Rudomain Iron Ore Treatment by High-Temperature Reduction
Abstract
:1. Introduction
2. Materials and Experimental Works
3. Results and Discussion
3.1. Physico-Chemical and Metallurgical Properties of Iron Ores
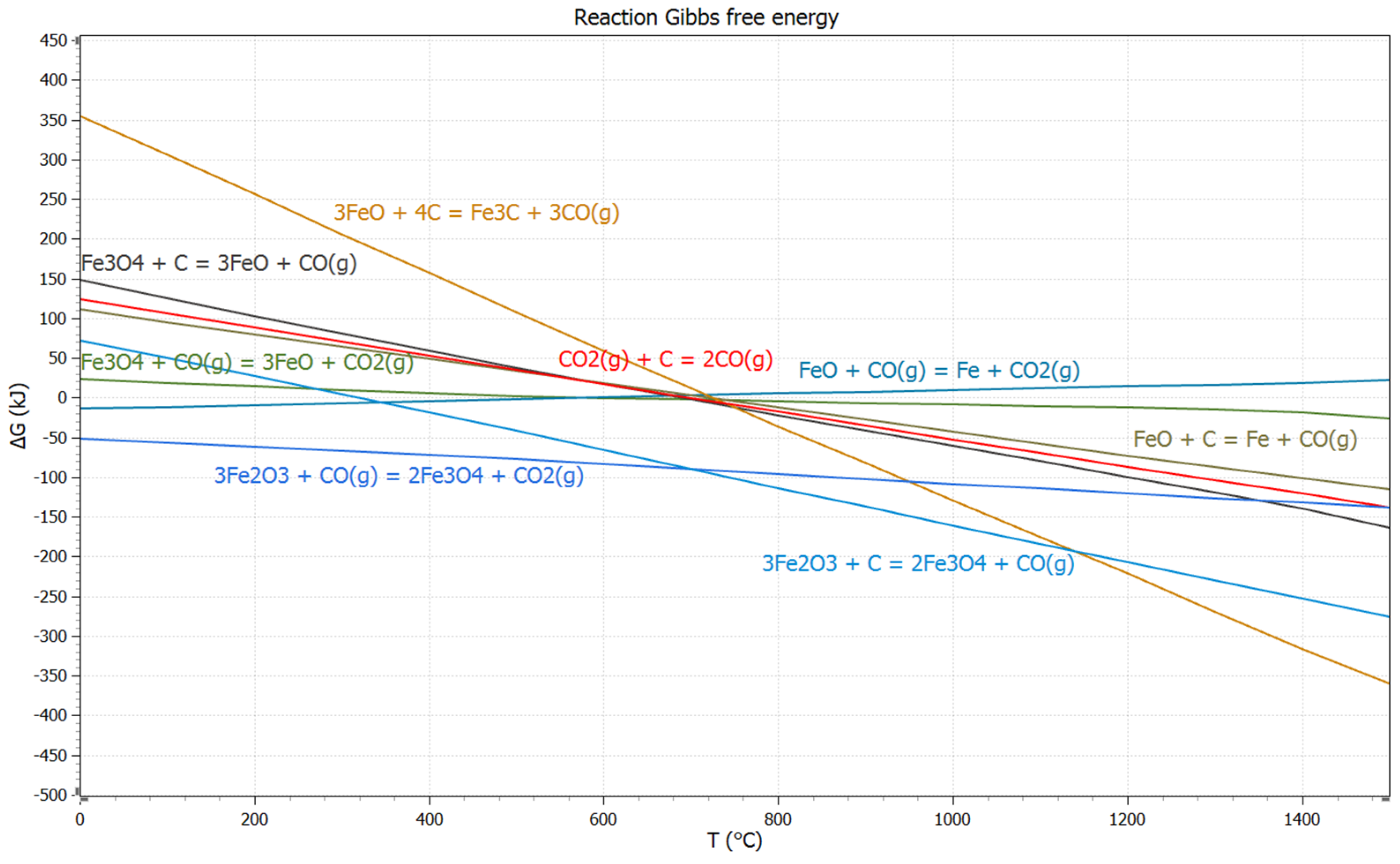
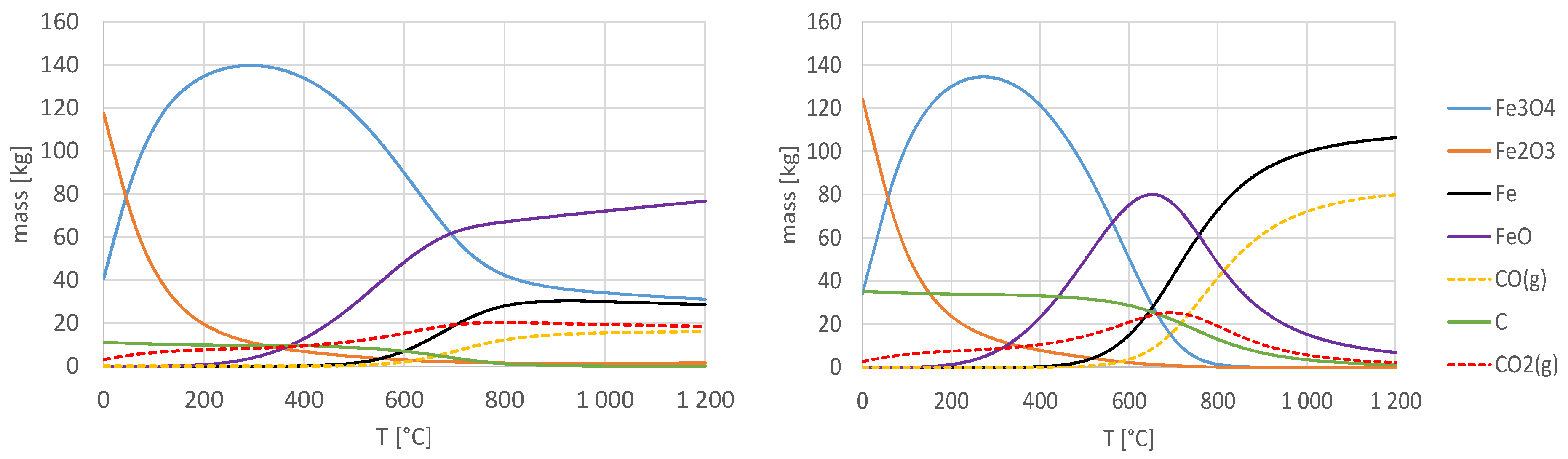
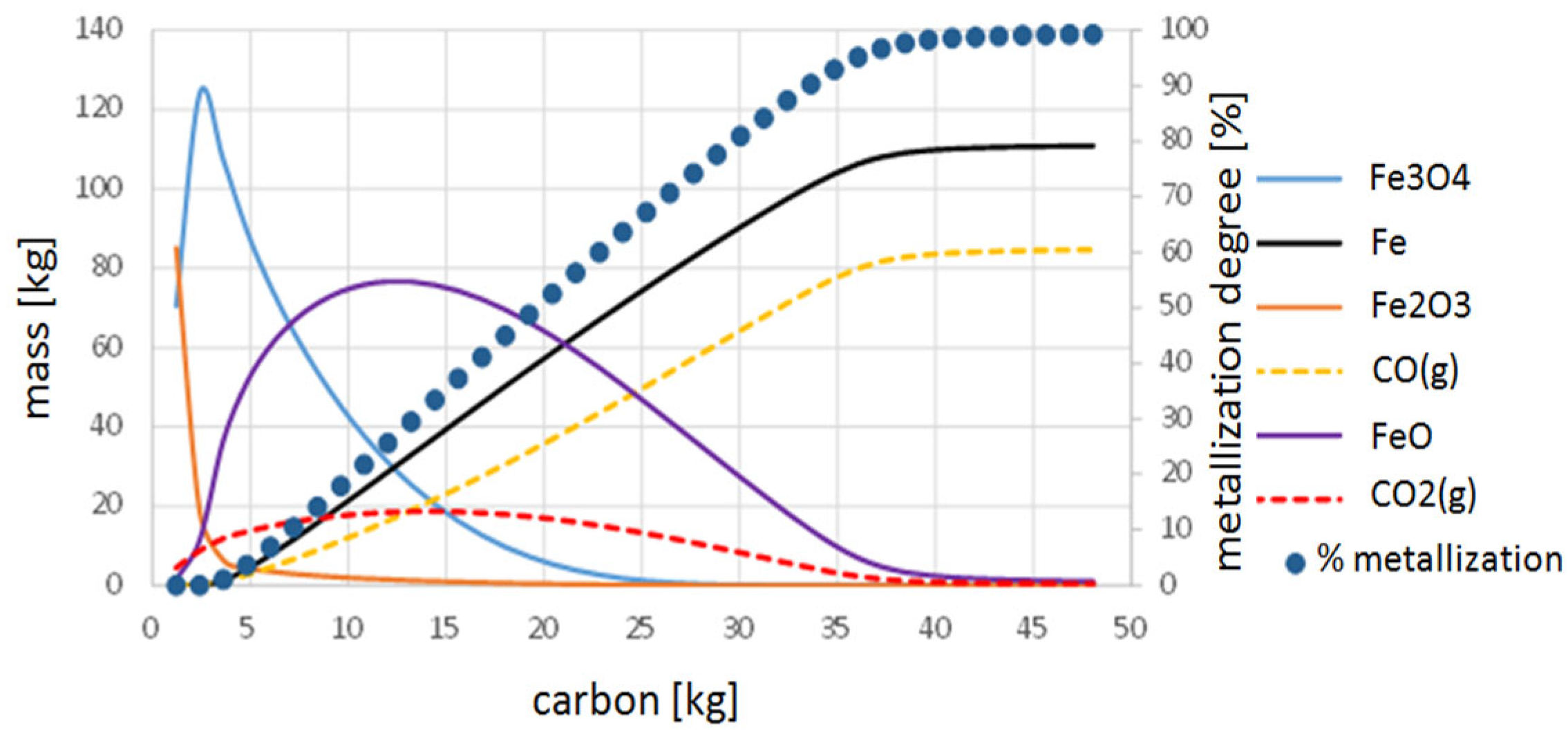
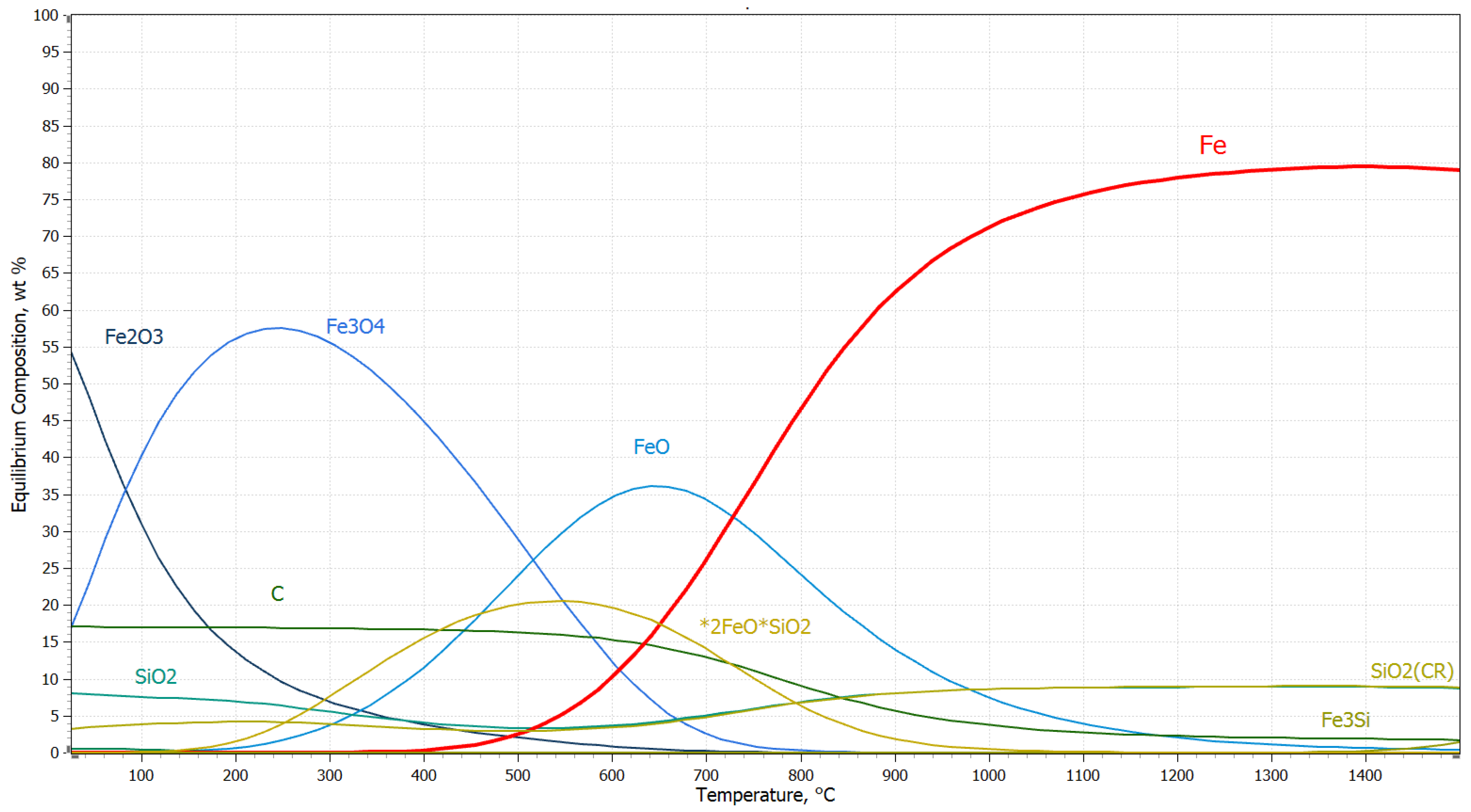
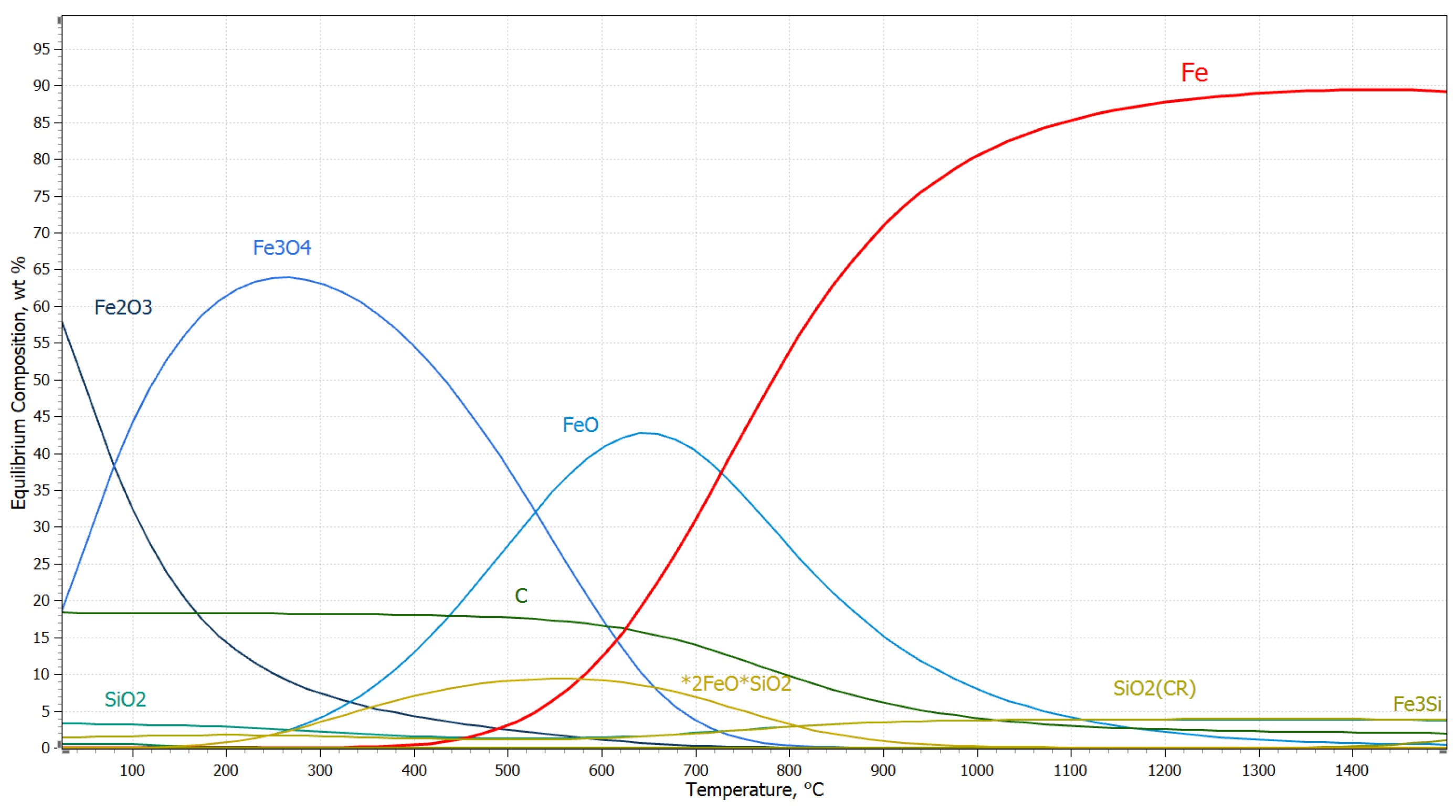
3.2. Thermodynamic Study
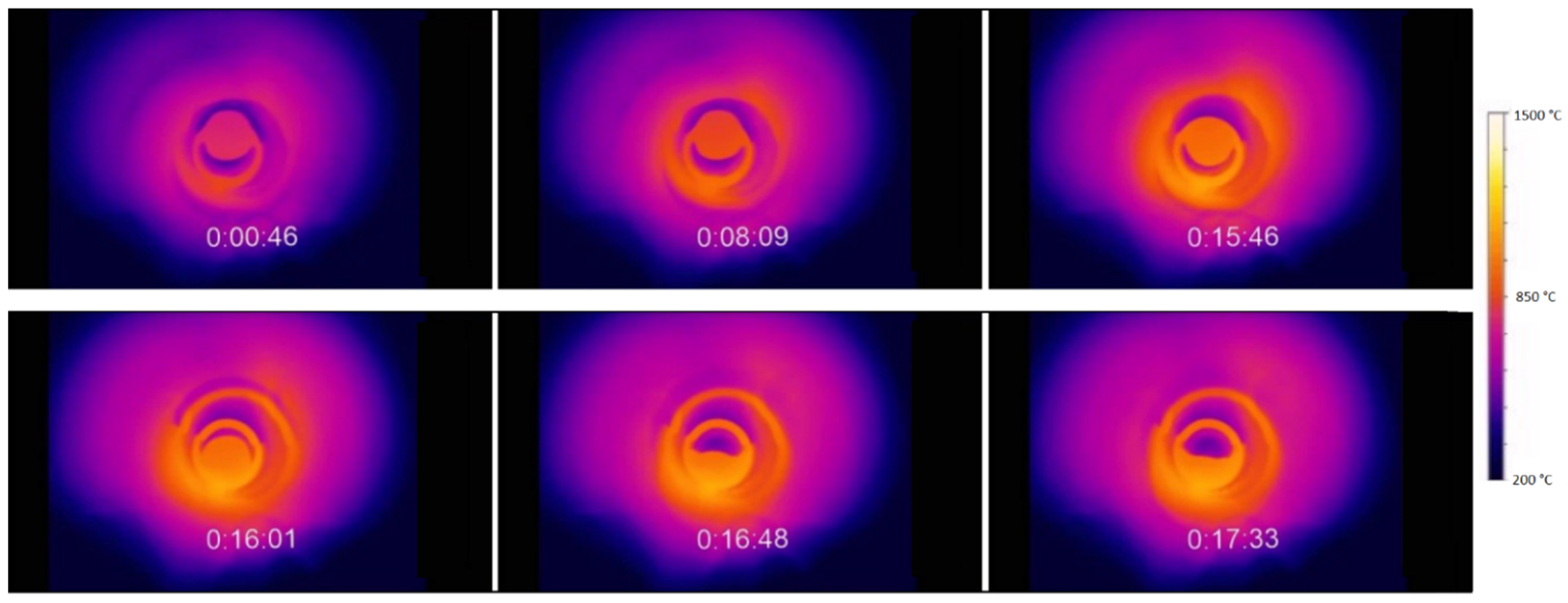
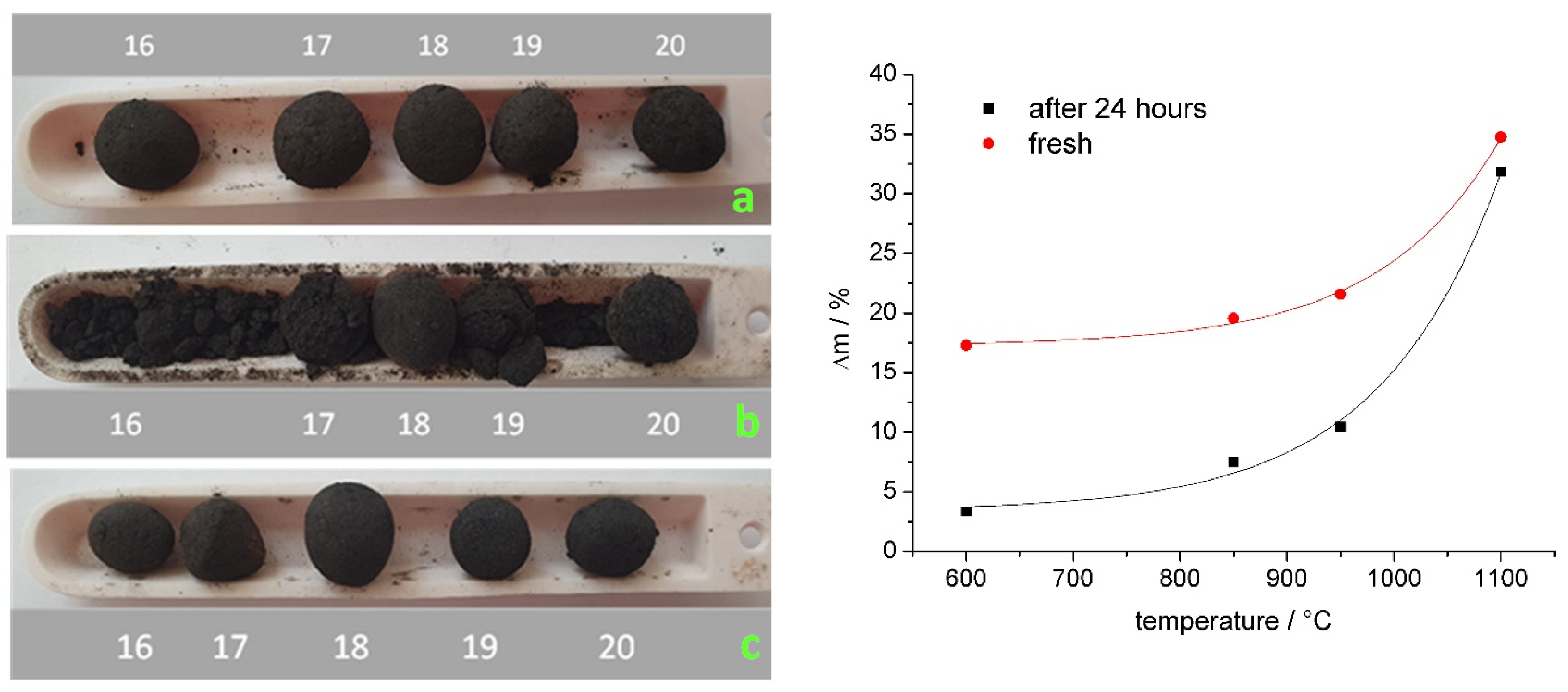
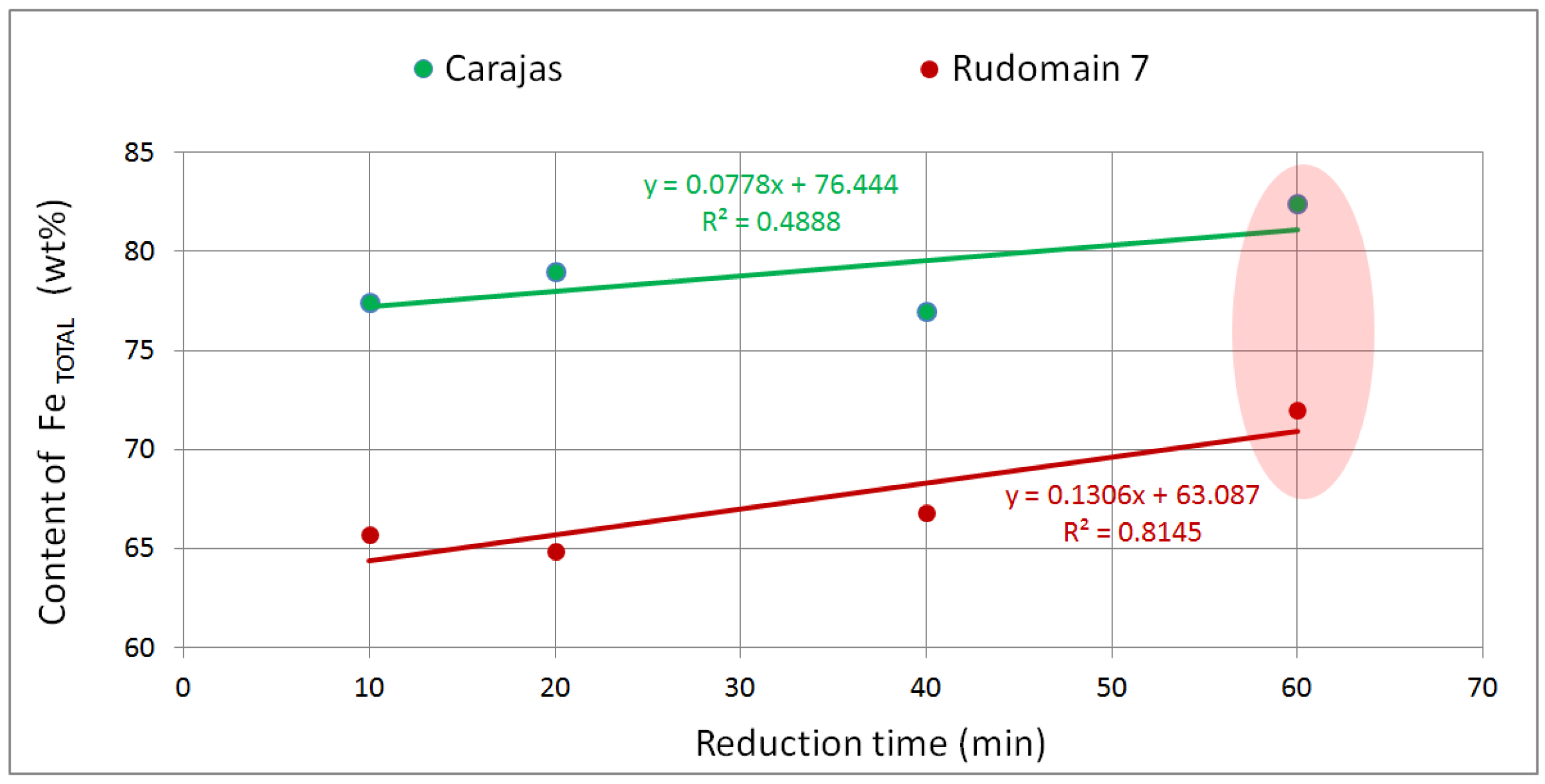
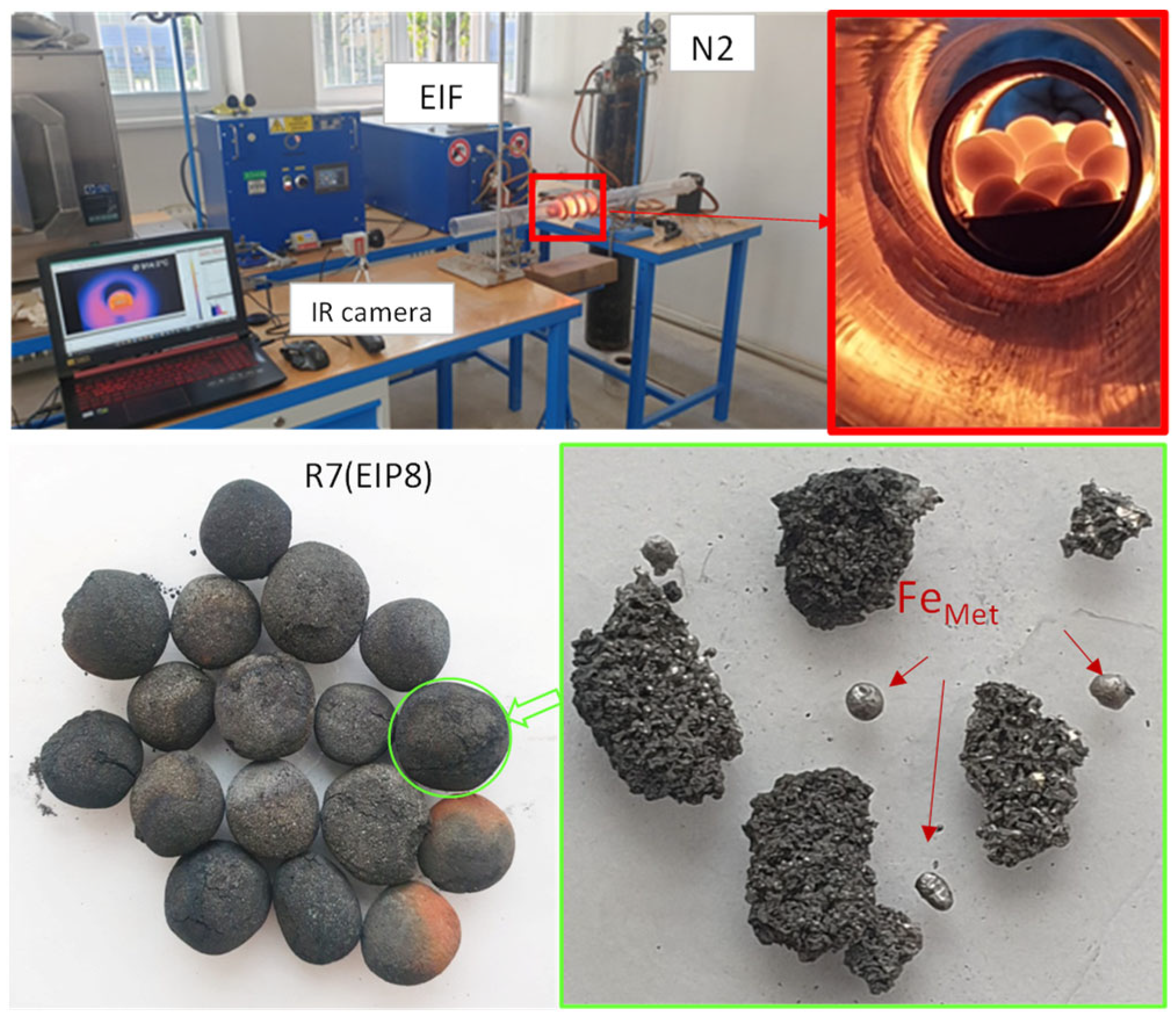
3.3. High-Temperature Experiments
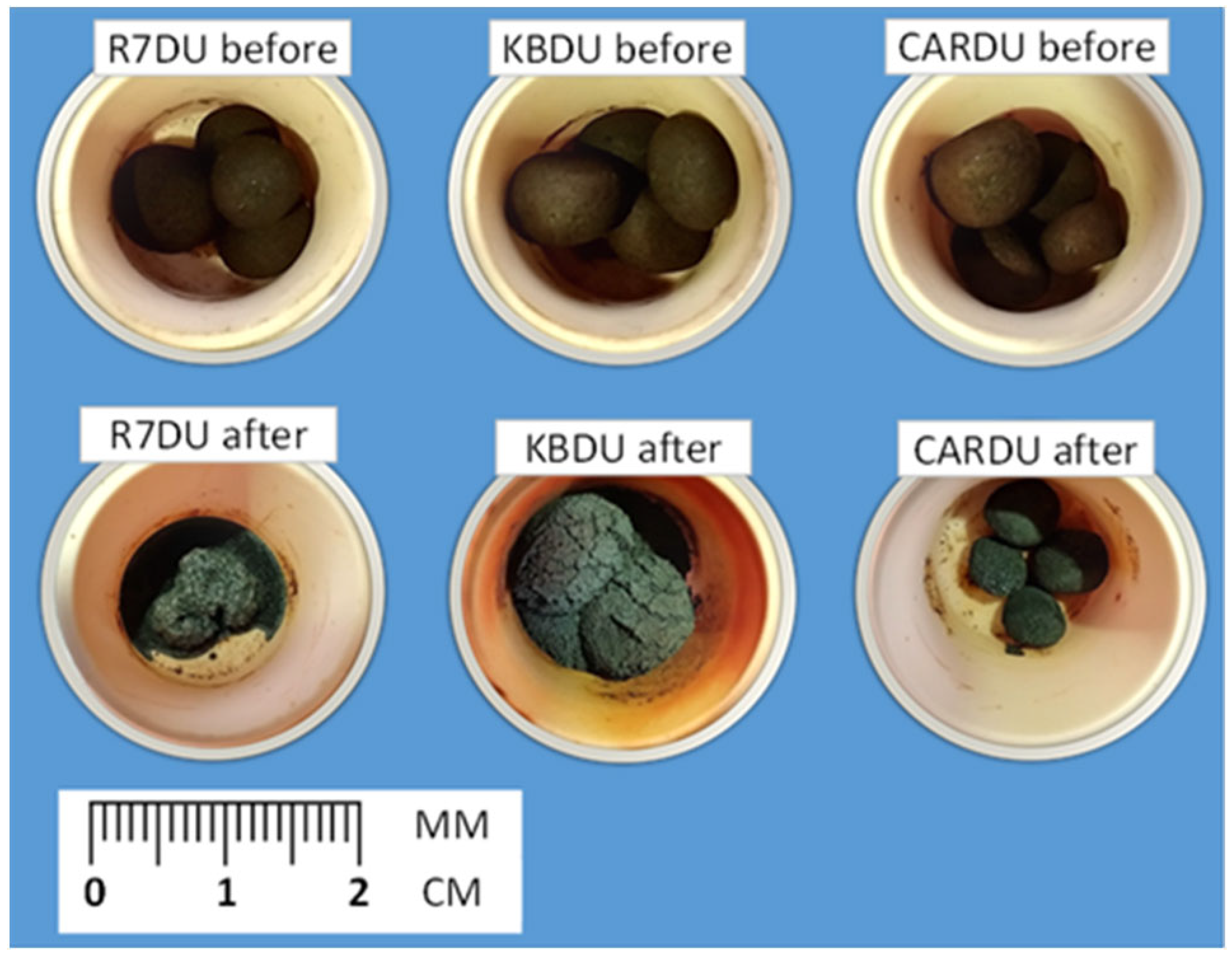
3.4. Material Research of Metallised DRI Pellets
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission. How Are Emissions of Greenhouse Gases by the EU Evolving? Available online: https://ec.europa.eu/eurostat/cache/infographs/energy/bloc-4a.html (accessed on 20 February 2023).
- McKinsey & Company. How Industry Can Move toward a Low-Carbon Future. 2018. Available online: https://www.mckinsey.com/business-functions/sustainability/our-insights/how-industry-can-move-toward-a-low-carbon-future (accessed on 20 February 2023).
- World Steel Association. Energy Use in the Steel Industry. Available online: https://www.worldsteel.org/en/dam/jcr:f07b864c-908e-4229-9f92-669f1c3abf4c/fact_energy_2019.pdf (accessed on 20 February 2023).
- International Energy Agency IEA. Iron and Steel Technology Roadmap. Available online: https://iea.blob.core.windows.net/assets/eb0c8ec1-3665-4959-97d0-187ceca189a8/Iron_and_Steel_Technology_Roadmap.pdf (accessed on 20 February 2023).
- IEA Iron and Steel—November 2021. Available online: https://www.iea.org/reports/iron-and-steel (accessed on 20 February 2023).
- Bailera, M.; Lisbona, P.; Peña, B.; Romeo, L.M. A review on CO2 mitigation in the Iron and Steel industry through Power to X processes. J. CO2 Util. 2021, 46, 101456. [Google Scholar] [CrossRef]
- Vogl, V.; Åhman, M.; Nilsson, L.J. The making of green steel in the EU: A policy evaluation for the early commercialization phase. Clim. Policy 2021, 21, 78–92. [Google Scholar] [CrossRef]
- Greensteel for Europe. Technology Assessment and Roadmapping. Available online: 210308-D1-2-Assessment-and-roadmapping-of-technologies-Publishable-version.pdf (accessed on 19 February 2023).
- Eurofer. A Steel Roadmap for Low Carbon EUROPE 2050. Available online: https://www.eurofer.eu/assets/publications/archive/archive-of-older-eurofer-documents/2013-Roadmap.pdf (accessed on 19 February 2023).
- Hubatka, S.; Branislav, B.; Dana, B.; Peter, D.; Lukáš, F.; Vladimír, Š. Decarbonisation of the Steel Industry–Pathway to Brighter Future–Slovakia and Czech Republic Case Study. In Proceedings of the Anniversary International Conference on Metallurgy and Materials, Brno, Czech Republic, 26–28 May 2021. [Google Scholar] [CrossRef]
- Decarbonization Challenge for Steel. Hydrogen as a Solution in Europe. Available online: https://www.mckinsey.com/~/media/McKinsey/Industries/Metals%20and%20Mining/Our%20Insights/Decarbonization%20challenge%20for%20steel/Decarbonization-challenge-for-steel.pdf (accessed on 19 February 2023).
- Technologies For a Sustainable Steel Industry. Available online: https://www.green-industrial-hydrogen.com/fileadmin/user_upload/Webinar_GrInHy2.0_-_Another_step_towards_hydrogen_based_steelmaking_Tenova.pdf (accessed on 19 February 2023).
- Greensteel for Europe. Collection of Possible Decarbonisation Barriers. Available online: 210308-GreenSteel-D1-5-Decarbonisation-Barriers-Publishable-version.pdf (accessed on 19 February 2023).
- Legemza, J.; Findorák, R.; Fröhlichová, M.; Džupková, M. Advances in Sintering of Iron Ores and Concentrates. In Iron Ores; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Dawson, P. Recent developments in iron ore sintering and sinter quality. Part 2 Research studies on sintering and sinter quality. Ironmak. Steelmak. 1994, 2, 137–143. [Google Scholar]
- Findorák, R. Železorudný Aglomerát a Faktory Vplývajúce na Jeho Kvalitu; Habilitation Work; Technical University of Košice: Košice, Slovakia, 2015. [Google Scholar]
- Jursova, S.; Pustejovska, P.; Brozova, S. Study on reducibility and porosity of metallurgical sinter. Alex. Eng. J. 2018, 57, 1657–1664. [Google Scholar] [CrossRef]
- Zhu, D.; Jiang, Y.; Pan, J.; Yang, C. Study of Mineralogy and Metallurgical Properties of Lump Ores. Metals 2022, 12, 1805. [Google Scholar] [CrossRef]
- Wu, S.; Han, H.; Xu, H.; Wang, H.; Liu, X. Increasing Lump Ores Proportion in Blast Furnace Based on the High-temperature Interactivity of Iron Bearing Materials. ISIJ Int. 2010, 50, 686–694. [Google Scholar] [CrossRef]
- Mousa, E.A.; Babich, A.; Senk, D. Reduction Behavior of Iron Ore Pellets with Simulated Coke Oven Gas and Natural Gas. Steel Res. Int. 2013, 84, 1085–1097. [Google Scholar] [CrossRef]
- Hashem, M.N.; Salah, B.A.; El-Hussiny, N.; Sayed, S.A.; Shalabi, M.E.H. Reduction Kinetics of Egyptian Iron Ore by Non Coking Coal. Int. J. Sci. Eng. Res. 2015, 6, 846–852. [Google Scholar]
- Sohn, H.Y.; Roy, S. Development of a Moving-Bed Ironmaking Process for Direct Gaseous Reduction of Iron Ore Concentrate. Metals 2022, 12, 1889. [Google Scholar] [CrossRef]
- Rane, K.; Date, P.; Srivatsan, T.S. Influence of Material and Process Parameters on Reduction-Swelling Characteristics of Sintered Iron Pellets. Metals 2023, 13, 141. [Google Scholar] [CrossRef]
- Sinha, K.M.K.; Sharma, T.; Haldar, D.D. Reduction of Iron Ore with Non Coking Coal. Int. J. Eng. Adv. Technol. (IJEAT) 2014, 3, 30–33. [Google Scholar]
- Rudomain. Available online: https://rudomain.com.ua/en/ (accessed on 19 February 2023).
- HSC Chemistry 9, Version 9. Thermodynamic Software for Process Simulation. Outokumpu Research Oy: Pori, Finland, 2018.
- STN ISO 540: 2010 (44 1363); Stanovenie Taviteľnosti Materiálov [Determination of the Fusibility of Materials]. Slovak Institute of Technical Standardization: Bratislava, Slovakia, 2010.





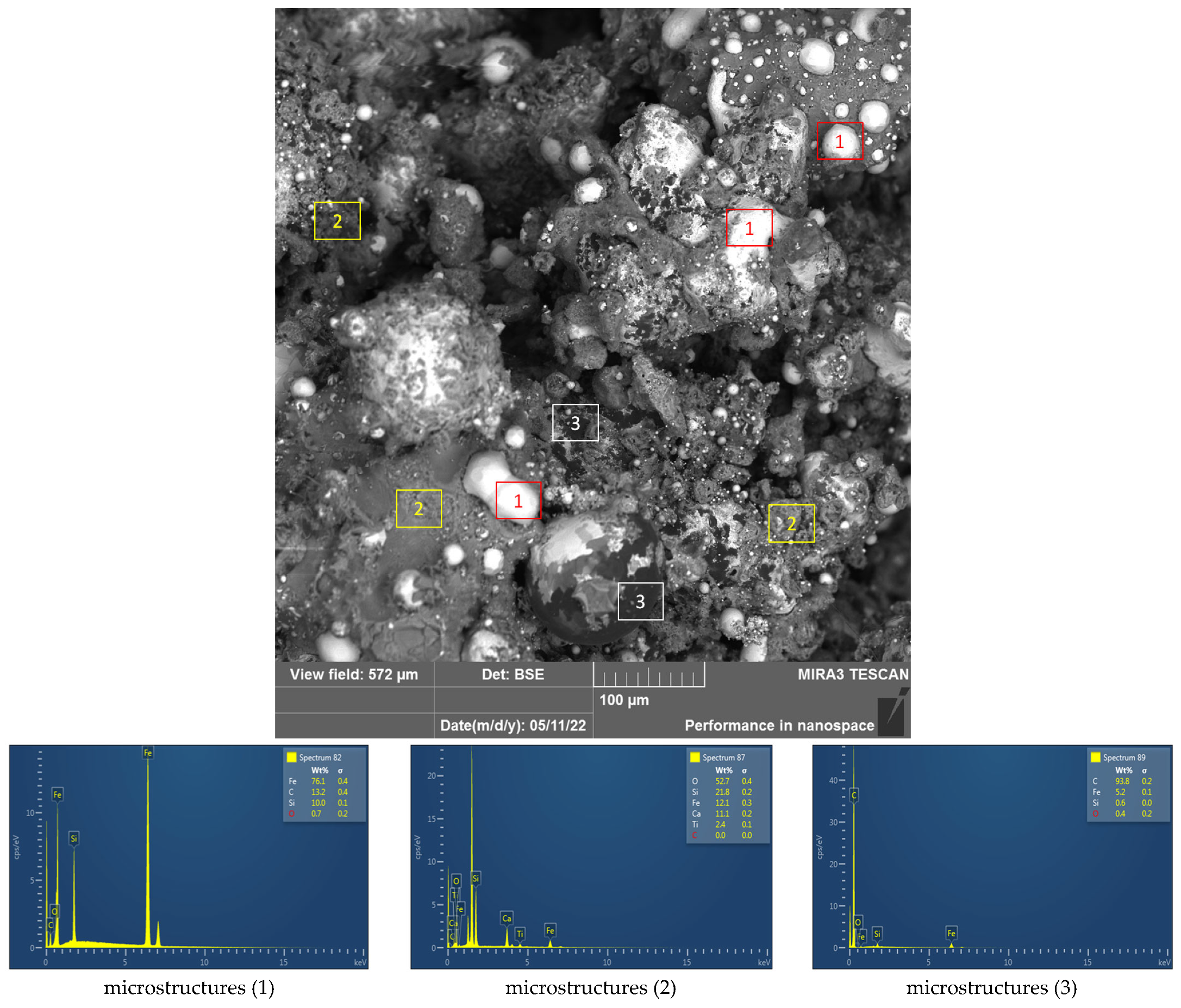
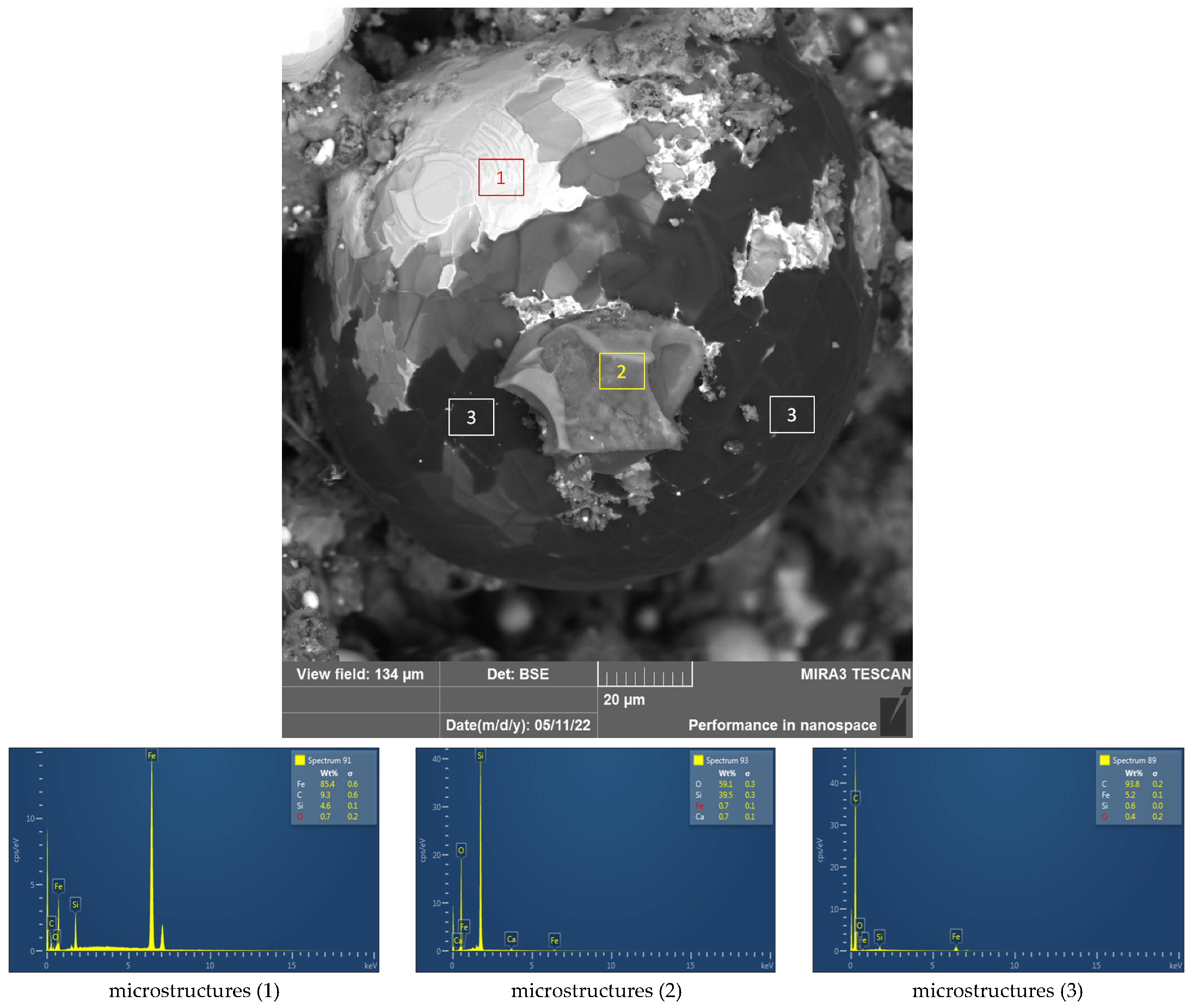

| Sample | Fe Total (%) | SiO2 (%) | Bulk Density (kg/m3) | True Density (kg/m3) |
|---|---|---|---|---|
| Rudomain 1 (R1) | 52.46 | 20.02 | 2435.60 | 3991.80 |
| Rudomain 7 (R7) | 58.20 | 13.40 | 2609.60 | 4412.66 |
| Rudomain 8 (R8) | 61.71 | 8.28 | 2678.80 | 4580.56 |
| Krivbas (KB) | 62.25 | 7.80 | 2810.20 | 4460.81 |
| Carajas (C) | 65.23 | 2.21 | 2606.40 | 4707.56 |
| H2O (%) | Ash (%) | Volatile Matter (%) | Carbon (%) | Sulphur (%) | Calorific Value (MJ/kg) | |||
|---|---|---|---|---|---|---|---|---|
| Charcoal properties | 4.90 | 3.50 | 8.20 | 88.30 | 0.05 | 30.46 | ||
| Ash composition (%) | ||||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | P2O5 | Others * | |
| 6.30 | 0.85 | 1.47 | 37.00 | 12.50 | 11.42 | 1.65 | 28.81 | |
| Equipment | Electrical resistance furnace 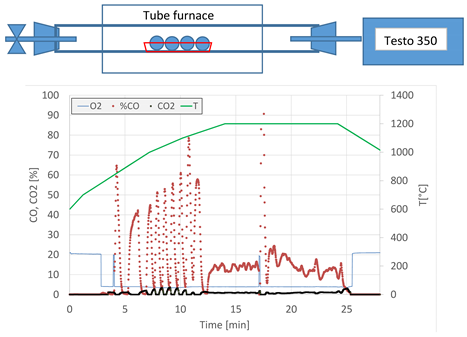 |
| Monitoring of exhaust gases and temperatures | |
| Measurements of temperature and exhaust gases | PtRh10%-Pt thermocouple TESTO 350 analyser |
| Boat material | Corundum |
| Atmosphere | Still air atmosphere or inert nitrogen atmosphere |
| Used temperatures | 1100–1200 °C |
| Holding time | 10–300 min |
| Sample | Raw iron ore pellets with an internal charcoal-based reducing agent (ore:charcoal = 4:1) |
| Equipment | Electric induction furnace with a retort |
| Retort material | A steel cylinder inside a retort made of silica glass |
| Atmosphere | Inert nitrogen atmosphere with a flow rate of 0.5–3 L/min. |
| Used temperatures (holding) | 1100–1200 °C |
| Monitored temperature | Contactless measurements using a thermal-imaging from MicroEpsylon camera (MICRO-EPSILON MESSTECHNIK GmbH & Co., KG, Ortenburg, Germany) |
| Holding time | 20–150 min |
| Sample | Raw iron ore pellets with an internal charcoal-based reducing agent (ore:charcoal = 4:1) |
| Iron Ore |  |  |  | |
|---|---|---|---|---|
| Krivbas (Ukraine) | Rudomain (Ukraine) | Carajas (Brazil) | ||
| Chemical composition (wt%) | FeTOTAL | 62.25 | 58.20 | 65.23 |
| MnTOTAL | 0.07 | 0.04 | 0.57 | |
| SiO2 | 7.80 | 13.40 | 2.21 | |
| Al2O3 | 1.33 | 1.08 | 1.05 | |
| CaO | 0.28 | 0.13 | 0.10 | |
| MgO | 0.60 | 0.24 | 0.10 | |
| P | 0.03 | 0.06 | 0.05 | |
| S | 0.03 | 0.01 | 0.01 | |
| Na2O | 0.180 | 0.150 | 0.005 | |
| K2O | 0.040 | 0.028 | 0.006 | |
| Others * | 27.39 | 26.66 | 30.67 | |
| Granulometric composition (%) | <0.1 mm | 1.55 | 6.05 | 0.35 |
| <0.5 mm | 23.64 | 41.75 | 12.85 | |
| <1 mm | 35.13 | 57.48 | 28.54 | |
| <5 mm | 62.18 | 86.84 | 55.03 | |
| <10 mm | 81.36 | 97.04 | 78.35 | |
| Moisture (%) | 2.35 | 1.75 | 1.55 | |
| Bulk specific gravity (kg.m−3) | ρA | 2820 | 2600 | 2680 |
| True specific gravity (kg.m−3) | ρ | 4460 | 4420 | 4710 |
| Melting point (°C) | 1472 | 1453 | 1540 | |
| Mineralogical composition | XRD | Hematite, hydrohematite, silica | Hematite, hydrohematite, hydrated iron silicate, magnetite cronstedtite, silica | Hematite, silica |
| Sample | Reduction Degree Rd (%) | Chemical Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FeTOT | SiO2 | Al2O3 | Na2O | C | S | P | Others * | ||
| R7 | - | 58.20 | 13.40 | 1.08 | 0.15 | - | 0.01 | 0.06 | 27.1 |
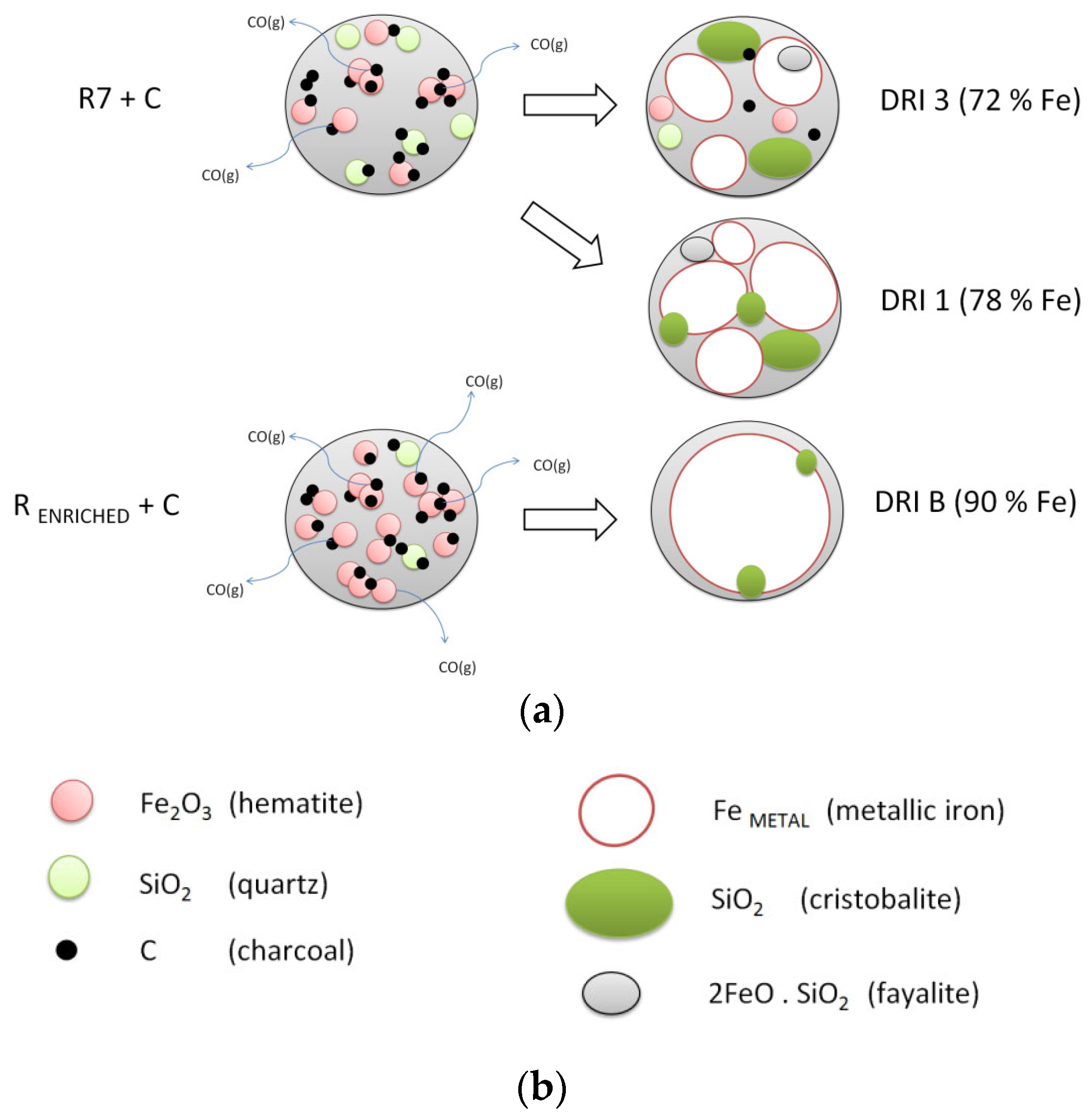
| DRI 1 | 95.60 | 78.08 | 19.05 | 1.70 | 0.02 | 0.68 | 0.02 | 0.02 | 0.43 |
| DRI 3 | 79.30 | 72.04 | 17.21 | 2.31 | - | - | 0.05 | 0.03 | 8.36 |
| Mineralogical Name | Phase Designation | Chemical Formula |
|---|---|---|
| Silicon oxide | Silica | SiO2 |
| Iron hydrogen oxide | Hydrohematite | Fe1.67H0.99O3 |
| Iron oxide | Hematite | Fe2O3 |
| Iron silicate hydroxide | Hydrated iron silicate | Fe3Si2O5(OH)4 |
| Silicon oxide | Silica | SiO2 |
| Iron silicate hydroxide | Cronstedtite | Fe3(Si,Fe)2O5(OH)4 |
| Iron oxide | Magnetite | Fe2.936O4 |
| Mineralogical Name | Phase Designation | Chemical Formula |
|---|---|---|
| Aluminium iron silicon | Al-ferrosilicon | Al0.7Fe3Si0.3 |
| Graphite | Graphite | C |
| Iron silicon | Ferrosilicon | Fe0.905Si0.095 |
| Iron | Iron | Fe |
| Carbon iron | Iron carbide | C0.055Fe1.945 |
| Silicon oxide | Silica | SiO2 |
| Silicon oxide | Cristobalite | SiO2 |
| DRI 1 | DRI 3 | Rudomain R10 (R7) | |
|---|---|---|---|
| Deformation temperature DT (°C) | 1455 | 1450 | 1438 |
| Shrinkage temperature ST [°C] | 1510 | 1492 | 1449 |
| Hemisphere temperature HT (°C) | NA | 1512 | 1453 |
| Flow temperature FT (°C) | NA | 1471 | 1455 |
| Melting interval (°C) | 1510–NA | 1492–1513 | 1449–1453 |
| Flow interval (°C) | NA | NA | 1453–1455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legemza, J.; Findorák, R.; Baricová, D.; Buľko, B.; Demeter, P.; Hubatka, S.; Karamanits, K. Rudomain Iron Ore Treatment by High-Temperature Reduction. Appl. Sci. 2023, 13, 10698. https://doi.org/10.3390/app131910698
Legemza J, Findorák R, Baricová D, Buľko B, Demeter P, Hubatka S, Karamanits K. Rudomain Iron Ore Treatment by High-Temperature Reduction. Applied Sciences. 2023; 13(19):10698. https://doi.org/10.3390/app131910698
Chicago/Turabian StyleLegemza, Jaroslav, Róbert Findorák, Dana Baricová, Branislav Buľko, Peter Demeter, Slavomír Hubatka, and Kostyantyn Karamanits. 2023. "Rudomain Iron Ore Treatment by High-Temperature Reduction" Applied Sciences 13, no. 19: 10698. https://doi.org/10.3390/app131910698








