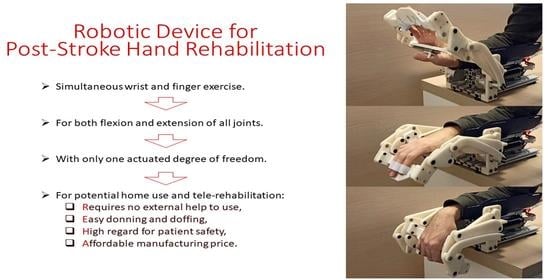
Robotic Device for Out-of-Clinic Post-Stroke Hand Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device’s Mechanism and Built
2.2. Actuation
2.3. Control System
- motor position control;
- communication with patient’s user interface, using Bluetooth connection;
- acquiring sensor data from:
- -
- strain gauge based force sensors,
- -
- incremental encoders of the motors,
- -
- torque measurements from the motors.
2.4. Experimental Validation
2.4.1. Data Acquisition
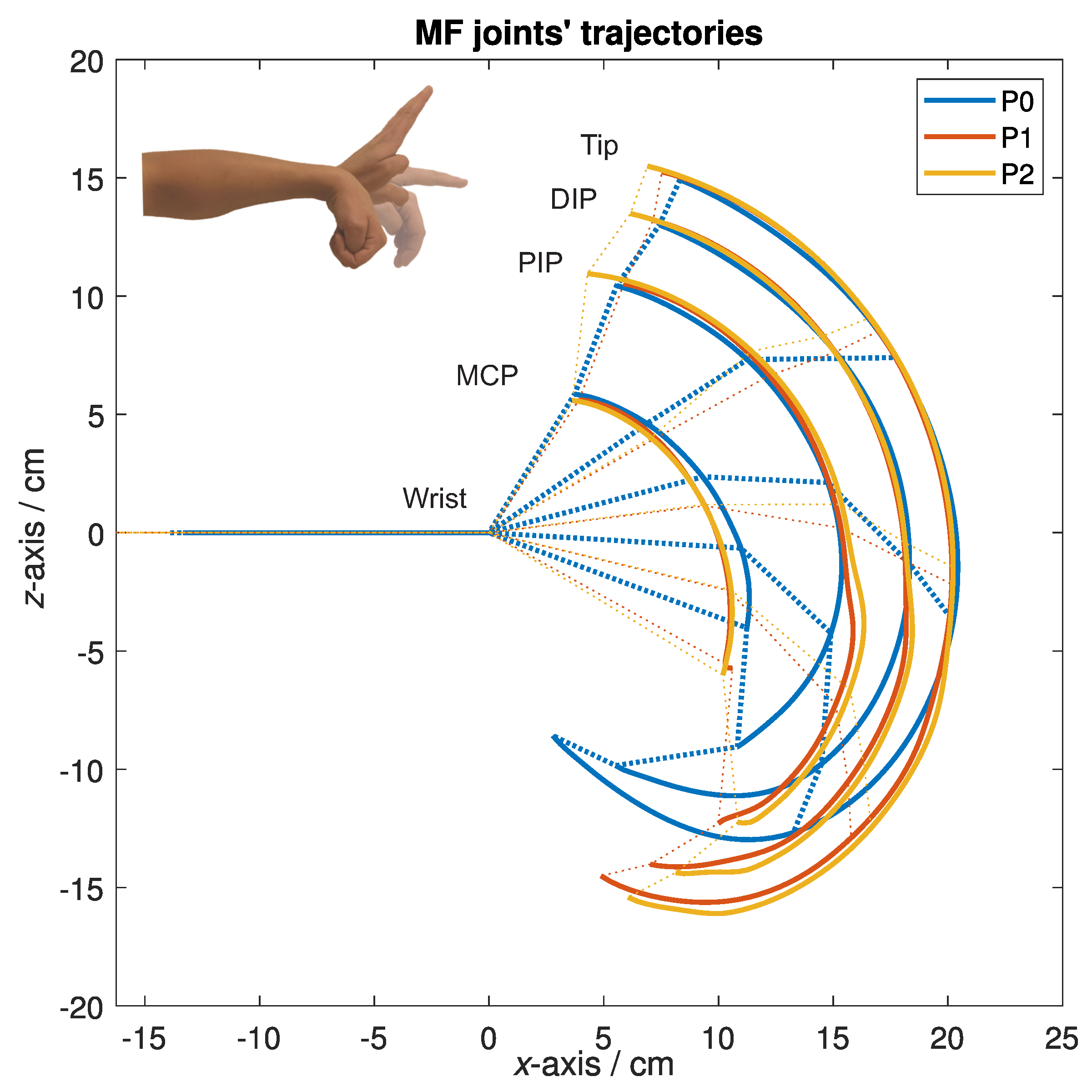
- P0 protocol—active movement through the available ROM, spanning from the full coupled flexion of the wrist and fingers to their full extension, with the thumb held in opposition, only using the device’s proximal part to have the forearm secured in the splint. The movement speed was optional;
- P1 protocol—active movement through the device’s available ROM with a passive device equipped. The movement speed was optional;
- P2 protocol—passive, actuated movement through the device’s available ROM, where only the device was actively contributing to the movement. The device was moving through its available ROM so that one cycle of the movement—from flexion into extension and back—lasted five seconds.
2.4.2. Data Analysis
3. Results
3.1. Form of the Motion Trajectories
3.2. Trajectory Deviations
4. Discussion
5. Conclusions
- is simple for donning and doffing, so that the patients could do it themselves, even in case of spasticity;
- is safe, so that the device can be used at home, in a nonclinical environment and also in the telerehabilitation applications;
- is adaptable to different patients in terms of their abilities and hand dimensions;
- is easy to use, portable, and has a relatively low manufacturing cost.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Analogue-to-digital converter |
| ADL | Activities of daily living |
| DC | Direct current |
| DIP | Distal interphalangeal (joint) |
| DOF | Degree of freedom |
| LED | Light-emitting diode |
| MCP | Metacarpophalangeal (joint) |
| MF | Middle finger |
| OS | Operating system |
| PF | Pinkie finger |
| PD | Proportional-derivative |
| PIP | Proximal interphalangeal (joint) |
| PWM | Pulse-width modulation |
| RMSD | Root-mean-square deviation |
| ROM | Range of motion |
| SD | Standard deviation |
| SPI | Serial peripheral interface |
References
- Dantes, E.; Axelerad, S.D.; Stroe, A.Z.; Axelerad, D.D.; Axelerad, A.D. The rehabilitation of hemiparesis after stroke. Ovidius Univ. Ann. Ser. Phys. Educ. Sport. Mov. Health 2020, 20, 5–10. [Google Scholar]
- Mackay, J.; Mensah, G.A.; Greenlund, K. The Atlas of Heart Disease and Stroke; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Luengo-Fernandez, R.; Violato, M.; Candio, P.; Leal, J. Economic burden of stroke across Europe: A population-based cost analysis. Eur. Stroke J. 2020, 5, 17–25. [Google Scholar] [CrossRef]
- Schaechter, J.D. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog. Neurobiol. 2004, 73, 61–72. [Google Scholar] [CrossRef]
- Li, S. Spasticity, motor recovery, and neural plasticity after stroke. Front. Neurol. 2017, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.L.; Hu, G.C. Post-stroke spasticity: A review of epidemiology, pathophysiology, and treatments. Int. J. Gerontol. 2018, 12, 280–284. [Google Scholar] [CrossRef]
- Lang, C.E.; Bland, M.D.; Bailey, R.R.; Schaefer, S.Y.; Birkenmeier, R.L. Assessment of upper extremity impairment, function, and activity after stroke: Foundations for clinical decision making. J. Hand Ther. 2013, 26, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feys, H.M.; De Weerdt, W.J.; Selz, B.E.; Cox Steck, G.A.; Spichiger, R.; Vereeck, L.E.; Putman, K.D.; Van Hoydonck, G.A. Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke: A single-blind, randomized, controlled multicenter trial. Stroke 1998, 29, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Dovat, L.; Lambercy, O.; Gassert, R.; Maeder, T.; Milner, T.; Leong, T.C.; Burdet, E. HandCARE: A cable-actuated rehabilitation system to train hand function after stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, H.T.; Van Limbeek, J.; Geurts, A.C.; Zwarts, M.J. Motor recovery after stroke: A systematic review of the literature. Arch. Phys. Med. Rehabil. 2002, 83, 1629–1637. [Google Scholar] [CrossRef]
- Nakayama, H.; Jørgensen, H.S.; Raaschou, H.O.; Olsen, T.S. Recovery of upper extremity function in stroke patients: The Copenhagen stroke study. Arch. Phys. Med. Rehabil. 1994, 75, 394–398. [Google Scholar] [CrossRef]
- Gresham, G.; Duncan, P.; Stason, W.; Adams, H.; Adelman, A.; Alexander, D.; Bishop, D.; Diller, L.; Donaldson, N.; Granger, C.; et al. Post-Stroke Rehabilitation; US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research: Rockville, MD, USA, 1995.
- Wissel, J.; Manack, A.; Brainin, M. Toward an epidemiology of poststroke spasticity. Neurology 2013, 80, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zorowitz, R.D.; Gillard, P.J.; Brainin, M. Post-stroke spasticity: Sequelae and burden on stroke survivors and caregivers. Neurology 2013, 80, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, J.; Guo, Y.; Tan, S. Prevalence and risk factors for spasticity after stroke: A systematic review and meta-analysis. Front. Neurol. 2021, 11, 1884. [Google Scholar] [CrossRef]
- Chino, N.; Melvin, J.L. Functional Evaluation of Stroke Patients; Springer: Tokyo, Japan, 1996. [Google Scholar]
- Prange, G.; Jannink, M.; Groothuis-Oudshoorn, C.; Hermens, H.; Ijzerman, M. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J. Rehabil. Res. Dev. 2009, 43, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Barreca, S.; Wolf, S.L.; Fasoli, S.; Bohannon, R. Treatment interventions for the paretic upper limb of stroke survivors: A critical review. Neurorehabilit. Neural Repair 2003, 17, 220–226. [Google Scholar] [CrossRef]
- Woldag, H.; Hummelsheim, H. Evidence-based physiotherapeutic concepts for improving arm and hand function in stroke patients. J. Neurol. 2002, 249, 518–528. [Google Scholar] [CrossRef]
- Van Der Lee, J.H.; Snels, I.A.; Beckerman, H.; Lankhorst, G.J.; Wagenaar, R.C.; Bouter, L.M. Exercise therapy for arm function in stroke patients: A systematic review of randomized controlled trials. Clin. Rehabil. 2001, 15, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Goljar, N. Klinične smernice za rehabilitacijo bolnikov po preboleli možganski kapi. Rehabilitacija 2014, 13, 12–18. [Google Scholar]
- Oujamaa, L.; Relave, I.; Froger, J.; Mottet, D.; Pelissier, J.Y. Rehabilitation of arm function after stroke. Literature review. Ann. Phys. Rehabil. Med. 2009, 52, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Sarakoglou, I.; Tsagarakis, N.G.; Caldwell, D.G. Occupational and physical therapy using a hand exoskeleton based exerciser. In Proceedings of the 2004 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) (IEEE Cat. No. 04CH37566), Sendai, Japan, 28 September–2 October 2004; Volume 3, pp. 2973–2978. [Google Scholar]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Boyd, R.N.; Ada, L. Physiotherapy management of spasticity. In Upper Motor Neuron Syndrome and Spasticity: Clinical Management and Neurophysiology; Cambridge University Press: Cambridge, UK, 2001; pp. 79–81. [Google Scholar]
- Coroian, F.; Jourdan, C.; Bakhti, K.; Palayer, C.; Jaussent, A.; Picot, M.C.; Mottet, D.; Julia, M.; Bonnin, H.Y.; Laffont, I. Upper limb isokinetic strengthening versus passive mobilization in patients with chronic stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2018, 99, 321–328. [Google Scholar] [CrossRef]
- Radomski, M.V.; Latham, C.A.T. Occupational Therapy for Physical Dysfunction; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1995. [Google Scholar]
- Volpe, B.T.; Ferraro, M.; Krebs, H.I.; Hogan, N. Robotics in the rehabilitation treatment of patients with stroke. Curr. Atheroscler. Rep. 2002, 4, 270–276. [Google Scholar] [CrossRef]
- Krebs, H.I.; Hogan, N.; Aisen, M.L.; Volpe, B.T. Robot-aided neurorehabilitation. IEEE Trans. Rehabil. Eng. 1998, 6, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Brackenridge, J.; Bradnam, L.V.; Lennon, S.; Costi, J.J.; Hobbs, D.A. A review of rehabilitation devices to promote upper limb function following stroke. Neurosci. Biomed. Eng. (Discontin.) 2016, 4, 25–42. [Google Scholar] [CrossRef]
- Meng, Q.; Xie, Q.; Yu, H. Upper-limb rehabilitation robot: State of the art and existing problems. In Proceedings of the 12th International Convention on Rehabilitation Engineering and Assistive Technology, Shanghai, China, 13–16 July 2018; pp. 155–158. [Google Scholar]
- Basteris, A.; Nijenhuis, S.M.; Stienen, A.H.; Buurke, J.H.; Prange, G.B.; Amirabdollahian, F. Training modalities in robot-mediated upper limb rehabilitation in stroke: A framework for classification based on a systematic review. J. Neuroeng. Rehabil. 2014, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Ates, S.; Haarman, C.J.; Stienen, A.H. SCRIPT passive orthosis: Design of interactive hand and wrist exoskeleton for rehabilitation at home after stroke. Auton. Robot. 2017, 41, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Hesse, S.; Werner, C.; Pohl, M.; Rueckriem, S.; Mehrholz, J.; Lingnau, M. Computerized arm training improves the motor control of the severely affected arm after stroke: A single-blinded randomized trial in two centers. Stroke 2005, 36, 1960–1966. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Wu, C.Y.; Lin, K.C.; Yao, G.; Wu, K.Y.; Chang, Y.J. Dose–response relationship of robot-assisted stroke motor rehabilitation: The impact of initial motor status. Stroke 2012, 43, 2729–2734. [Google Scholar] [CrossRef] [Green Version]
- Lambercy, O.; Dovat, L.; Gassert, R.; Burdet, E.; Teo, C.L.; Milner, T. A haptic knob for rehabilitation of hand function. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 356–366. [Google Scholar] [CrossRef]
- Hesse, S.; Schulte-Tigges, G.; Konrad, M.; Bardeleben, A.; Werner, C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch. Phys. Med. Rehabil. 2003, 84, 915–920. [Google Scholar] [CrossRef]
- Stroke, P. Robotic devices and brain: Machine interfaces for hand rehabilitation post-stroke. J. Rehabil. Med. 2017, 49, 449–460. [Google Scholar]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef]
- Timmermans, A.A.; Seelen, H.A.; Willmann, R.D.; Kingma, H. Technology-assisted training of arm-hand skills in stroke: Concepts on reacquisition of motor control and therapist guidelines for rehabilitation technology design. J. Neuroeng. Rehabil. 2009, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Goljar Kregar, N.; Kotnik, S. Ocenjevanje funkcije roke pri bolnikih po možganski kapi s Southamptonskim testom SHAP. Rehabilitacija 2015, 14, 4–10. [Google Scholar]
- Paik, Y.R.; Kim, S.K.; Lee, J.S.; Jeon, B.J. Simple and task-oriented mirror therapy for upper extremity function in stroke patients: A pilot study. Hong Kong J. Occup. Ther. 2014, 24, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the state-of-the-art and areas of application. JMIR Rehabil. Assist. Technol. 2017, 4, e7511. [Google Scholar] [CrossRef]
- Suso-Martí, L.; La Touche, R.; Herranz-Gómez, A.; Angulo-Díaz-Parreño, S.; Paris-Alemany, A.; Cuenca-Martínez, F. Effectiveness of Telerehabilitation in Physical Therapist Practice: An Umbrella and Mapping Review with Meta–Meta-Analysis. Phys. Ther. 2021, 101, pzab075. [Google Scholar] [CrossRef]
- Fausti, D.; Seneci, C. A Hand Rehabilitation Device. European Patent EP2549971B1A, 21 March 2011. Available online: https://patents.google.com/patent/EP2549971B1/de?oq=EP2549971B1 (accessed on 16 July 2021).
- Saringer, J.H. Device for Imparting Continuous Passive Motion to Human Joints. U.S. Patent US4665900A, 31 December 1984. Available online: https://patents.google.com/patent/US4665900A/en?oq=US4665900A (accessed on 16 July 2021).
- Weinzweig, N. Removable Flexible Finger Covering with Fingertip Connector Clip. U.S. Patent US5261393A, 17 September 1992. Available online: https://patents.google.com/patent/US5261393A/en?oq=US5261393A (accessed on 16 July 2021).
- Brook, A.H.; Carian, P.J.; Katzin, L.; Landsinger, E.E.; Moore, J.D.; Rotter, L.D.; Stanley, S. Continuous Passive Motion Devices and Methods. U.S. Patent US4875469A, 13 June 1988. Available online: https://patents.google.com/patent/US4875469A/en?oq=US4875469A (accessed on 16 July 2021).
- Moon, I.; Bae, J. Wearable Excercise Device for Upper Limb Rehabilitation. Korean Patent KR101546882B1, 6 September 2013. Available online: https://patents.google.com/patent/KR101546882B1/en?oq=KR101546882B1 (accessed on 16 July 2021).
- Kim, Y.; Moon, I. KR101126637B1: Upper-Limb Rehabilitation Robot and Parallel Link Mechanism. Korean Patent KR101126637B1, 10 September 2009. Available online: https://patents.google.com/patent/KR101126637B1/en?oq=KR101126637B1 (accessed on 16 July 2021).
- Koeneman, E.J.; Koeneman, J.B.; Herring, D.E.; Schultz, R.S. System and Method for Neuromuscular Reeducation. U.S. Patent US8214029B2, 12 April 2010. Available online: https://patents.google.com/patent/US8214029B2/en?oq=US8214029B2 (accessed on 16 July 2021).
- States, R.; Pappas, E. Precision and repeatability of the Optotrak 3020 motion measurement system. J. Med Eng. Technol. 2006, 30, 11–16. [Google Scholar] [CrossRef]
- Brokaw, E.B.; Holley, R.J.; Lum, P.S. Hand spring operated movement enhancer HandSOME device for hand rehabilitation after stroke. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5867–5870. [Google Scholar]








| Sex | Age/Years | Weight/kg | Height/cm | DA | W-MF MCP/cm | W-PF MCP/cm | LMF/cm | LPF/cm | HW/cm | |
|---|---|---|---|---|---|---|---|---|---|---|
| Subject A | M | 25 | 75 | 194 | R | 10.5 | 9.6 | 11.0 | 7.9 | 8.0 |
| Subject B | M | 27 | 88 | 186 | R | 10.3 | 8.3 | 10.7 | 7.9 | 8.6 |
| Subject C | F | 28 | 60 | 164 | R | 9.4 | 7.4 | 9.6 | 7.2 | 7.8 |
| Subject D | M | 25 | 65 | 174 | R | 9.8 | 8.8 | 11.0 | 8.0 | 8.2 |
| Subject E | F | 27 | 52 | 169 | R | 9.5 | 8.6 | 9.8 | 7.6 | 7.5 |
| Subject F | M | 43 | 100 | 186 | R | 9.8 | 8.8 | 11.0 | 8.0 | 10.0 |
| MF MCP | MF Tip | |||
|---|---|---|---|---|
| Flexion/cm | Extension/cm | Flexion/cm | Extension/cm | |
| Subject A | 0.3 ± 0.1 | 0.6 ± 0.1 | 2.9 ± 1.3 | 0.7 ± 0.4 |
| Subject B | 1.7 ± 0.1 | 1.4 ± 0.1 | 1.1 ± 0.2 | 0.8 ± 0.1 |
| Subject C | 0.5 ± 0.1 | 0.2 ± 0.1 | 1.3 ± 0.6 | 0.4 ± 0.2 |
| Subject D | 0.7 ± 0.2 | 0.4 ± 0.1 | 1.8 ± 0.6 | 0.2 ± 0.1 |
| Subject E | 0.7 ± 0.3 | 0.4 ± 0.2 | 2.0 ± 0.6 | 0.9 ± 0.3 |
| Subject F | 0.5 ± 0.1 | 0.2 ± 0.2 | 5.3 ± 1.0 | 1.6 ± 0.1 |
| Average | 0.7 ± 0.5 | 0.5 ± 0.4 | 2.4 ± 1.6 | 0.7 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandeljc, A.; Rajhard, A.; Munih, M.; Kamnik, R. Robotic Device for Out-of-Clinic Post-Stroke Hand Rehabilitation. Appl. Sci. 2022, 12, 1092. https://doi.org/10.3390/app12031092
Mandeljc A, Rajhard A, Munih M, Kamnik R. Robotic Device for Out-of-Clinic Post-Stroke Hand Rehabilitation. Applied Sciences. 2022; 12(3):1092. https://doi.org/10.3390/app12031092
Chicago/Turabian StyleMandeljc, Ana, Aleksander Rajhard, Marko Munih, and Roman Kamnik. 2022. "Robotic Device for Out-of-Clinic Post-Stroke Hand Rehabilitation" Applied Sciences 12, no. 3: 1092. https://doi.org/10.3390/app12031092
APA StyleMandeljc, A., Rajhard, A., Munih, M., & Kamnik, R. (2022). Robotic Device for Out-of-Clinic Post-Stroke Hand Rehabilitation. Applied Sciences, 12(3), 1092. https://doi.org/10.3390/app12031092