Changes of Physical and Mechanical Properties of Coral Reef Limestone under CO2–Seawater–Rock Interaction
Abstract
:1. Introduction
2. Materials and Methods
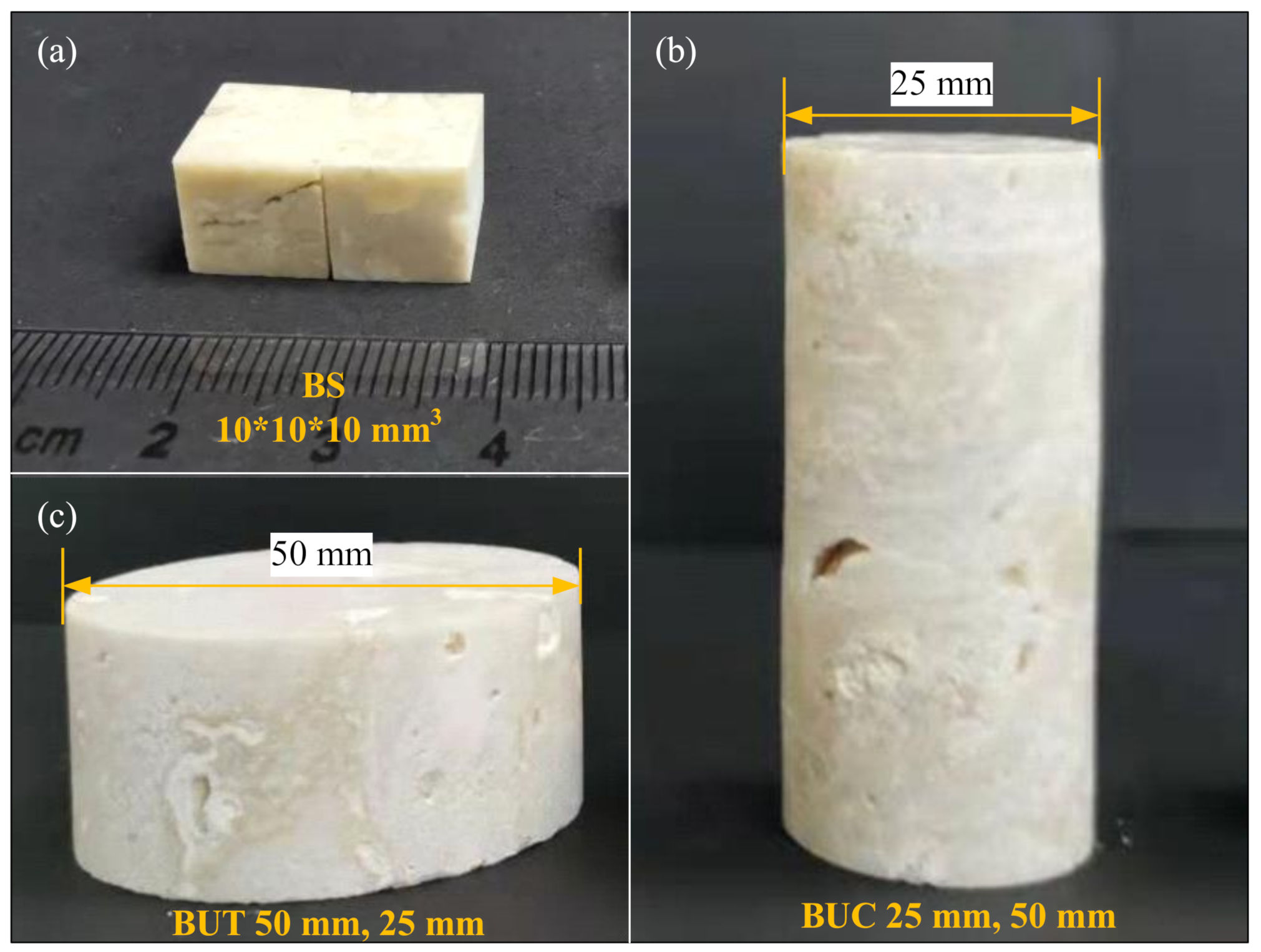
2.1. Materials
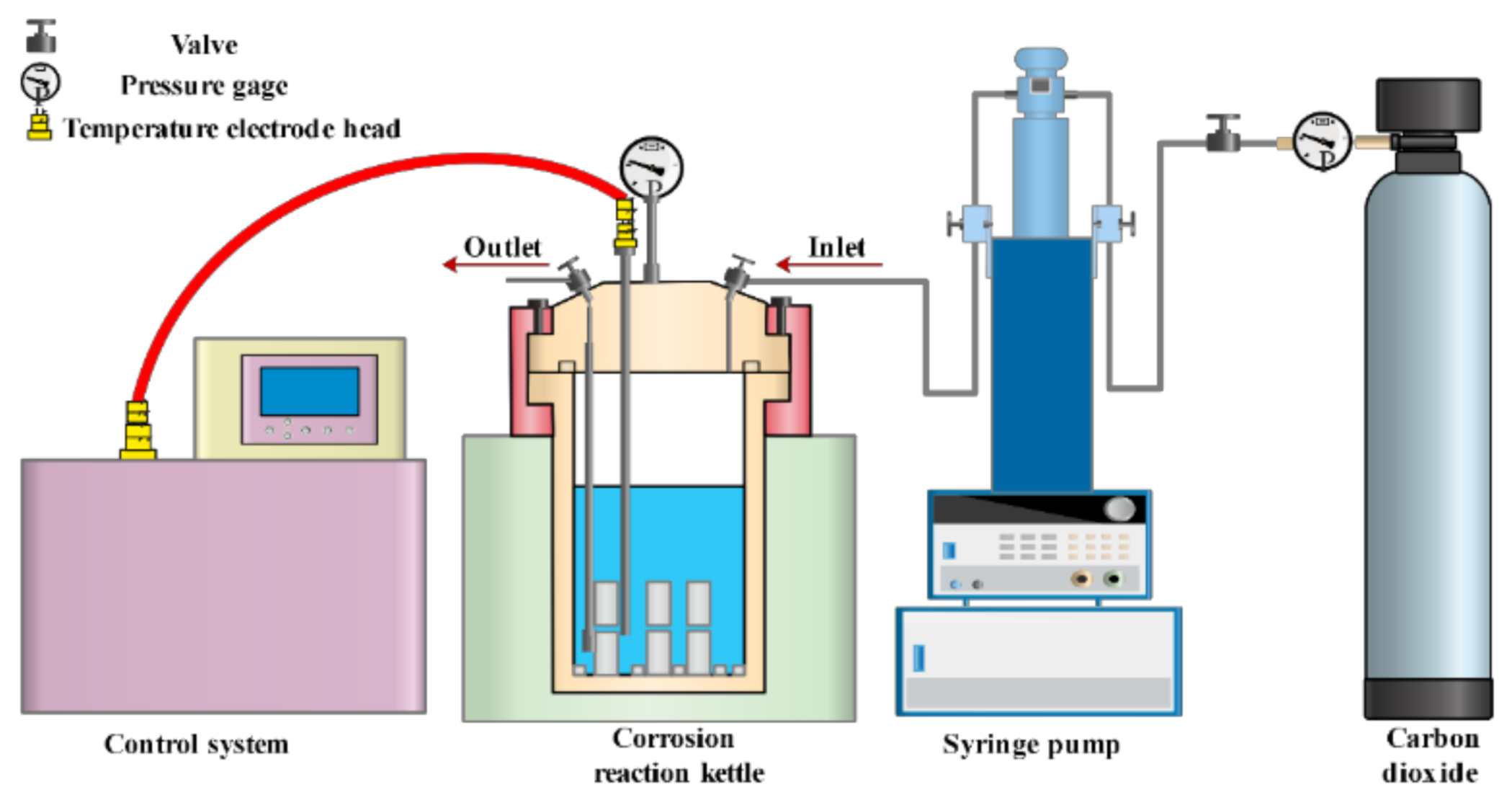
2.2. Apparatus and Methodology
2.2.1. Water Absorption
2.2.2. Volume Measurement and Porosity Calculated
2.2.3. Permeability Measurement
- (1)
- The sample was wrapped in a heat shrink tube and placed vertically into the core holder. The confining pressure pump was used to deliver the ultrapure water into the pressure chamber of the core holder where a confining pressure of 1 MPa was applied.
- (2)
- In order to remove the air of the lines of the pore system, the vacuum pump was applied to vacuum the system for 6 h. After that, the pore pump was set at 0.2 MPa to maintain a constant sample pore pressure.
- (3)
- The temperature of the whole system was stabilized at the level of 30 °C using a water bath. After the whole system had stabilized (where the temperature, the pore pressure and confining pressure were stable), a pressure pulse (20 kPa) was applied. The pressure decay curve was recorded constantly by the differential pressure gauge. The permeability could be calculated through the formulas (2) and (3).
- (4)
- The permeability of the sample under the different confining pressures could be obtained by repeating steps (2)–(3) and changing the confining pressure in step (1). In this study, the permeabilities of the cylinder specimens were measured under the confining pressures of 1 MPa, 3 MPa, 5 MPa and 7 MPa.

2.2.4. CO2–Water–Rock Interaction
- (1)
- The solution was prepared, and its concentration of Ca2+ before interaction was measured by a compact calcium ion meter (HORIBA Ca-11, Kyoto, Japan). The initial Ca2+ concentration of the solution is 460 ppm.
- (2)
- All samples were dried and weighed prior to testing. Then, the volume and permeability of the cylinder specimens were measured,
- (3)
- All samples were placed into the hydrothermal reactor, and 800 mL seawater was added. All samples were immersed by the solution. Then, CO2 was injected into the reactor by the syringe pump. The pressure and temperature were held at 7MPa and 30 °C, respectively.
- (4)
- The solution was analyzed for dissolved Ca2+ concentration during the reaction. After 3 days of reaction time, the specimens were taken out and washed repeatedly with distilled water to avoid influence from salt crystals after drying. Then, they were oven-dried to study the changes of mass. The effective volume and permeability of the cylinder specimens were tested by the methods introduced above. One of the cube specimens was taken and prepared for SEM analysis.
- (5)
- The specimens (except the sample analyzed by SEM) were placed back into the reactor, and the same amount of seawater was added to continue the next stage of reaction. Then, steps (3)–(4) were repeated.
- (6)
- After 15 days, all the samples were taken out from the reactor to investigate the evolution of the dry mass, effective volume, permeability, and the change of surface properties and mechanical behavior.
2.2.5. Mechanical Tests
3. Results and Discussion
3.1. Changes of Physical Properties
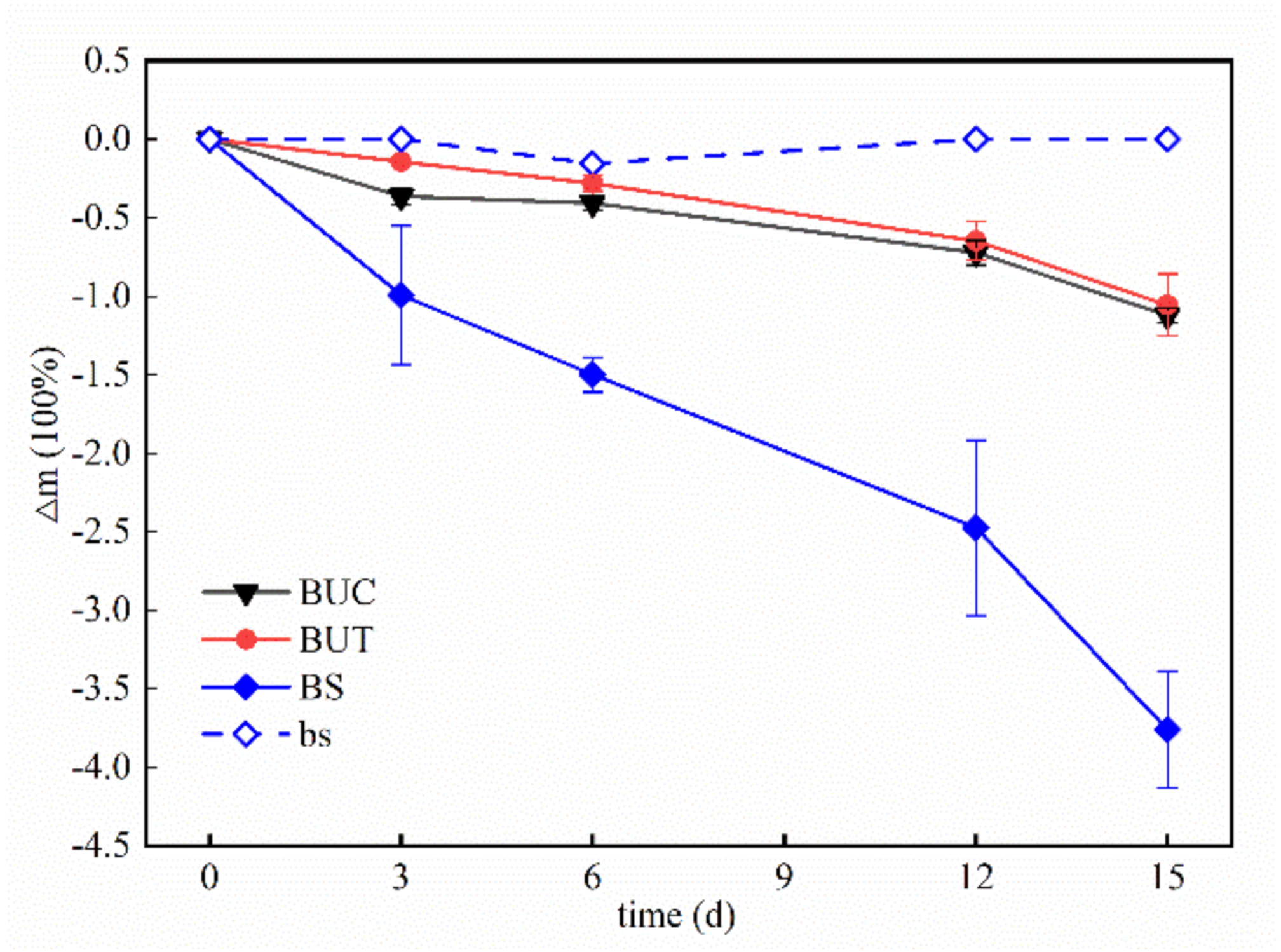
3.1.1. Mass Loss
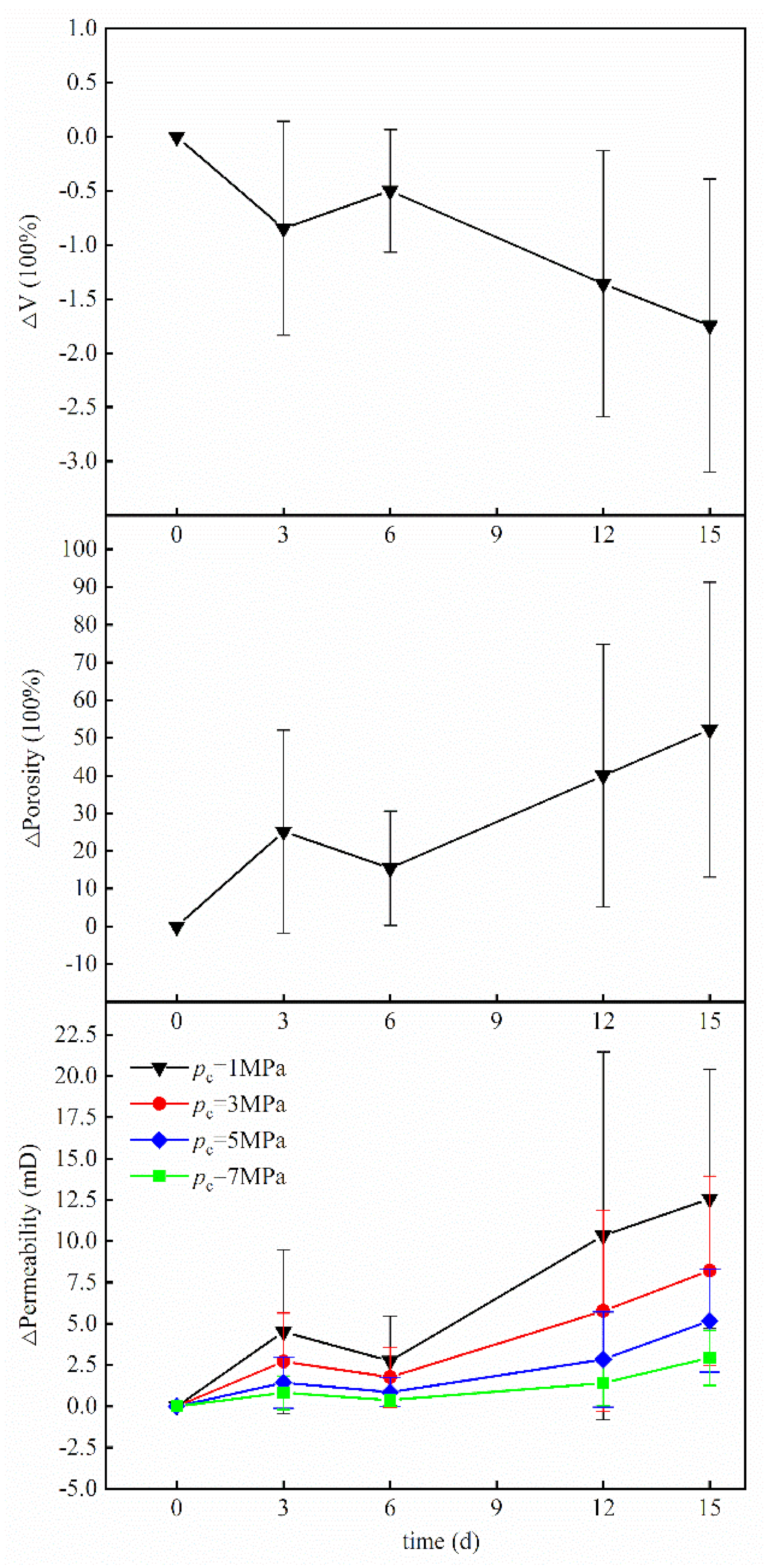
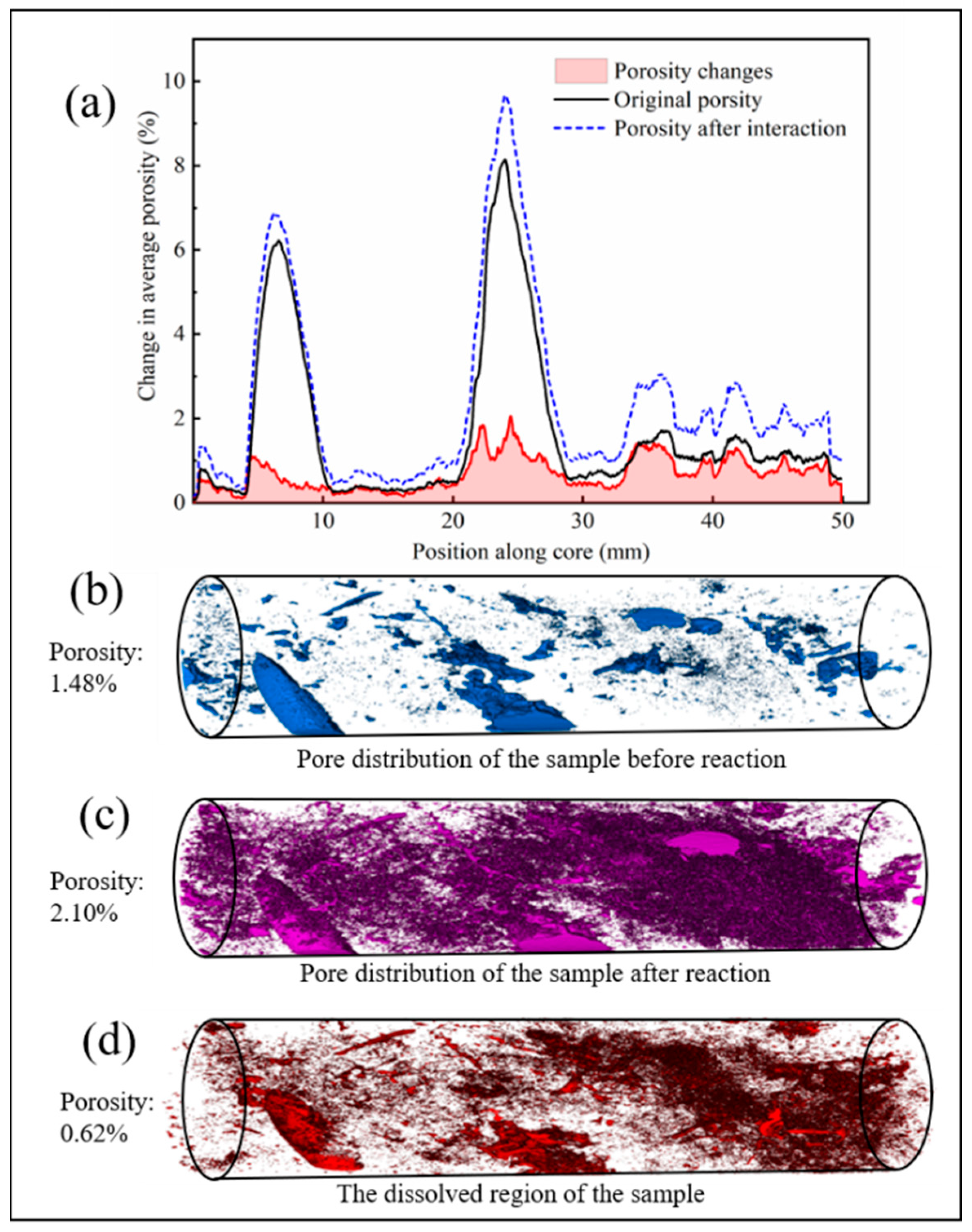
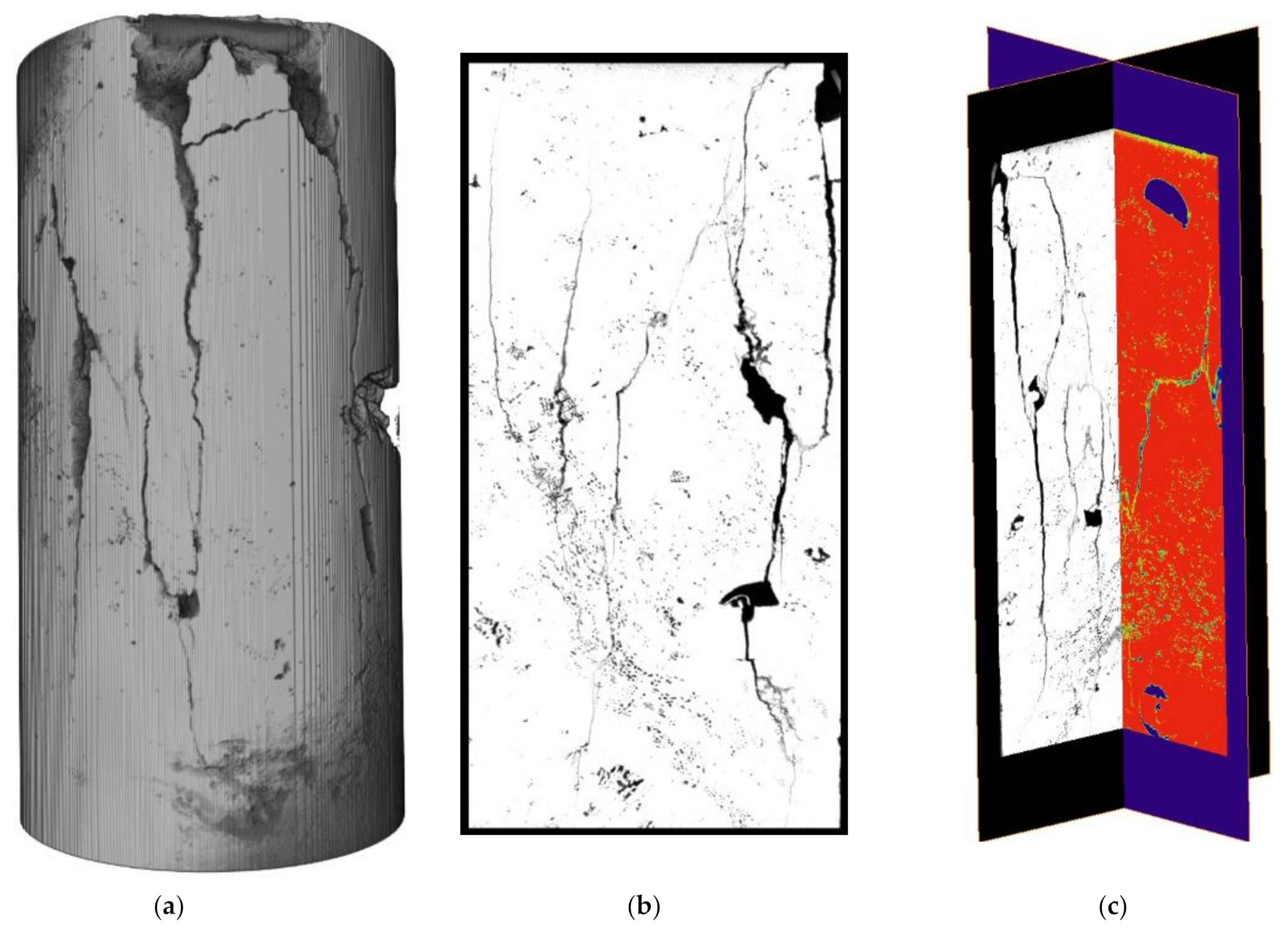
3.1.2. Changes in Volume, Porosity and Permeability
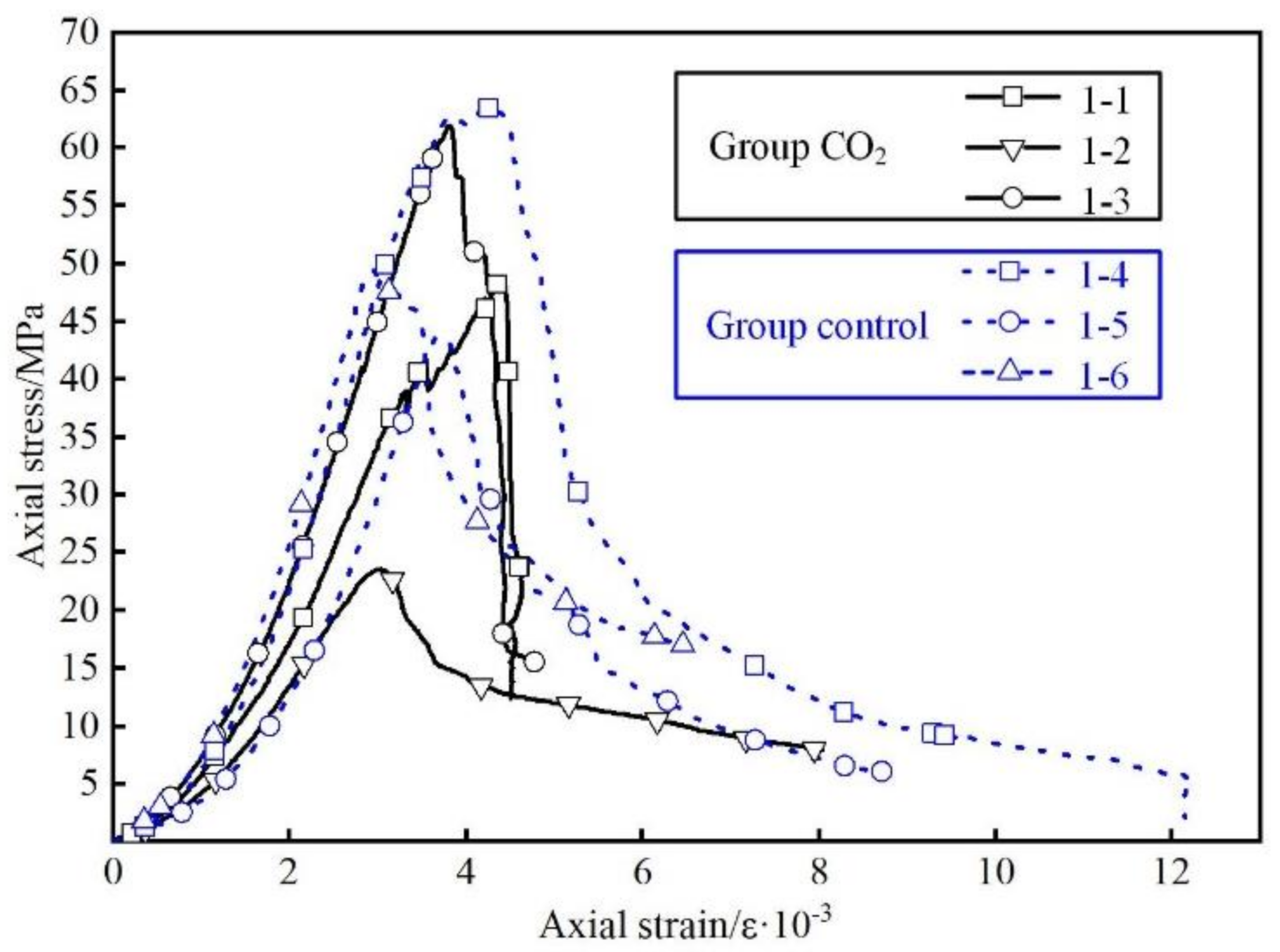
3.2. Change of Mechanical Property
4. Conclusions
- (1)
- The coral reef limestones dissolved when immersed in seawater supersaturated with carbon dioxide, which caused the changes of the physical properties of the rock samples. The mass and volume of reef limestones decreased during the reaction, and the permeability increased during the reaction process.
- (2)
- Although the reaction was mainly a dissolution process, there was a small amount of precipitation produced after 15 days of reaction time, which had a big impact on the permeability of the rock.
- (3)
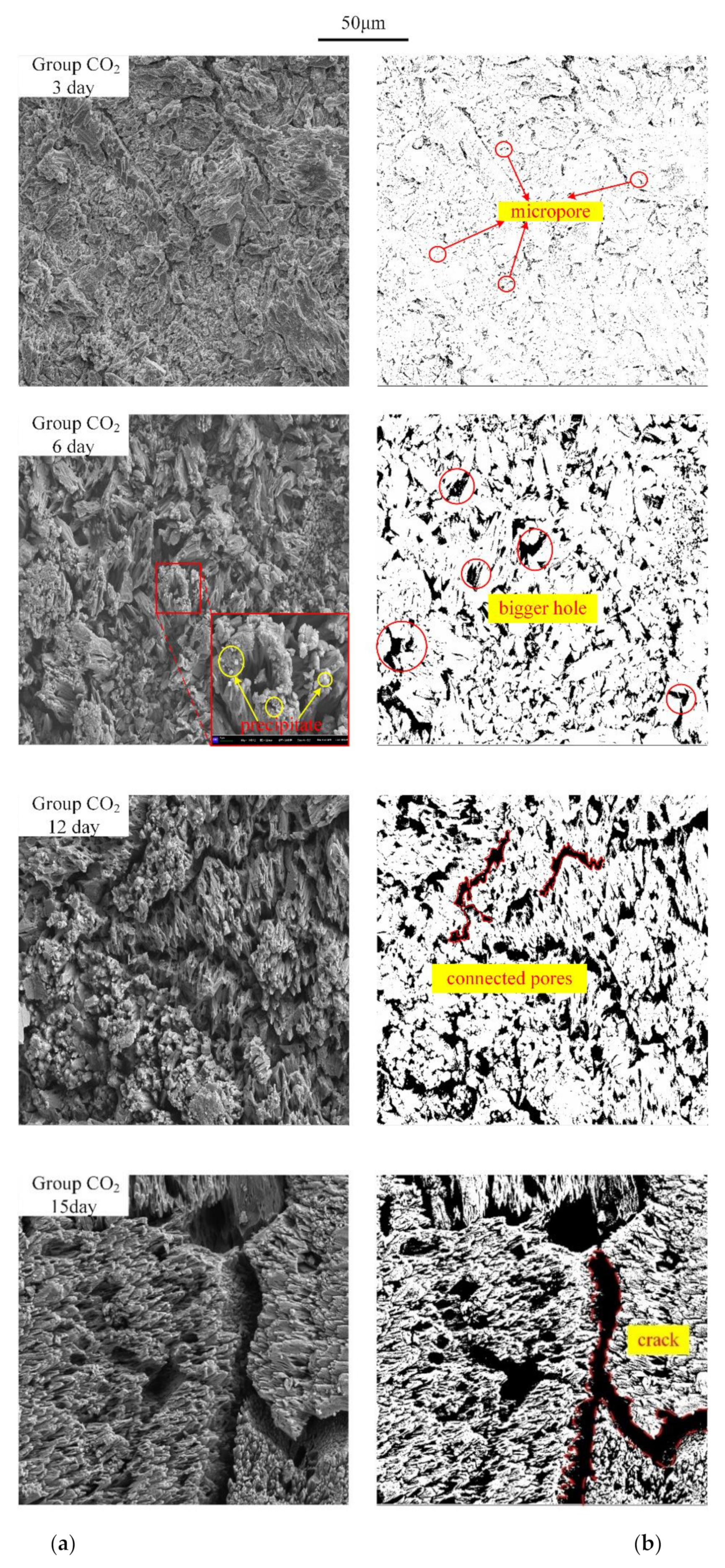
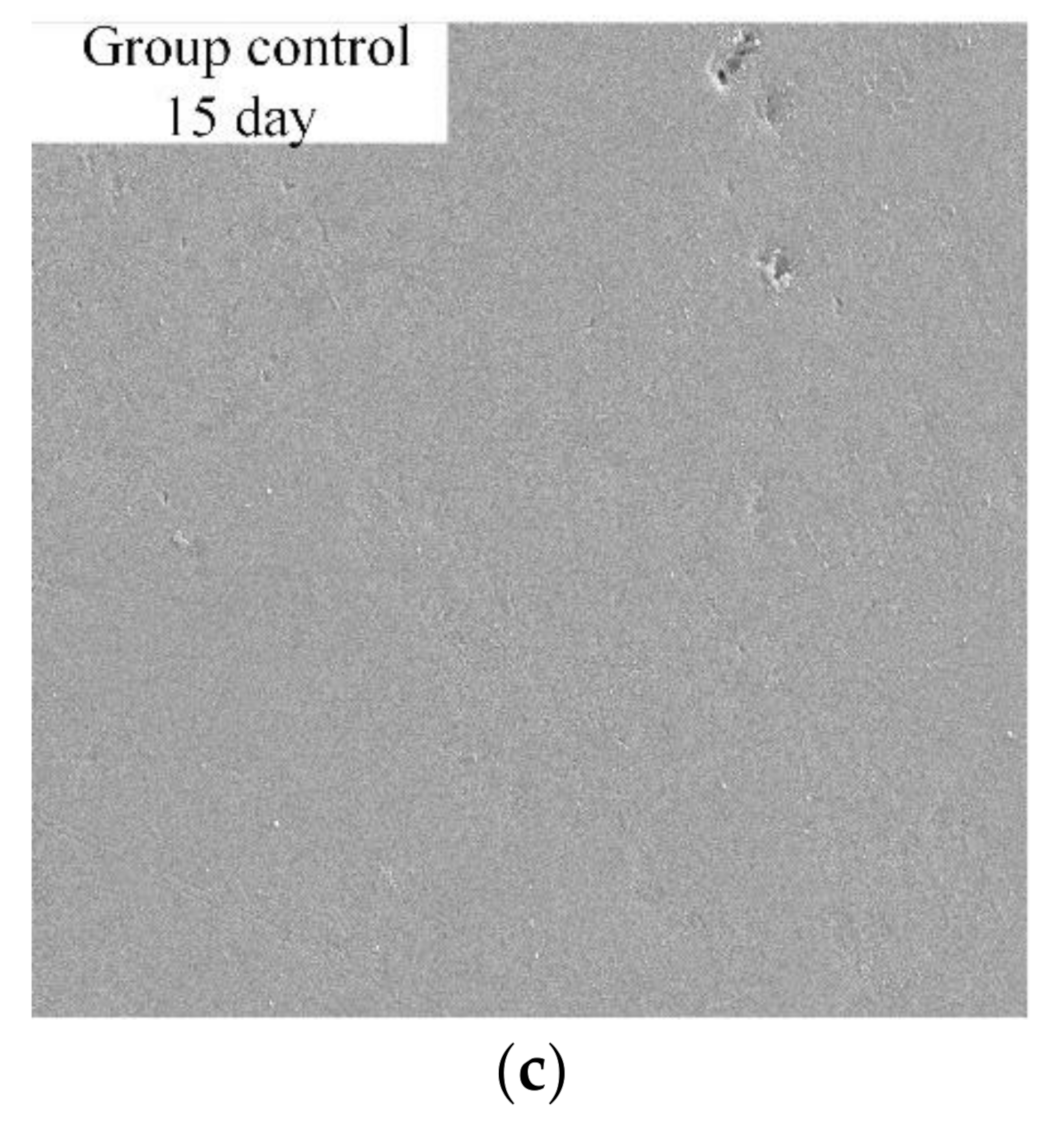
- As the reaction time increased, the reaction rate increased, and the microscopic pore structure of the reaction surface changed significantly during the reaction process. At the beginning of the reaction, a large amount of micropores were generated. Then, the micropores grew bigger and connected with each other as the reaction continued. In this way, the reaction contact surface increased, making the reaction rate increase with time.
- (4)
- Because the rocks were soaked for only 15 days, the total cumulative amount of calcium carbonate dissolved was less, and the mechanical properties were not affected.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC: Summary for Policymakers in Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 3–30. [Google Scholar]
- Orr, J.; Fabry, V.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Melzner, F.; Mark, F.C.; Seibel, B.A.; Tomanek, L. Ocean Acidification and Coastal Marine Invertebrates: Tracking CO2 Effects from Seawater to the Cell. Annu. Rev. Mar. Sci. 2020, 12, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, J.P.; Hansson, L. Ocean Acidification; Oxford University Press: Oxford, UK, 2011; pp. 129–131. [Google Scholar]
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Wang, J.H.; Sun, X.X.; Xu, X.M.; Wu, C.F.; Peng, J.; Yuan, J.B. Marine Carbon Sequestration: Current Situation, Problems and Future. Adv. Earth Sci. 2015, 30, 17–25. [Google Scholar]
- Caldeira, K.; Wickett, M.E. Oceanography: Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tynan, S.; Opdyke, B.N. Effects of lower surface ocean pH upon the stability of shallow water carbonate sediments. Sci. Total Environ. 2011, 409, 1082–1086. [Google Scholar] [CrossRef]
- Morse, J.W.; Zullig, J.J.; Bernstein, L.D.; Millero, F.J.; Milne, P.; Mucci, A.; Choppin, G.R. Chemistry of calcium carbonate-rich shallow water sediments in The Bahamas. Am. J. Sci. 1985, 285, 2. [Google Scholar] [CrossRef]
- Muehllehner, N.; Langdon, C.; Venti, A.; Kadko, D. Dynamics of carbonate chemistry, production, and calcification of the Florida Reef Tract (2009–2010): Evidence for seasonal dissolution. Glob. Biogeochem. Cycles 2016, 30, 661–688. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Rodolfo-Metalpa, R.; Martin, S.; Ransome, E.; Fine, M.; Turner, S.M.; Rowley, S.J.; Tedesco, D.; Buia, M. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 2008, 454, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Marubini, F.; Ferrier-Pages, C.; Cuif, J.P. Suppression of skeletal growth in scleractinian corals by decreasing ambient carbonate-ion concentration: A cross-family comparison. Proc. R. Soc. B Biol. Sci. 2003, 270, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Marubini, F.; Barnett, H.; Langdon, C.; Atkinson, M.J. Dependence of calcification on light and carbonate ion concentration for the hermatypic coral Porites compressa. Mar. Ecol. Prog. Ser. 2001, 220, 153–162. [Google Scholar] [CrossRef]
- Drupp, P.S.; De Carlo, E.H.; Mackenzie, F.T. Porewater CO2-carbonic acid system chemistry in permeable carbonate reef sands. Mar. Chem. 2016, 185, 48–64. [Google Scholar] [CrossRef]
- Wang, R.; Song, C.J. Reef Engineering Geology of Nansha Islands; Science Press: Beijing, China, 1997. [Google Scholar]
- Zhang, W.; Cao, Y.; Yuan, G. Experiment of interaction between calcite and fluid saturated with CO2 under different heating rates. Pet. Geol. Recovery Effic. 2017, 24, 57–65. [Google Scholar]
- Ketzer, J.M.; Iglesias, R.; Einloft, S.; Dullius, J.; Ligabue, R.; De Lima, V. Water-rock-CO2 interactions in saline aquifers aimed for carbon dioxide storage: Experimental and numerical modeling studies of the Rio Bonito Formation (Permian), southern Brazil. Appl. Geochem. 2009, 24, 760–767. [Google Scholar] [CrossRef]
- Lyu, Q.; Ranjith, P.G.; Long, X.P.; Ji, B. Experimental Investigation of Mechanical Properties of Black Shales after CO₂-Water-Rock Interaction. Materials 2016, 9, 663. [Google Scholar] [CrossRef]
- Sampath, K.H.S.M.; Sin, I.; Perera, M.S.A.; Matthai, S.K.; Ranjith, P.G.; Dong-Yin, L. Effect of supercritical-CO2 interaction time on the alterations in coal pore structure. J. Nat. Gas Sci. Eng. 2020, 76, 103214. [Google Scholar] [CrossRef]
- Liteanu, E.; Niemeijer, A.; Spiers, C.J.; Peach, C.J.; De Bresser, J.H.P.J. The effect of CO2 on creep of wet calcite aggregates. Geophys. Res. Solid Earth 2012, 117, B03211. [Google Scholar]
- Li, S.; Wu, Y.; Huo, R.; Song, Z.; Fujii, Y.; Shen, Y. Mechanical Properties of Acid-corroded Sandstone Under Uniaxial Compression. Rock Mech. Rock Eng. 2020, 54, 289–302. [Google Scholar] [CrossRef]
- Duan, Y.T.; Li, X.; Ranjith, P.G.; Wu, Y.F. An investigation of the evolution of the internal structures and failure modes of Longmaxi shale using novel X-ray microscopy. J. Pet. Sci. Eng. 2020, 184, 106479. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.; Ranjith, P.G.; Tan, J.; Kang, Y.; Wang, Z. Experimental investigation on the mechanical properties of a low-clay shale with different adsorption times in sub-/super-critical CO2. Energy 2018, 147, 1288–1298. [Google Scholar] [CrossRef]
- Madland, M.V.; Finsnes, A.; Alkafadgi, A.; Risnes, R.; Austad, T. The influence of CO2 gas and carbonate water on the mechanical stability of chalk. J. Pet. Sci. Eng. 2006, 51, 149–168. [Google Scholar] [CrossRef]
- Angeli, M.; Soldal, M.; Skurtveit, E.; Aker, E. Experimental percolation of supercritical CO2 through a caprock. Energy Procedia 2009, 1, 3351–3358. [Google Scholar] [CrossRef]
- Ojala, I.O. The effect of CO2 on the mechanical properties of reservoir and cap rock. Energy Procedia 2011, 4, 5392–5397. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.C.; Vanorio, T. The rock physics and geochemistry of carbonates exposed to reactive brines. J. Geophys. Res. Solid Earth 2016, 121, 1497–1513. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhu, C.; Zheng, K.; Ma, C.; Yi, M. Crack Initiation and Damage Evolution of Micritized Framework Reef Limestone in the South China Sea. Rock Mech. Rock Eng. 2021, 54, 5591–5601. [Google Scholar] [CrossRef]
- Zhao, H.T.; Sha, Q.A.; Zhu, Y.Z. Quaternary Coral Reef Geology of Yongshu Reef, Nansha Islands; China Ocean Press: Beijing, China, 1992. [Google Scholar]
- Ministry of Water Resources of the People’s Republic of China. Testing Procedures of Water Conservancy and Hydropower Engineering to Rock Experiments; The Publishing House of Water Conservancy and Hydropower Engineering: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Guo, X.Q.; Zhang, G.Y.; Song, F.Y.; He, P.L. The investigation of the pore structure of macroporous ion exchange resin and polymeric adsorbent I. The determination of their apparent density. Ion Exch. Adsorpt. 1987, 1, 20–24. [Google Scholar]
- Sander, R.; Pan, Z.; Connell, L.D. Laboratory measurement of low permeability unconventional gas reservoir rocks: A review of experimental methods. J. Nat. Gas Sci. Eng. 2017, 37, 248–279. [Google Scholar] [CrossRef]
- Guo, J. Analysis of Characters and Mechanisms of the Mixed Layer Salinity Variation in South China Sea. Master’s Thesis, State Oceanic Administration, Qingdao, China, 2012. [Google Scholar]
- Soleimani, P.; Shadizadeh, S.R.; Kharrat, R. Carbonate and sandstone rocks dissolution investigation during injection of smart carbonated water. Int. J. Oil Gas Coal Technol. 2021, 26, 382–404. [Google Scholar] [CrossRef]
- Sun, J.G. Fundamentals of Rock Physics; Geological Publishing House: Beijing, China, 2006. [Google Scholar]
- Yang, Y.; Bruns, S.; Rogowska, M.; Hakim, S.S.; Hammel, J.U.; Stipp, S.L.; Sørensen, H.O. Retraction of the dissolution front in natural porous media. Sci. Rep. 2018, 8, 5693. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Chu, Y.; Li, S.; Yang, Y.; Bai, X.; Ye, C.; Wen, D. Petrophysical characterization of high-rank coal by nuclear magnetic resonance: A case study of the Baijiao coal reservoir, SW China. R. Soc. Open Sci. 2018, 5, 181411. [Google Scholar] [CrossRef] [Green Version]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Pape, H.; Clauser, C.; Iffland, J. Permeability prediction based on fractal pore-space geometry. Geophysics 1999, 64, 1447–1460. [Google Scholar] [CrossRef]
- Wang, J.; Schweizer, D.; Liu, Q.; Su, A.; Hu, X.; Blum, P. Three-dimensional landslide evolution model at the Yangtze River. Eng. Geol. 2021, 292, 106275. [Google Scholar] [CrossRef]
- Li, S.P.; Wu, D.X.; Xie, W.H.; Li, Y.S.; Wu, Z.Y.; Zhou, G.; Zhao, H.Y. Effect of confining presurre, pore pressure and specimen dimension on permeability of Yinzhuang Sandstone. Int. J. Rock Mech. Min. Sci. 1997, 34, e1–e175. [Google Scholar] [CrossRef]
- Bacci, G.; Korre, A.; Durucan, S. An experimental and numerical investigation into the impact of dissolution/precipitation mechanisms on CO2 injectivity in the wellbore and far field regions. Int. J. Greenh. Gas Control. 2011, 5, 579–588. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Zhang, F.E. Laboratory simulation experiment on dissolution of limestone under hydrodynamic pressure. Carbonates Evaporites 2013, 28, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.C.; Macfarlane, J.; Vanorio, T. Permeability Evolution of a Cemented Volcanic Ash during Carbonation and CO2 Depressurization. J. Geophys. Res.-Solid Earth 2018, 123, 8409–8427. [Google Scholar] [CrossRef]
- Wang, X.; Shan, H.; Wang, X.; Zhu, C.Q. Strength Characteristics of Reef Limestone for Different Cementation Types. Geotech. Geol. Eng. 2020, 38, 79–89. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, R.; Li, Q.; Wei, H.Z.; Li, X.Y. Physical and engineering characteristics of reef limestone: A review. Sci. Technol. Rev. 2020, 38, 57–70. [Google Scholar]
- Park, J.; Baek, K.; Lee, M.; Chung, C.W.; Wang, S. The Use of the Surface Roughness Value to Quantify the Extent of Supercritical CO2 Involved Geochemical Reaction at a CO2 Sequestration Site. Appl. Sci.-Basel 2017, 7, 572. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zheng, K.; Zhu, C.Q.; Meng, Q.S.; Wu, W.J. Brittleness evaluation of coral reef limestone base on stress-strain curve. Rock Soil Mech. 2021, 42, 673–680. [Google Scholar]






| Group | Test Condition | Number of Sample | Form | Water Absorption Coefficient (%) | Mass (g) | ||
|---|---|---|---|---|---|---|---|
| Before | After | ΔM | |||||
| CO2 | Injection of CO2 Pressure of 7 Mpa 30 °C | 1-1 | BUC | 0.71 | 64.04 | 63.36 | −0.68 |
| 1-2 | 0.88 | 63.59 | 62.86 | −0.73 | |||
| 1-3 | 0.78 | 65.57 | 64.82 | −0.75 | |||
| CT1-2 | BUT | 0.71 | 128.46 | 126.85 | −1.60 | ||
| B1-1 | 0.86 | 126.65 | 125.31 | −1.34 | |||
| B1-2 | 0.61 | 127.71 | 126.62 | −1.09 | |||
| V | BS | 1.10 | 2.74 | 2.64 | −0.10 | ||
| Control | Without CO2 Constant temperature 30 °C | 1-4 | buc | 1.14 | 60.52 | 60.50 | −0.02 |
| 1-5 | 1.06 | 60.30 | 60.24 | −0.06 | |||
| 1-6 | 1.17 | 59.72 | 59.70 | −0.02 | |||
| b1-1 | but | 0.67 | 126.83 | 126.78 | −0.05 | ||
| b1-2 | 0.56 | 125.74 | 125.60 | −0.14 | |||
| b1-3 | 0.56 | 125.21 | 125.09 | −0.12 | |||
| 11 | bs | 1.38 | 2.52 | 2.52 | 0.00 | ||
| Group | Number of Sample | Size | Volume(cm3) | Porosity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | ΔV(cm3) | Before | After | ΔP | |||
| CO2 | 1-1 | BUC | 24.45 | 23.66 | −0.79 | 3.05 | 6.20 | 3.15 |
| 1-2 | 23.59 | 23.45 | −0.14 | 6.98 | 7.54 | 0.57 | ||
| 1-3 | 24.53 | 24.19 | −0.34 | 2.96 | 4.31 | 1.34 | ||
| Control | 1-4 | buc | 22.82 | 22.76 | −0.02 | 5.32 | 5.58 | 0.26 |
| 1-5 | 22.56 | 22.52 | −0.06 | 5.63 | 5.82 | 0.19 | ||
| 1-6 | 22.29 | 22.24 | −0.02 | 7.70 | 7.90 | 0.21 | ||
| Group | Number of Sample | Pc (MPa) | Permaebility (mD) | ||||
|---|---|---|---|---|---|---|---|
| 0 Day | 3 Day | 6 Day | 12 Day | 15 Day | |||
| CO2 | 1-1 | 1 | 0.65 | 2.51 | 2.29 | 5.92 | 11.40 |
| 3 | 0.48 | 1.68 | 1.55 | 3.65 | 8.83 | ||
| 5 | 0.28 | 0.91 | 0.86 | 1.94 | 5.56 | ||
| 7 | 0.12 | 0.34 | 0.38 | 0.94 | 3.29 | ||
| 1-2 | 1 | 3.28 | 13.52 | 9.12 | 26.39 | 24.47 | |
| 3 | 2.48 | 8.57 | 6.31 | 15.22 | 16.36 | ||
| 5 | 1.61 | 4.80 | 3.44 | 7.73 | 9.86 | ||
| 7 | 0.84 | 2.83 | 1.60 | 3.82 | 5.31 | ||
| 1-3 | 1 | 0.06 | 1.47 | 0.82 | 2.68 | 5.83 | |
| 3 | 0.07 | 0.87 | 0.43 | 1.50 | 2.47 | ||
| 5 | 0.06 | 0.48 | 0.21 | 0.77 | 2.07 | ||
| 7 | 0.02 | 0.24 | 0.10 | 0.43 | 1.18 | ||
| Control | 1-4 | 1 | 0.45 | - | - | - | 0.45 |
| 3 | 0.21 | - | - | - | 0.21 | ||
| 5 | 0.09 | - | - | - | 0.09 | ||
| 7 | 0.05 | - | - | - | 0.05 | ||
| 1-5 | 1 | 0.36 | - | - | - | 0.35 | |
| 3 | 0.14 | - | - | - | 0.14 | ||
| 5 | 0.08 | - | - | - | 0.07 | ||
| 7 | 0.04 | - | - | - | 0.03 | ||
| 1-6 | 1 | 0.27 | - | - | - | 0.26 | |
| 3 | 0.11 | - | - | - | 0.11 | ||
| 5 | 0.06 | - | - | - | 0.05 | ||
| 7 | 0.03 | - | - | - | 0.03 | ||
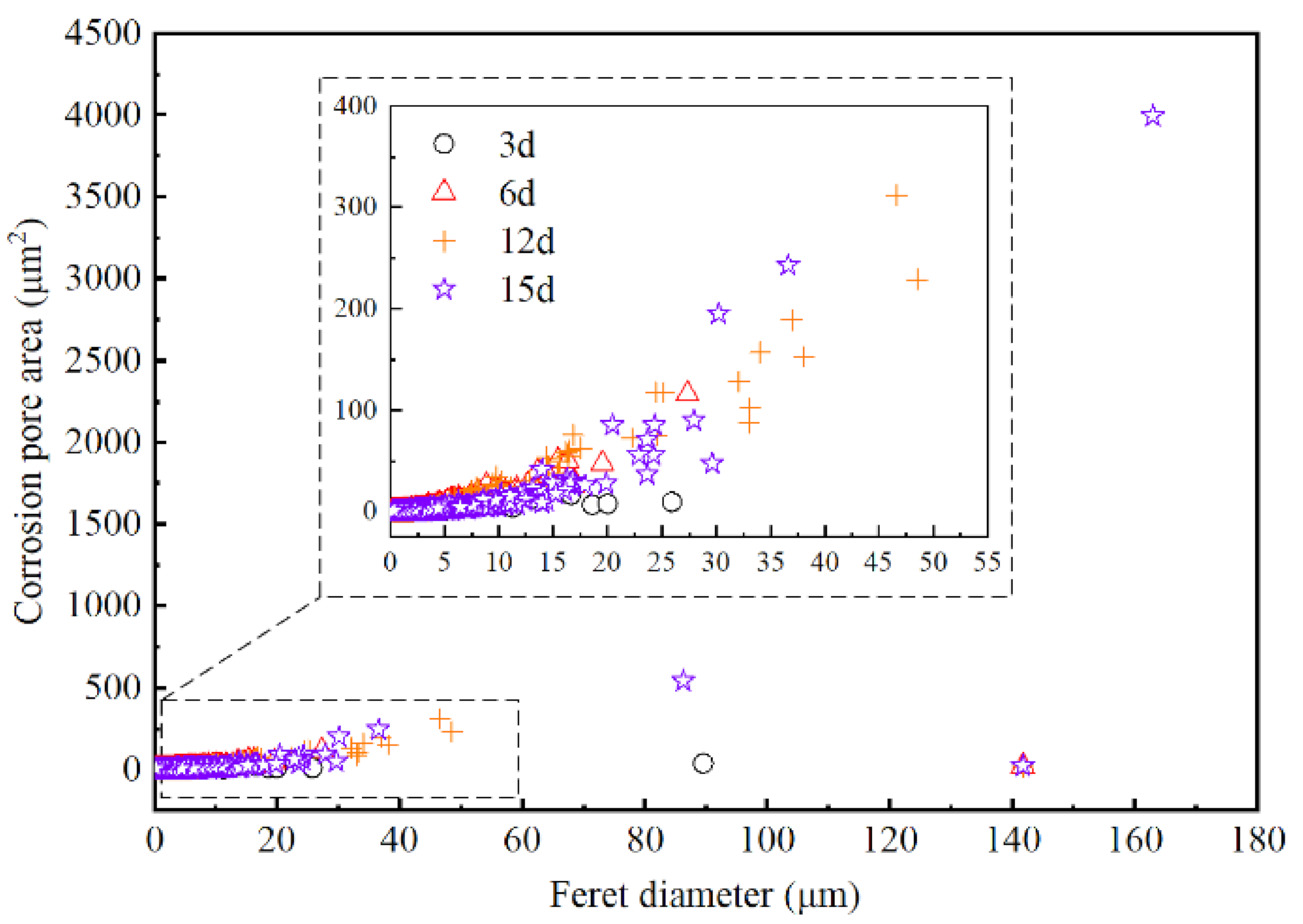
| Reaction Time (Day) | Pore Count | Average Size (μm2) | Corrosion Area (μm2) | Surface Porosity (%) |
|---|---|---|---|---|
| 3 | 11,897 | 0.15 | 1837.92 | 6.08 |
| 6 | 3388 | 0.74 | 2491.90 | 17.73 |
| 12 | 3381 | 1.20 | 4043.15 | 28.76 |
| 15 | 5059 | 1.38 | 6979.61 | 49.65 |
| Group | Number of Sample | Form | Compressive Strength (MPa) | Average Compressive Strength (MPa) | Elastic Modulus (GPa) | Average Elastic Modulus (GPa) |
|---|---|---|---|---|---|---|
| CO2 | 1-1 | BUC | 48.57 | 44.65 | 4.53 | 4.56 |
| 1-2 | 23.52 | 3.54 | ||||
| 1-3 | 61.87 | 5.62 | ||||
| Control | 1-4 | buc | 63.38 | 52.14 | 4.59 | 4.60 |
| 1-5 | 49.35 | 6.04 | ||||
| 1-6 | 43.68 | 3.16 |
| Group | Number of Sample | Form | Tensile Strength (MPa) | Average Tensile Strength (MPa) |
|---|---|---|---|---|
| CO2 | CT1-2 | BUT | 6.94 | 7.04 |
| B1-1 | 6.50 | |||
| B1-2 | 7.67 | |||
| Control | b1-1 | but | 5.86 | 6.42 |
| b1-2 | 5.75 | |||
| b1-3 | 7.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Li, Q.; Wang, R.; Yao, T. Changes of Physical and Mechanical Properties of Coral Reef Limestone under CO2–Seawater–Rock Interaction. Appl. Sci. 2022, 12, 4105. https://doi.org/10.3390/app12094105
Zhong Y, Li Q, Wang R, Yao T. Changes of Physical and Mechanical Properties of Coral Reef Limestone under CO2–Seawater–Rock Interaction. Applied Sciences. 2022; 12(9):4105. https://doi.org/10.3390/app12094105
Chicago/Turabian StyleZhong, Yu, Qi Li, Ren Wang, and Ting Yao. 2022. "Changes of Physical and Mechanical Properties of Coral Reef Limestone under CO2–Seawater–Rock Interaction" Applied Sciences 12, no. 9: 4105. https://doi.org/10.3390/app12094105
APA StyleZhong, Y., Li, Q., Wang, R., & Yao, T. (2022). Changes of Physical and Mechanical Properties of Coral Reef Limestone under CO2–Seawater–Rock Interaction. Applied Sciences, 12(9), 4105. https://doi.org/10.3390/app12094105







