Preliminary Investigation of Possible Biochar Use as Carbon Source in Polyacrylonitrile Electrospun Fiber Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Production
2.2. Biochar Preparation
- Set frequency of vibration to 25.0 Hz;
- Mill for 2 min;
- Cool down the vessels for 5 min to reduce the powder temperature;
- Mill for 2 more minutes (as-received) or 3 min (clean);
- Cool down the vessels for 5 min to reduce the powder temperature;
- Sieve with the 32 µm sieve (as-received); wet-sieve with the 32 µm sieve (clean).
2.3. Fibers Preparation
- 100% PAN: 1.5 g PAN + 10 mL DMF;
- 90% PAN/10% Biochar: 1.35 g PAN + 0.15 g Biochar + 10 mL DMF;
- 75% PAN/25% Biochar: 1.125 g PAN + 0.375 g Biochar + 10 mL DMF;
- 50% PAN/50% Biochar: 0.75 g PAN + 0.75 g Biochar + 10 mL DMF.
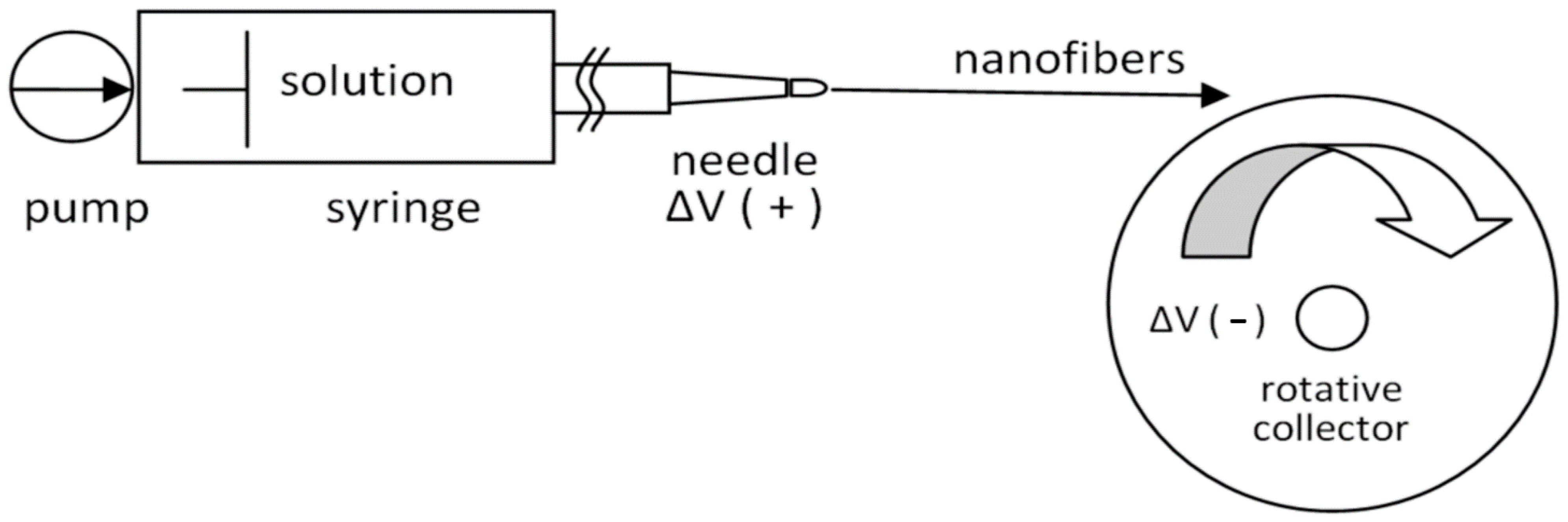
2.4. Electrospinning Process
2.5. Stabilization/Cyclization
2.6. Carbonization
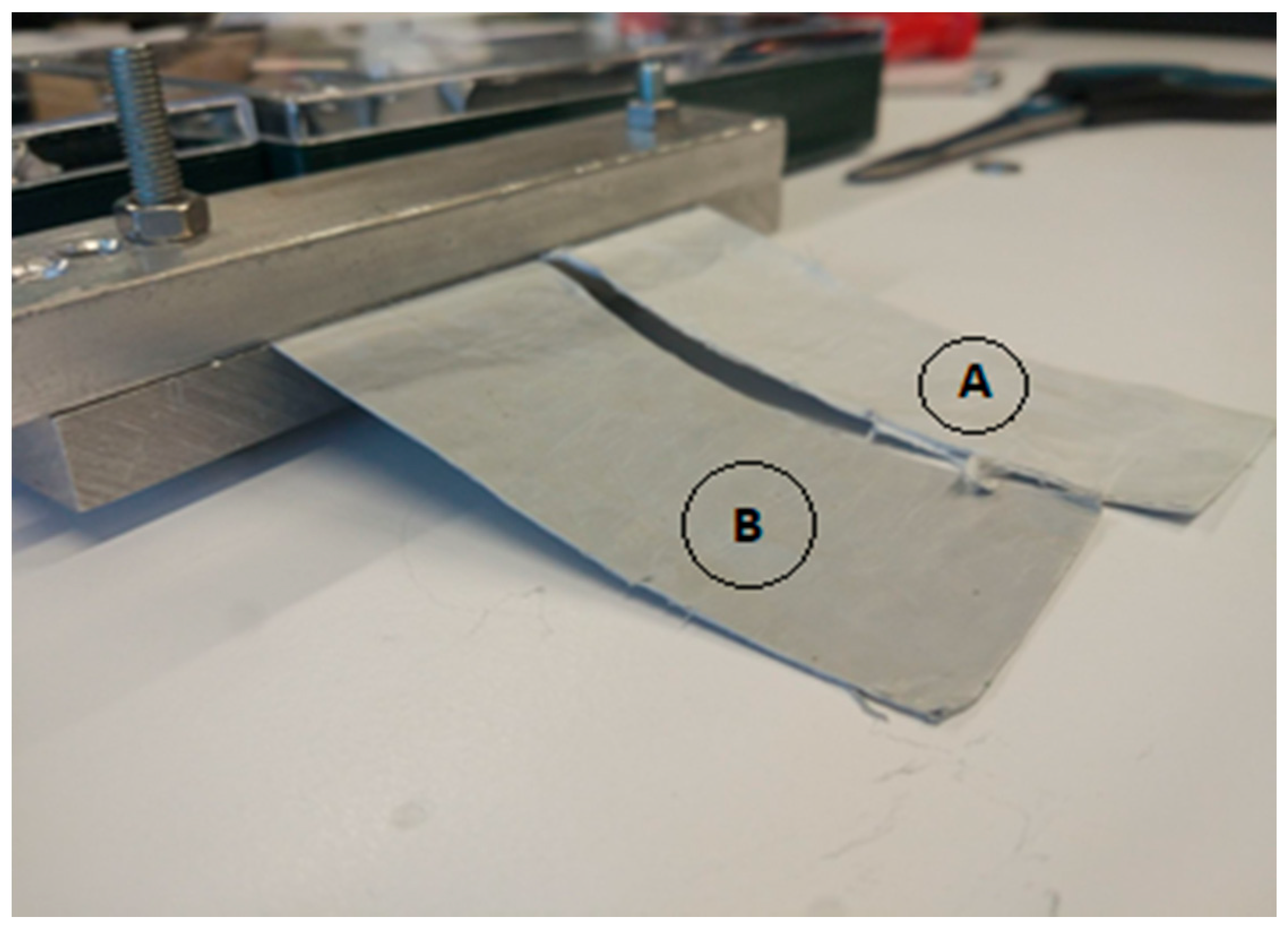
2.7. Samples’ Characterization Techniques
- -
- The Environmental Scanning Electron Microscope (ESEM, FEI Quanta-200) was used for microstructural characterizations. Scanning electron microscope imaging uses a focused electron beam to scan the sample surface. The signals that originate from the electron-surface interactions provide the information regarding the sample micromorphology. ESEM was used in high-vacuum mode, applying the secondary electron detector, Everhart–Thornley type (ETD), to obtain micro-morphological data on sample surfaces.
- -
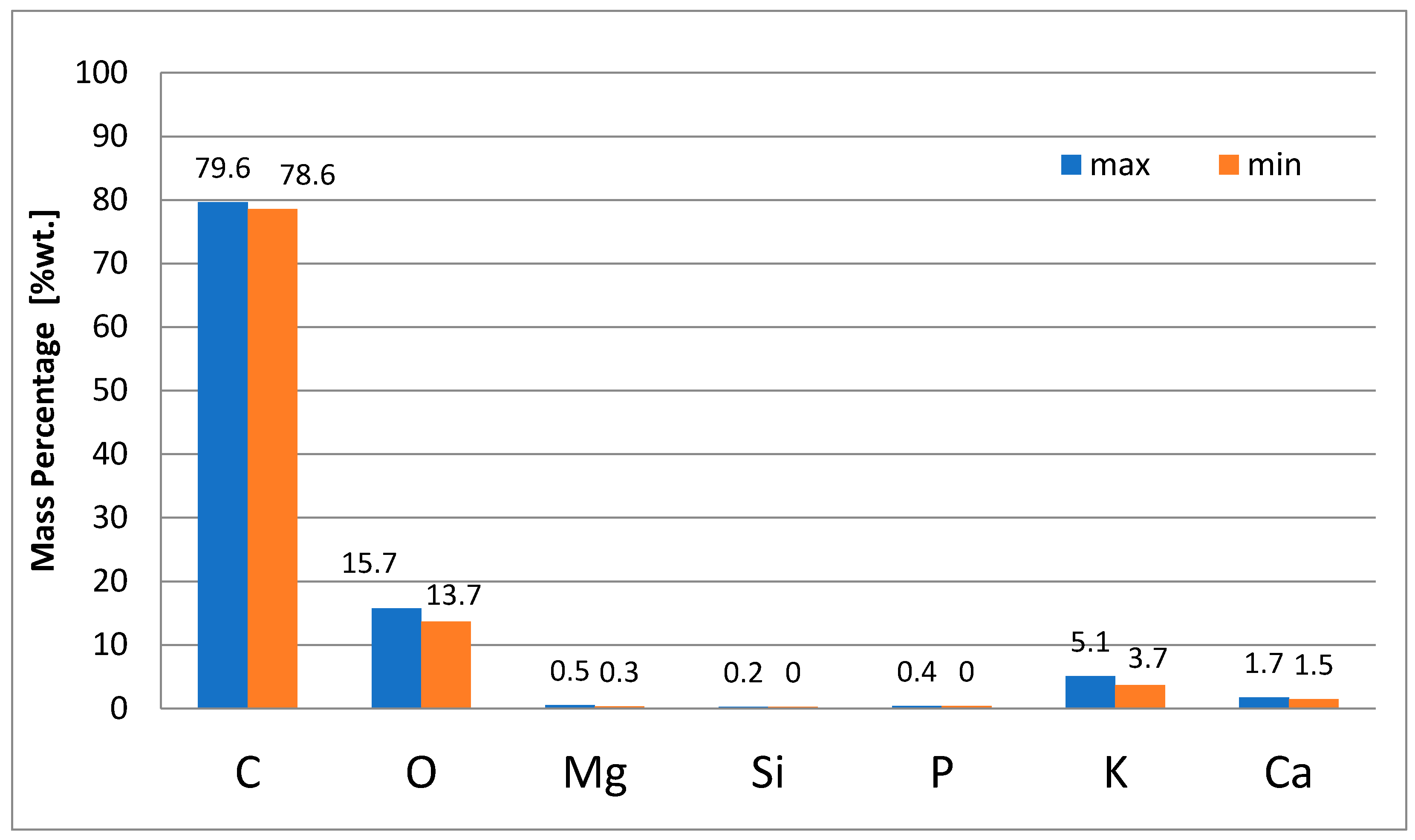
- Energy-dispersive X-ray spectroscopy (X-EDS) was applied to carry out semi-quantitative chemical analysis. This analytical technique is often provided by the ESEM apparatus due to back-scattered electron imaging. X-EDS provides a chemical characterization of the sample, analyzing the specific electromagnetic emission spectrum, which identifies each element. Data were acquired and elaborated through X-EDS Oxford INCA-350 software. More spectra were acquired for each sample to obtain a significant statistical analysis. Chemical information about elemental composition was expressed as weight elemental percentage.
- -
- Fourier-transform infrared spectroscopy (FT-IR): measuring the absorption and emission infrared spectrum of the samples, it is possible to identify compounds, gaining fundamental information about the chemical bonds. The instrument used in this work was FT-IR (Bruker Tensor 27 IR). IR analysis was conducted at ACI-LUH, Hannover.
3. Results and Discussion
3.1. Biochar Analyses
Imaging of Prepared Biochar and Milled Biochar
3.2. Imaging of PAN/Biochar Fibers though the Preparation Process
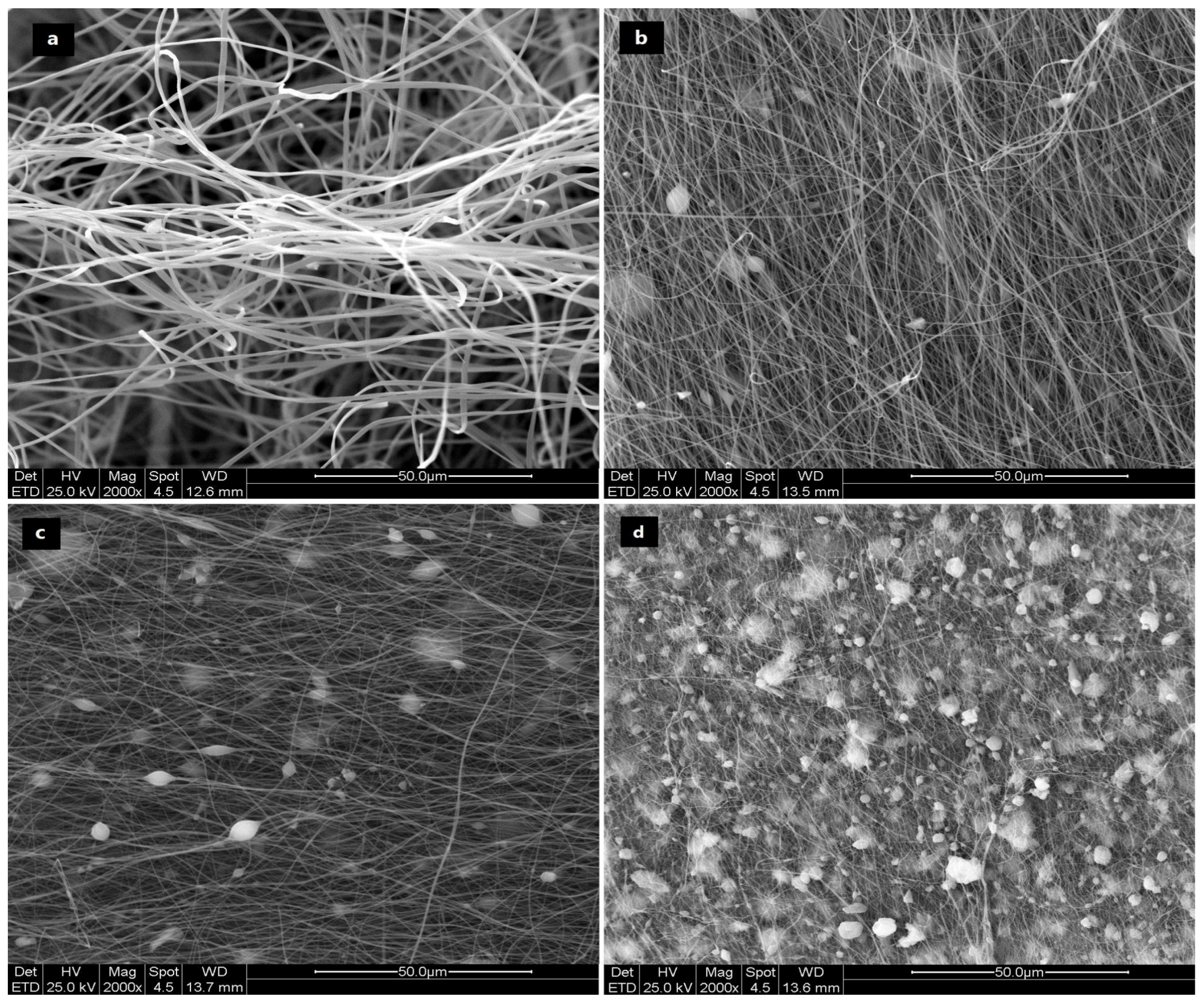
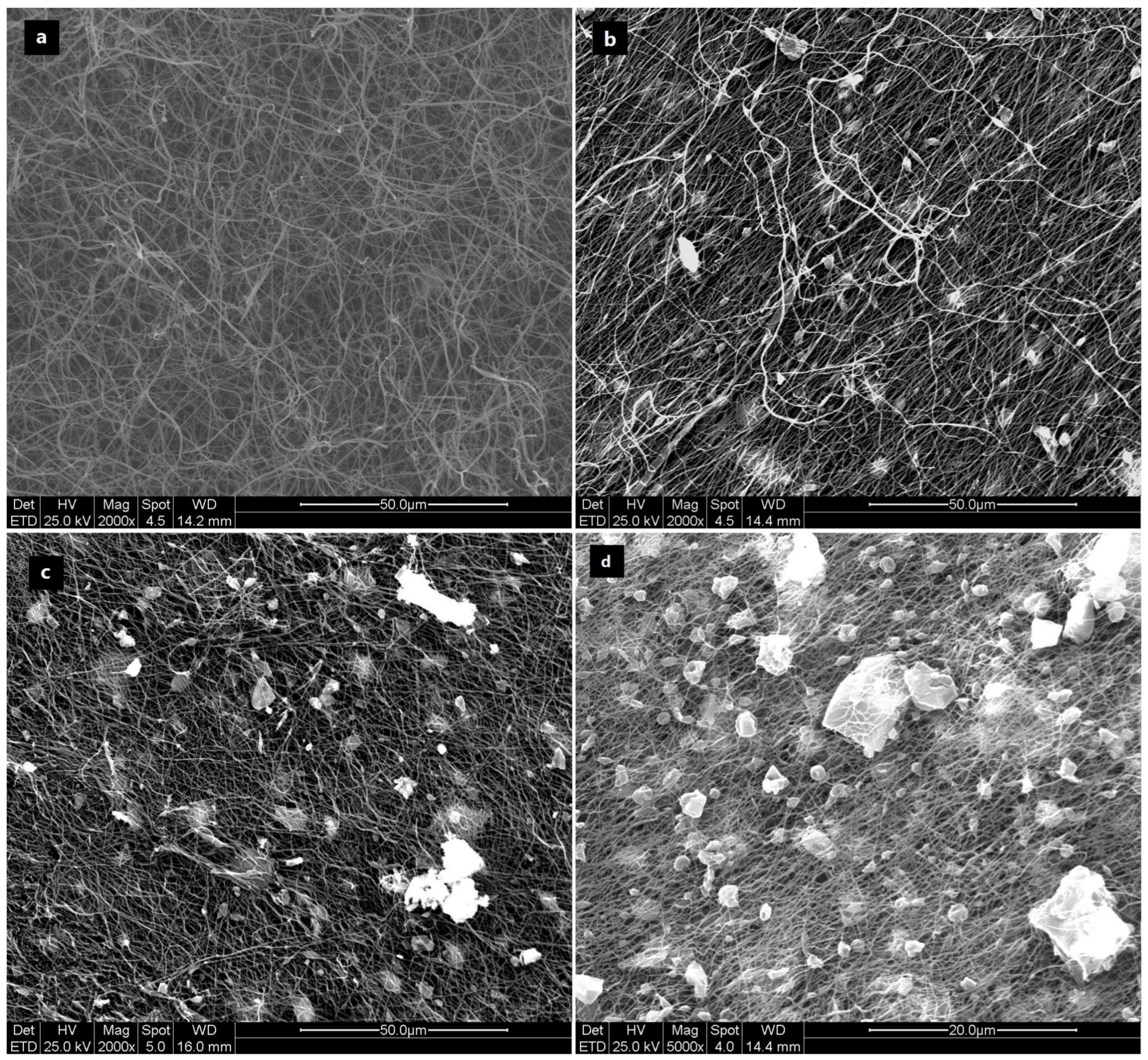
3.2.1. ESEM Imaging of “as-Spun” Fibers
3.2.2. ESEM Imaging of Carbonized Fibers
3.2.3. EDS Analyses of “as-Spun” Fibers
3.2.4. EDS Analyses of Carbonized Fibers
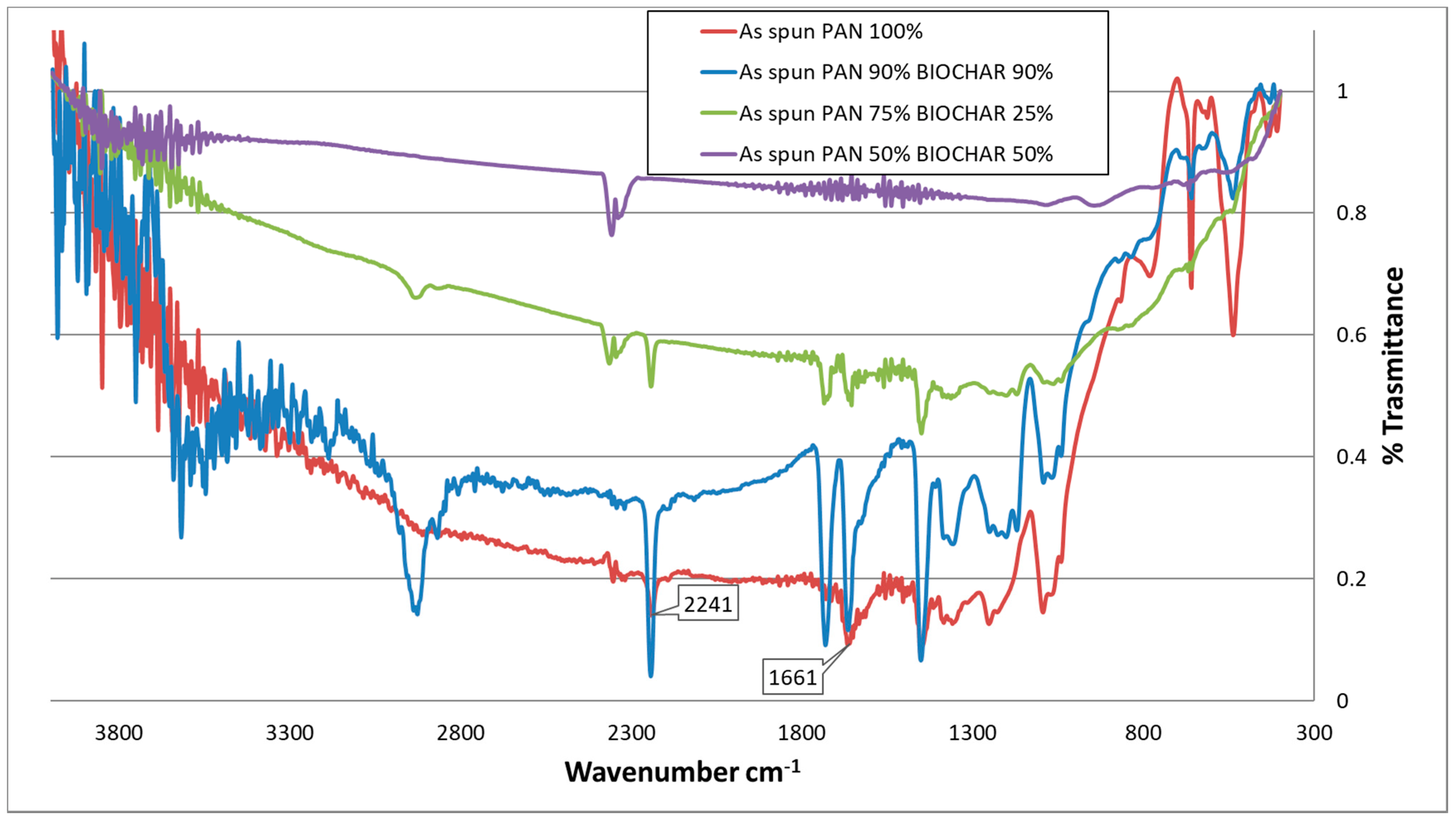
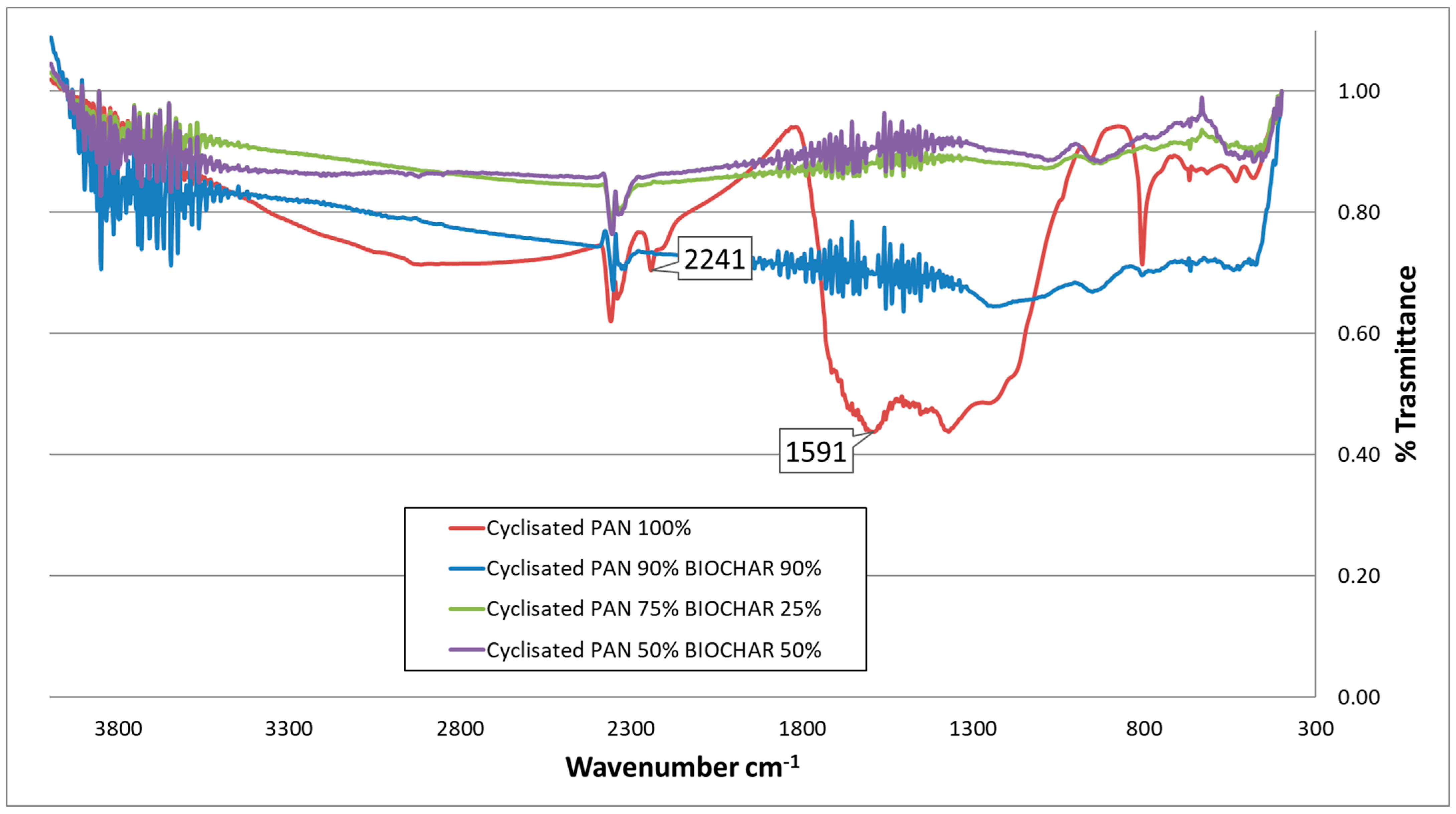
3.2.5. FT-IR Spectroscopy of “as-Spun” and Cyclized Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2018. [Google Scholar]
- Piccolo, F.; Andreola, F.; Barbieri, L.; Lancellotti, I. Synthesis and Characterization of Biochar-Based Geopolymer Materials. Appl. Sci. 2021, 11, 10945. [Google Scholar] [CrossRef]
- Farges, R.; Gharzouni, A.; Ravier, B.; Jeulin, P.; Rossignol, S. Insulating foams and dense geopolymers from biochar by-products. J. Ceram. Sci. Technol. 2018, 9, 193–200. [Google Scholar] [CrossRef]
- Araya, M.; Rivas, J.; Sepúlveda, G.; Espinoza-González, C.; Lira, S.; Meynard, A.; Blanco, E.; Escalona, N.; Ginocchio, R.; Garrido-Ramírez, E.; et al. Effect of Pyrolysis Temperature on Copper Aqueous Removal Capability of Biochar Derived from the Kelp Macrocystis pyrifera. Appl. Sci. 2021, 11, 9223. [Google Scholar] [CrossRef]
- Mansouri, F.E.; Farissi, H.E.; Zerrouk, M.H.; Cacciola, F.; Bakkali, C.; Brigui, J.; Lovillo, M.P.; Esteves da Silva, J.C.G. Dye Removal from Colored Textile Wastewater Using Seeds and Biochar of Barley (Hordeum vulgare L.). Appl. Sci. 2021, 11, 5125. [Google Scholar] [CrossRef]
- Enaime, G.; Lübken, M. Agricultural Waste-Based Biochar for Agronomic Applications. Appl. Sci. 2021, 11, 8914. [Google Scholar] [CrossRef]
- Ali, L.; Xiukang, W.; Naveed, M.; Ashraf, S.; Nadeem, S.M.; Haider, F.U.; Mustafa, A. Impact of Biochar Application on Germination Behavior and Early Growth of Maize Seedlings: Insights from a Growth Room Experiment. Appl. Sci. 2021, 11, 11666. [Google Scholar] [CrossRef]
- Liou, Y.; Huang, W. A Process for Preparing High Graphene Sheet Content Carbon Materials from Biochar Materials. In Electroplating of Nanostructures; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2015; Available online: https://www.intechopen.com/chapters/48922 (accessed on 14 April 2022). [CrossRef] [Green Version]
- Saha, J.K.; Dutta, A. A Review of Graphene: Material Synthesis from Biomass Sources. Waste Biomass Valorization 2022, 13, 1385–1429. [Google Scholar] [CrossRef]
- Tang, J.; Lv, H.; Gong, Y.; Huang, Y. Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal. Bioresour. Technol. 2015, 196, 355–363. [Google Scholar] [CrossRef]
- Moulefera, I.; Trabelsi, M.; Mamun, A.; Sabantina, L. Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends. Polymers 2021, 13, 1071. [Google Scholar] [CrossRef]
- Vélez, D.C.P.; Magalhães, W.L.E.; Capobianco, G. Carbon fiber from fast pyrolysis bio-oil. Sci. Technol. Mater. 2018, 30, 16–22. [Google Scholar] [CrossRef]
- Nan, W.; Zhao, Y.; Ding, Y.; Shende, A.R.; Fong, H.; Shende, R.V. Mechanically flexible electrospun carbon nanofiber mats derived from biochar and polyacrylonitrile. Mater. Lett. 2017, 205, 206–210. [Google Scholar] [CrossRef]
- Li, X.; Xu, T.; Liang, Z.; Amar, V.S.; Huang, R.; Maddipudi, B.K.; Shende, R.V.; Fong, H. Simultaneous Electrospinning and Electrospraying for the Preparation of a Precursor Membrane Containing Hydrothermally Generated Biochar Particles to Produce the Value-Added Product of Carbon Nanofibrous Felt. Polymers 2021, 13, 676. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio. Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing Co. Pte. Ltd.: Danvers, MA, USA, 2005; pp. 5–300. [Google Scholar]
- Lee, C.S. Carbon Nanofibers Synthesis, Applications and Performance, 1st ed.; Nova Science Pub Inc.: New York, NY, USA, 2018; pp. 4–5. [Google Scholar]
- Figueiredo, J.L.; Bernardo, C.A.; Baker, R.T.K.; Hüttinger, K.J. Carbon Fibers Filaments and Composites, 1st ed.; Springer: Berlin, Germany, 1989; pp. 50–250. [Google Scholar]
- Dang, W.; Liu, J.; Wang, X.; Yan, K.; Zhang, A.; Yang, J.; Chen, L.; Liang, J. Structural Transformation of Polyacrylonitrile (PAN) Fibers during Rapid Thermal Pretreatment in Nitrogen Atmosphere. Polymers 2020, 12, 63. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-Based Nanofiber—Nanoparticle Hybrids and Their Medical Applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef]
- Khan, Z.; Kafiah, F.; Shafi, H.Z.; Nufaiei, F.; Furquan, S.A.; Matin, A. Morphology, Mechanical Properties and Surface Characteristics of Electrospun Polyacrylonitrile (PAN) Nanofiber Mats. Int. J. Adv. Eng. Nano Technol. 2015, 2, 15–22. [Google Scholar]
- Callister, W.D., Jr.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering, 1st ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. 70–163. [Google Scholar]
- Qin, Y. Medical Textile Materials; Woodhead Publishing—Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–266. [Google Scholar] [CrossRef]
- Gulrajani, M.L. Advances in the Dyeing and Finishing of Technical Textiles, 1st ed.; Woodhead Publishing—Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Morgan, P. Carbon Fibers and Their Composites; Taylor and Francis CRC Press: Boca Raton, FL, USA, 2005; pp. 340–621. [Google Scholar]
- Puglia, M.; Torri, G.; Martinelli, V.; Tartarini, P. Vine Prunings Agro-Energetic Chain: Experimental and Economical Assessment of Vine Pellets Use in Gasification Power Plants. In Proceedings of the 28th European Biomass Conference and Exhibition, Online, 6–9 July 2020. [Google Scholar]
- Allesina, G.; Pedrazzi, S. Barriers to Success: A Technical Review on the Limits and Possible Future Roles of Small Scale Gasifiers. Energies 2021, 14, 6711. [Google Scholar] [CrossRef]
- All Power Labs Inc. PP30 Gasifier Datasheet. 2021. Available online: https://www.allpowerlabs.com/pp30-power-pallet (accessed on 14 April 2022).
- Pedrazzi, S.; Santunione, G.; Mustone, M.; Cannazza, G.; Citti, C.; Francia, E.; Allesina, G. Techno-economic study of a small scale gasifier applied to an indoor hemp farm: From energy savings to biochar effects on productivity. Energy Convers. Manag. 2021, 228, 113645. [Google Scholar] [CrossRef]
- Pedrazzi, S.; Allesina, G.; Sebastianelli, L.; Puglia, M.; Morselli, N.; Tartarini, P. Chemically enhanced char for syngas filtering purposes. In Proceedings of the 26th European Biomass Conference and Exhibition Proceedings, Copenhagen, Denmark, 14–17 May 2018. [Google Scholar]
- Tsekos, C.; Anastasakis, K.; Schoenmakers, P.L.; De Jong, W. PAH sampling and quantification from woody biomass fast pyrolysis in a pyroprobe reactor with a modified tar sampling system. J. Anal. Appl. Pyrolysis 2020, 147, 104802. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Knoef, H.A.M.; Buffinga, G.J.; Zielke, U.; Sjöström, K.; Brage, C.; Hasler, P.; Smell, P.A.; Suomalainen, M.; Dorrington, M.A.; et al. Guideline for Sampling and Analysis of Tars and Particles in Biomass Producer Gases. In Progress in Thermochemical Biomass Conversion; Bridgwater, A.V., Ed.; Blackwell Science Ltd.: London, UK, 2001; pp. 162–175. [Google Scholar] [CrossRef]
- Little, A.; Cash, M. High Temperature Corrosion of Silicon-Based Ceramics in a Simulated Coal Gasification Environment. In Proceedings of the CORROSION 98, San Diego, CA, USA, 22–27 March 1998. [Google Scholar]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.B.; Dreyer, B.; Renz, F.; Tegenkamp, C.; Sindelar, R. Electrospun Polyacrylonitrile Based Carbon Nanofibers: The Role of Creep Stress towards Cyclization and Graphitization. J. Mater. Sci. Eng. 2018, 7, 1000493. [Google Scholar] [CrossRef]
- Juhász, L.; Moldován, K.; Gurikov, P.; Liebner, F.; Fábián, I.; Kalmár, J.; Cserháti, C. False Morphology of Aerogels Caused by Gold Coating for SEM Imaging. Polymers 2021, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Di Blasi, C.; Buonanno, F.; Branca, C. Reactivities of some biomass chars in air. Carbon 1999, 37, 1227–1238. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Huang, H.; Li, Y.; Ma, T. A facile and general procedure to hyperporous carbons: Carbonization of organic zinc salts. Mater. Today Energy 2020, 17, 100446. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Development of adsorptive membranes by confinement of activated biochar into electrospun nanofibers. Beilstein J. Nanotechnol. 2016, 7, 1556–1563. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chung, Y.S.; Choi, H.S.; Jin, F.L.; Park, S.J. Preparation and Characterization of PAN-based Superfined Carbon Fibers for Carbon-paper Applications. Bull. Korean Chem. Soc. 2013, 34, 3733–3737. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.B.; Slawig, D.; Schlosser, A.; Koch, J.; Bigall, N.C.; Renz, F.; Tegenkamp, C.; Sindelar, R. Polyacrylonitrile (PAN) based electrospun carbon nanofibers (ECNFs): Probing the synergistic effects of creep assisted stabilization and CNTs addition on graphitization and low dimensional electrical transport. Carbon 2021, 172, 283–295. [Google Scholar] [CrossRef]
- Ali, A.B.; Renz, F.; Koch, J.; Tegenkamp, C.; Sindelar, R. Graphene Nanoplatelet (GNPs) Doped Carbon Nanofiber (CNF) System: Effect of GNPs on the Graphitic Structure of Creep Stress and Non-Creep Stress Stabilized Polyacrylonitrile (PAN). Nanomaterials 2020, 10, 351. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Song, Y.; Bian, Y.; Wang, F.; Gu, C.; Yang, X.; Jiang, X. Effects of root exudates on the sorption of polycyclic aromatic hydrocarbons onto biochar. Environ. Pollut. Bioavailab. 2019, 31, 156–165. [Google Scholar] [CrossRef]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Mechanical, Chemical, and Physical Properties of Wood and Perennial Grass Biochars for Possible Composite Application. Bioresources 2016, 11, 1334–1348. [Google Scholar] [CrossRef]
- Šupić, S.; Malešev, M.; Radonjanin, V.; Bulatović, V.; Milović, T. Reactivity and Pozzolanic Properties of Biomass Ashes Generated by Wheat and Soybean Straw Combustion. Materials 2021, 14, 1004. [Google Scholar] [CrossRef] [PubMed]



| % Ash | % C | % H | % N | % S | |
|---|---|---|---|---|---|
| Vine prunings biochar | 32.48 | 67.78 | 0.82 | 0.57 | N.D. |
| Size: | >710 µm | 500–710 µm | 125–500 µm | 63–125 µm | 32–63 µm | <32 µm |
| mass % | 71.44 | 7.11 | 13.22 | 2.4 | 2.4 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirra, A.; Ali, A.B.; Renz, F.; Sindelar, R.; Pedrazzi, S.; Allesina, G. Preliminary Investigation of Possible Biochar Use as Carbon Source in Polyacrylonitrile Electrospun Fiber Production. Appl. Sci. 2022, 12, 4441. https://doi.org/10.3390/app12094441
Schirra A, Ali AB, Renz F, Sindelar R, Pedrazzi S, Allesina G. Preliminary Investigation of Possible Biochar Use as Carbon Source in Polyacrylonitrile Electrospun Fiber Production. Applied Sciences. 2022; 12(9):4441. https://doi.org/10.3390/app12094441
Chicago/Turabian StyleSchirra, Aaron, Annas Bin Ali, Franz Renz, Ralf Sindelar, Simone Pedrazzi, and Giulio Allesina. 2022. "Preliminary Investigation of Possible Biochar Use as Carbon Source in Polyacrylonitrile Electrospun Fiber Production" Applied Sciences 12, no. 9: 4441. https://doi.org/10.3390/app12094441









