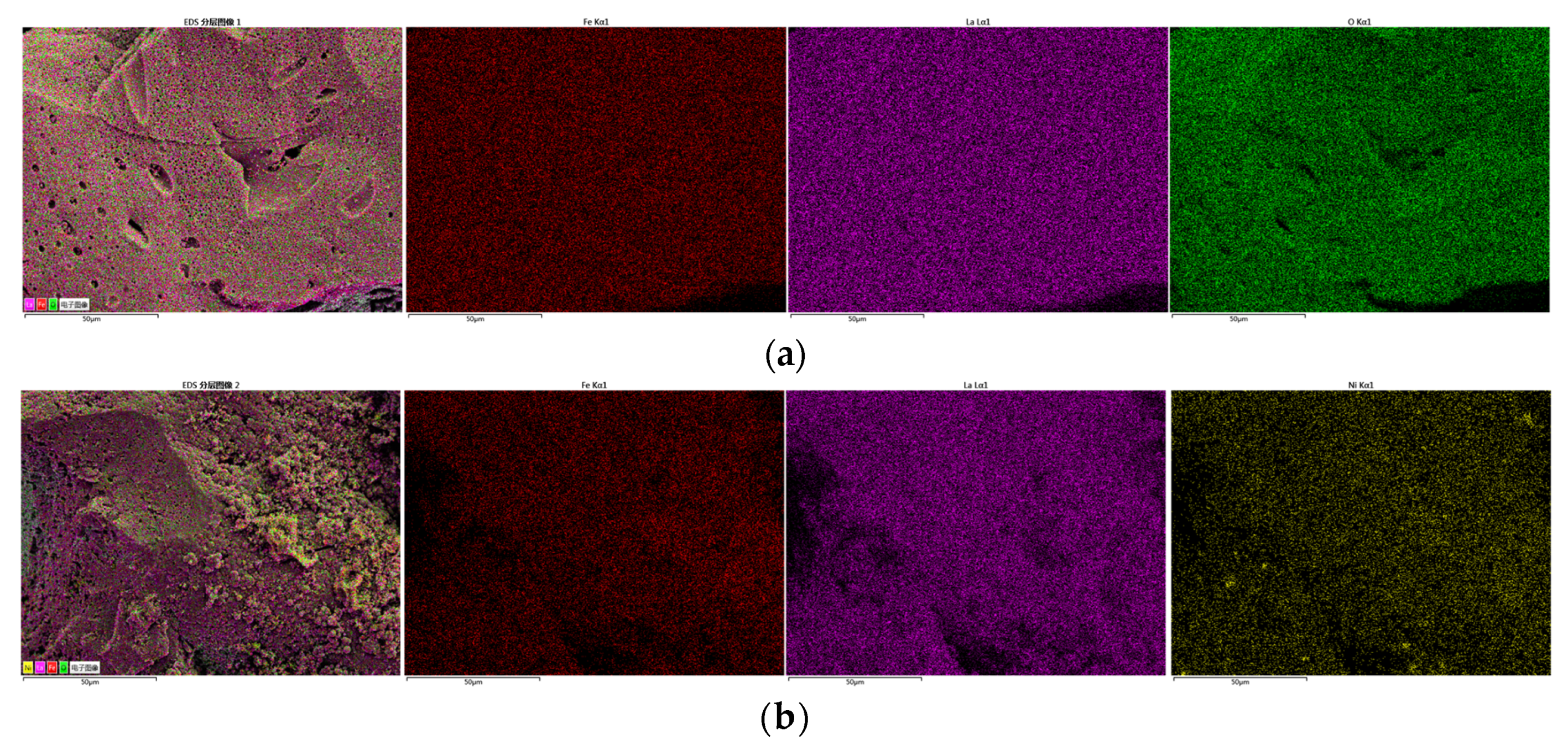
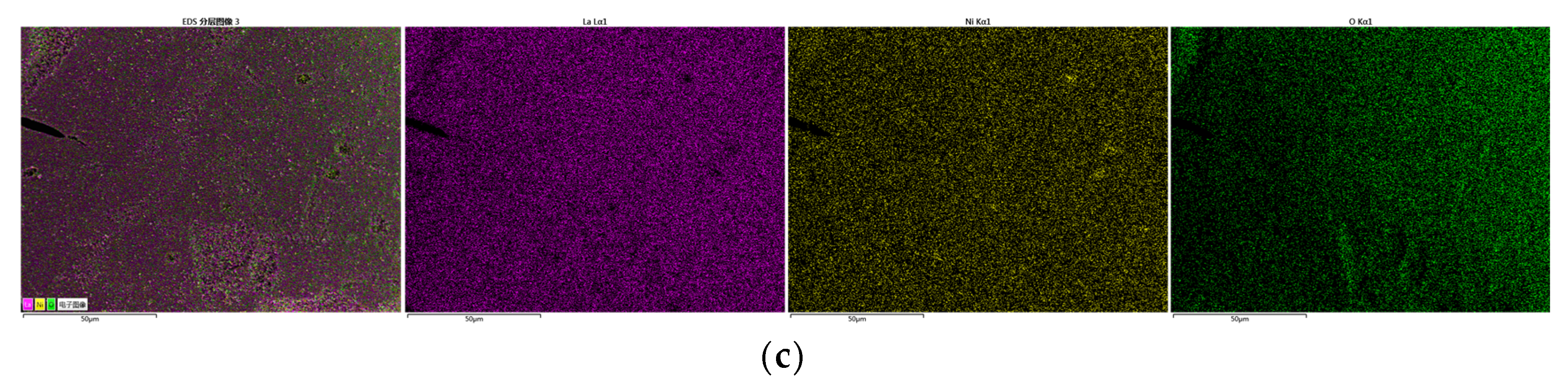


| Test | Oxygen Carrier | Toluene | Steam | Temperature | Purpose |
|---|---|---|---|---|---|
| g/min | S/C | °C | |||
| 1 | None | 0.286 | - | 750–1000 | Reference condition |
| 2 | LaM (M = Fe, Ni, Mn, Co and Cu) | 0.286 | - | 900 | Evaluate the effect of transition mental on toluene conversion |
| 3 | LaFe1-xNix (x = 0–1) | 0.286 | - | 900 | Optimize the ferrite with Ni substitution to enhance catalytic effect |
| 4 | LaFe1-xNix (x = 0–1) | 0.286 | 0.8 | 900 | Promote yield of C1 and H2 by steam addition |
| 5 | LaFe0.6Ni0.4 | 0.286 | 0.8–2 | 800–950 | Optimize S/C and temperature, and evaluate oxygen carrier stability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Yang, J.; Song, G.; Cui, X.; Niu, M.; Zhao, S. LaFe1-xNix as a Robust Catalytic Oxygen Carrier for Chemical Looping Conversion of Toluene. Appl. Sci. 2022, 12, 391. https://doi.org/10.3390/app12010391
Gu H, Yang J, Song G, Cui X, Niu M, Zhao S. LaFe1-xNix as a Robust Catalytic Oxygen Carrier for Chemical Looping Conversion of Toluene. Applied Sciences. 2022; 12(1):391. https://doi.org/10.3390/app12010391
Chicago/Turabian StyleGu, Haiming, Juan Yang, Guohui Song, Xiaobo Cui, Miaomiao Niu, and Shanhui Zhao. 2022. "LaFe1-xNix as a Robust Catalytic Oxygen Carrier for Chemical Looping Conversion of Toluene" Applied Sciences 12, no. 1: 391. https://doi.org/10.3390/app12010391






