Char from Pyrolysis of Waste Tires to Increase Bitumen Performances
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Char Characterization
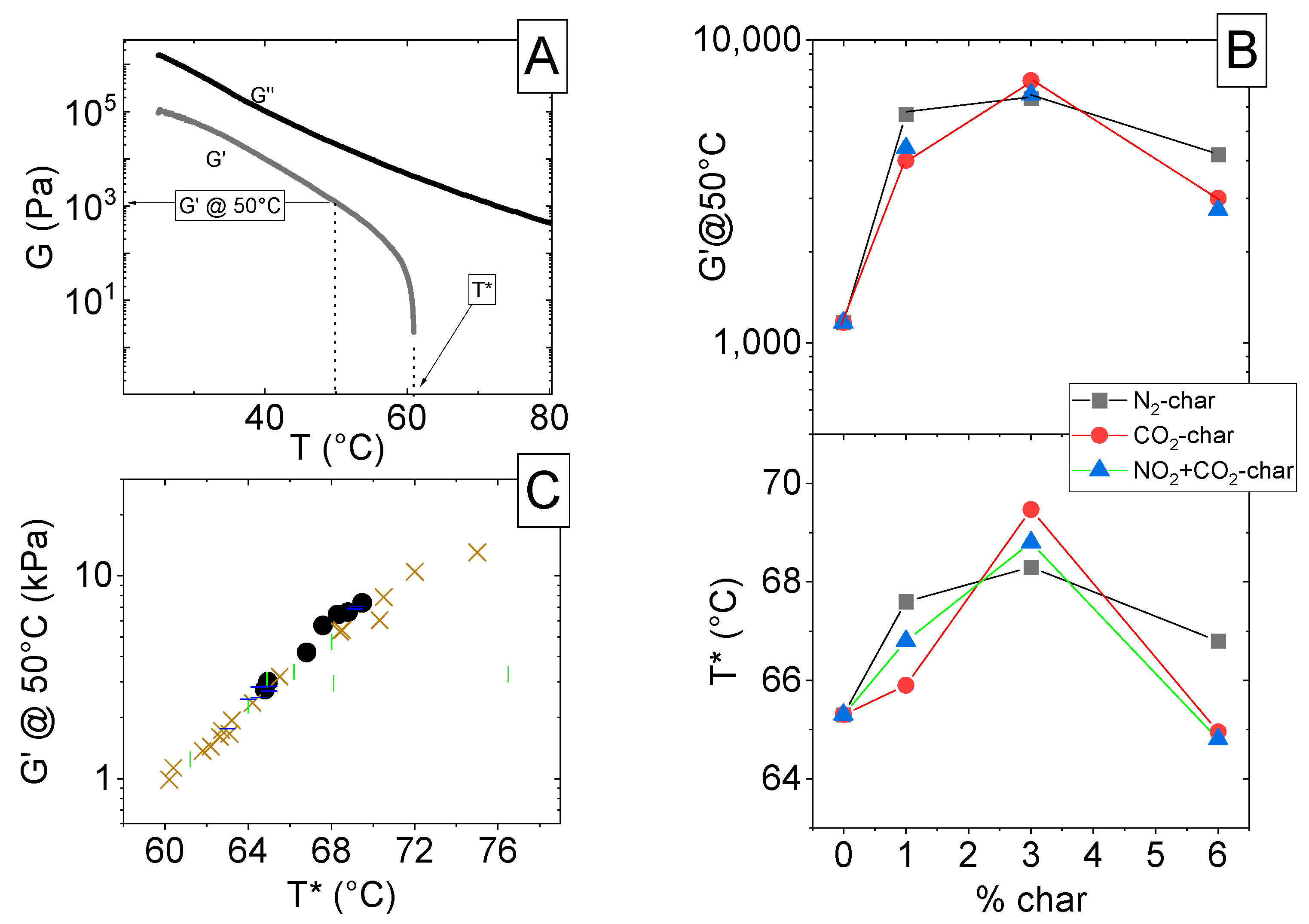
3.2. Bitumen Characterization
- -
- 58 for bitumen modified with N2-char;
- -
- 57 for bitumen modified with CO2-char;
- -
- 58 for bitumen modified with N2+CO2-char.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frederika, P.P.; Donatella, D.; Clara, S.; Sossio, C.; Aneta, V. Viscoelastic properties and morphological characteristics of polymer-modified bitumen blends. J. Appl. Polym. Sci. 2010, 118, 1320–1330. [Google Scholar]
- Becker, Y.; Méndez, M.P.; Rodríguez, Y. Polymer modified asphalt. Vis. Tecnol. 2001, 9, 39–50. [Google Scholar]
- Mashaan, N.S.; Ali, A.H.; Karim, M.R.; Abdelaziz, M. Effect of crumb rubber concentration on the physical and rheological properties of rubberised bitumen binders. Int. J. Phys. Sci. 2011, 6, 684–690. [Google Scholar]
- Moreno-Navarro, F.; Rubio-Gámez, M.C.; Jiménez Del Barco-Carrión, A. Tire crumb rubber effect on hot bituminous mixtures fatigue-cracking behaviour. J. Civ. Eng. Manag. 2016, 22, 65–72. [Google Scholar] [CrossRef]
- Sipra, A.T.; Gao, N.; Sarwar, H. Municipal solid waste (MSW) pyrolysis for bio-fuel production A review ofeffects of MSW components and catalysts. Fuel Process. Technol. 2018, 175, 131–147. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Guillermo, S.M.; Madrid, S.J. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. Anal. Appl. Pyrolysis 2007, 79, 415–423. [Google Scholar] [CrossRef]
- Kadlimatti, H.M.; Mohan, B.R.; Saidutta, M.B. Bio-oil from microwave assisted pyrolysis of food waste-optimization using response surface methodology. Biomass Bioenergy 2019, 123, 25–33. [Google Scholar] [CrossRef]
- Romeo, I.; Olivito, F.; Tursi, A.; Algieri, V.; Beneduci, A.; Chidichimo, G.; Maiuolo, L.; Sicilia, E.; De Nino, A. Totally green cellulose conversion into bio-oil and cellulose citrate using molten citric acid in an open system: Synthesis, characterization and computational investigation of reaction mechanisms. RSC Adv. 2020, 10, 34738–34751. [Google Scholar] [CrossRef]
- Maiuolo, L.; Olivito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Tursi, A.; Sposato, C.; Feo, A.; De Nino, A. Synthesis, Characterization and Mechanical Properties of Novel Bio-Based Polyurethane Foams Using Cellulose-Derived Polyol for Chain Extension and Cellulose Citrate as a Thickener Additive. Polymers 2021, 13, 2802. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Hossain, A.K.; Davies, P.A. Pyrolysis liquids and gases as alternative fuels in internal combustion engines—A review. Renew Sustain. Energy Rev. 2010, 21, 165–189. [Google Scholar] [CrossRef]
- Resentia, N.K.; Subagio, B.S.; Hariyadi, E.S.; Pradoto, R.G.K. Rheological Characteristic of Petroleum Asphalt Binder Modified by Natural Nano Silica and Nano Fly ash. Int. J. Pavement Res. Technol. 2023. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, B.; Ye, X.P.; Shu, X.; Jia, X. Utilizing bio-char as a bio-modifier for asphalt cement: A sustainable application of bio-fuel by-product. Fuel 2014, 133, 52–62. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Guarieiro, L.L.N.; Rezende, M.J.C.; Barbosa, W.T.; da Rocha, G.O.; Pereira, P.A.P.; Fernandes, D.R.; Lopes, W.A.; Mota, C.J.A.; de Andrade, J.B. Reaching Circular Economy through Circular Chemistry: The Basis for Sustainable Development. J. Braz. Chem. Soc. 2022, 33, 1353–1374. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- European Commission. “Manifesto for a Resource Efficient Europe”. Available online: https://ec.europa.eu/commission/presscorner/detail/en/MEMO_12_989 (accessed on 21 January 2013).
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015. [Google Scholar]
- Loise, V.; Calandra, P.; Abe, A.A.; Porto, M.; Rossi, C.O.; Davoli, M.; Caputo, P. Additives on aged bitumens: What probe to distinguish between rejuvenating and fluxing effects? J. Mol. Liq. 2021, 339, 116742. [Google Scholar] [CrossRef]
- Petrauskas, D.; Saleem, U.; Read, J.; Whiteoak, D. The Shell Bitumen Handbook; Telford, T., Ed.; ICE Publishing: London, UK, 2003; p. 29. [Google Scholar] [CrossRef]
- Nazary, M.; Kofteci, S. Effect of filler types and asphalt penetration grade on properties of asphalt mastic. Pet. Sci. Technol. 2018, 36, 1503–1509. [Google Scholar] [CrossRef]
- Kaliszewski, M.; Zgrzebnicki, M.; Kałamaga, A.; Pinjara, S.; Wróbel, R.J. Commercial Kevlar derived activated carbons for CO2 and C2H4 sorption. Pol. J. Chem. Technol. 2021, 23, 81–87. [Google Scholar] [CrossRef]
- Shaffie, E.; Arshad, A.K.; Alisibramulisi, A.; Ahmad, J.; Hashim, W.; Abd Rahman, Z.; Jaya, R.P. Effect of mixing variables on physical properties of modified bitumen using natural rubber latex. Int. J. Civ. Eng. Technol. 2018, 9, 1812–1821. [Google Scholar]
- Frolov, I.N.; Okhotnikova, E.S.; Ziganshin, M.A.; Firsin, A.A. Cold crystallization event on DSC heating curves of bitumen. J. Therm. Anal. Calorim. 2022, 147, 5269–5278. [Google Scholar] [CrossRef]
- Policicchio, A.; Florent, M.; Attia, M.F.; Whitehead, D.C.; Jagiello, J.; Bandosz, T.J. Effect of the incorporation of functionalized cellulose nanocrystals into UiO-66 on composite porosity and surface heterogeneity alterations. Adv. Mater. Interfaces 2020, 7, 1902098. [Google Scholar] [CrossRef]
- Hunter, R.; Self, A.; Read, J. The Shell Bitumen Handbook, 6th ed.; ICE Publishing: London, UK, 2015. [Google Scholar]
- Remišová, E.; Zatkaliková, V.; Schlosser, F. Study of Rheological Properties of Bituminous Binders in Middle and High Temperatures. Civ. Environ. Eng. 2018, 12, 13–20. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, S.; Xu, S.; Dong, N.; Yu, H. Rheological Behavior of Warm Mix Asphalt Modified with Foaming Process and Surfactant Additive. Crystals 2021, 11, 410. [Google Scholar] [CrossRef]
- Barnes, H.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989; ISBN 0-444-87469-0. [Google Scholar]
- Lushinga, N.; Cao, L.; Dong, Z. Effect of Silicone Oil on Dispersion and Low-Temperature Fracture Performance of Crumb Rubber Asphalt. Adv. Mater. Sci. Eng. 2019, 2019, 8602562. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Haul, D.H.E.L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 1217–1230. [Google Scholar]
- Padula, L.; da Silveira Balestrin, L.B.; de Oliveira Rocha, N.; de Carvalho, C.H.M.; Westfahl, H., Jr.; Cardoso, M.B.; Sabadini, E.; Loh, W. Role of Asphaltenes and Additives on the Viscosity and Microscopic Structure of Heavy Crude Oils. Energy Fuels 2016, 30, 3644–3651. [Google Scholar] [CrossRef]
- Caputo, P.; Ventruti, G.; Calandra, P.; Porto, M.; Teltayev, B.; Angelico, R.; Rossi, C.O. Searching effective indicators of microstructural changes in bitumens during aging: A multi-technique approach. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128529. [Google Scholar] [CrossRef]
- Xing, X.; Pei, J.; Li, R.; Tan, X. Effect and mechanism of calcium carbonate whisker on asphalt binder. Mater. Res. Express 2019, 6, 055306. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; De Filpo, G.; Rossi, C.O.; Calandra, P. Polysaccharides-Reinforced Bitumens: Specificities and Universality of Rheological Behavior. Appl. Sci. 2019, 9, 5564. [Google Scholar] [CrossRef]
- Caputo, P.; Algieri, V.; Maiuolo, L.; De Nino, A.; Sicilia, E.; Ponte, F.; Calandra, P.; Rossi, C.O. Waste additives as biopolymers for the modification of bitumen: Mechanical performance and structural analysis characterization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131079. [Google Scholar] [CrossRef]
- Calandra, P.; Quaranta, S.; Figueira, B.A.M.; Caputo, P.; Porto, M.; Rossi, C.O. Mining wastes to improve bitumen performances: An example of circular economy. J. Colloid Interface Sci. 2022, 614, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, E.; Bocci, E. A Review on Bitumen Aging and Rejuvenation Chemistry: Processes, Materials and Analyses. Sustainability 2021, 13, 6523. [Google Scholar] [CrossRef]
- Tauste, R.; Moreno-Navarro, F.; Sol-Sánchez, M.; Rubio-Gámez, M.C. Understanding the bitumen ageing phenomenon: A review. Constr. Build. Mater. 2018, 192, 593–609. [Google Scholar] [CrossRef]
- Reza, M.S.; Yun, C.S.; Afroze, S.; Radenahmad, N.; Bakar, M.S.A.; Saidur, R.; Taweekun, J.; Azad, A.K. Preparation of activated carbon from biomass and its’ applications in water and gas purification, a review. Arab J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Chandra, S.; Jagdale, P.; Medha, I.; Tiwari, A.K.; Bartoli, M.; De Nino, A.; Olivito, F. Biochar-supported TiO2-based nanocomposites for the photocatalytic degradation of sulfamethoxazole in water—A review. Toxics 2021, 9, 313. [Google Scholar] [CrossRef]
- Rajib, A.; Saadeh, S.; Katawal, P.; Mobasher, B.; Fini, E.H. Enhancing Biomass Value Chain by Utilizing Biochar as a Free Radical Scavenger to Delay Ultraviolet Aging of Bituminous Composites Used in Outdoor Construction. Resour. Conserv. Recycl. 2021, 168, 105302. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, R.; Kumar, A. Characterization of thermal storage stability of waste plastic pyrolytic char modified asphalt binders with sulfur. PLoS ONE 2021, 16, e0248465. [Google Scholar] [CrossRef]
- Somé, C.; Pavoine, A.; Chailleux, E.; Andrieux, L.; DeMArco, L.; Philippe, D.-S.; Stephan, B. Rheological behavior of vegetable oil-modified asphaltite binders and mixes. In Proceedings of the 6th Eurasphalt & Eurobitume Congress, Prague, Czech Republic, 1–3 June 2016. [Google Scholar]
- Kennedy, T.W.; Huber, G.; Harrigan, E.T.; Cominsky, R.J.; Hughes, C.S.; Von Quintus, H.L.; Moulthrop, J.S. Superior Performing Asphalt Pavements (Superpave): The Product of The Shrp Asphalt Research Program; National Research Council: Ottawa, ON, Canada, 1994. [Google Scholar]
- Gargiulo, V.; Alfè, M.; Ruoppolo, G.; Cammarota, F.; Oliviero Rossi, C.; Loise, V.; Porto, M.; Calandra, P.; Pochylski, M.; Gapinski, J.; et al. How char from waste pyrolysis can improve bitumen characteristics and induce anti-aging effects. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132199. [Google Scholar] [CrossRef]
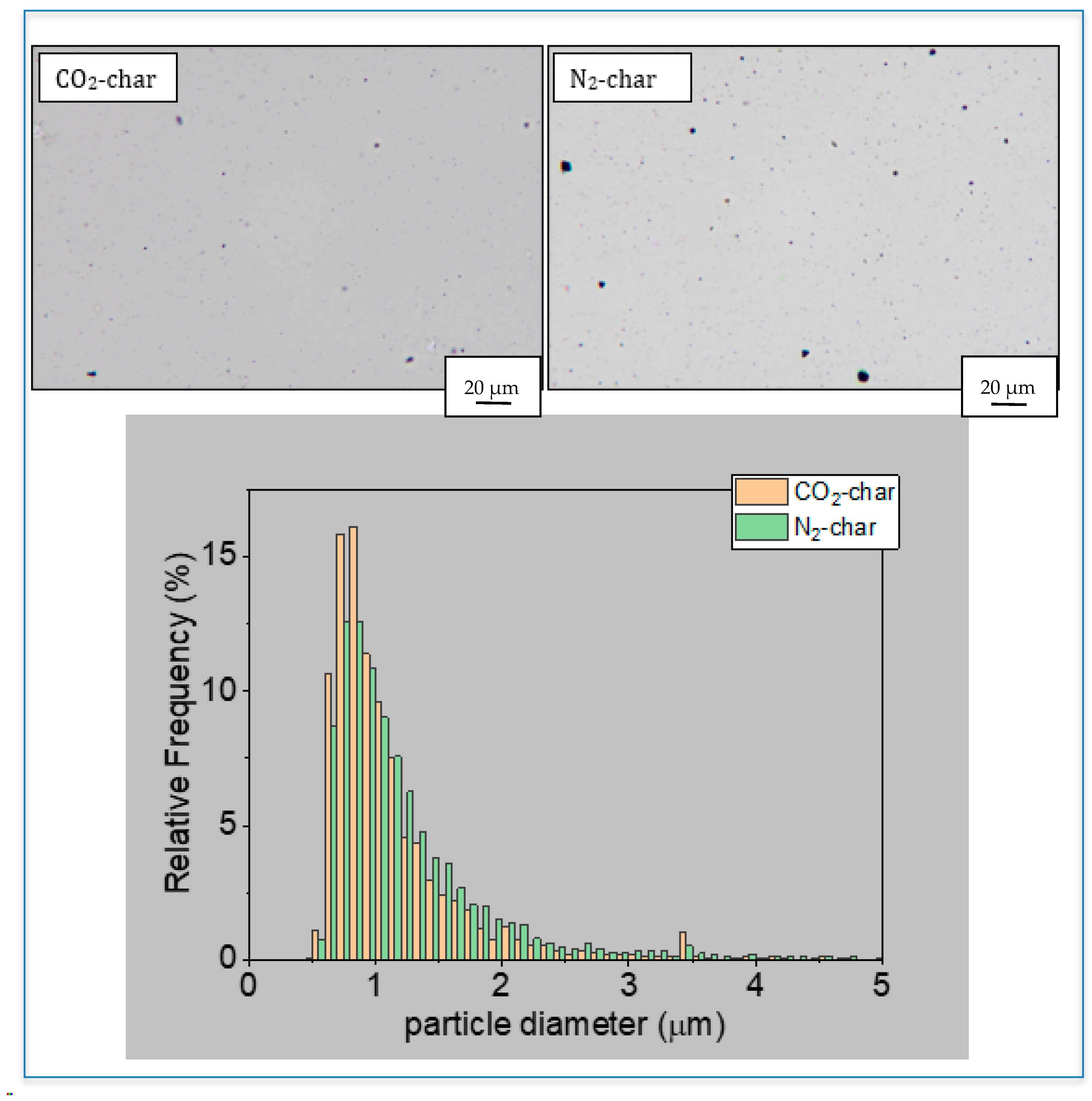
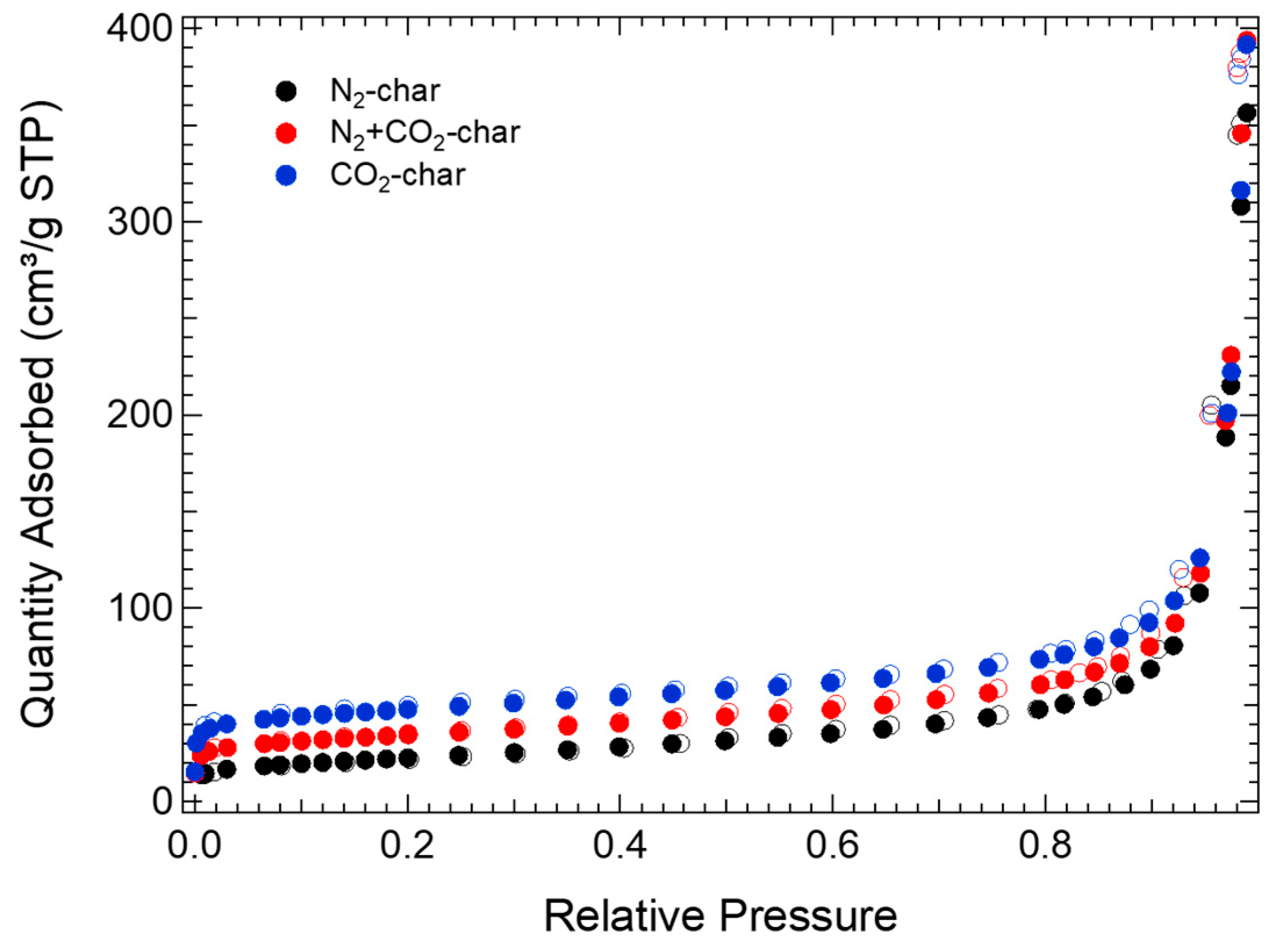
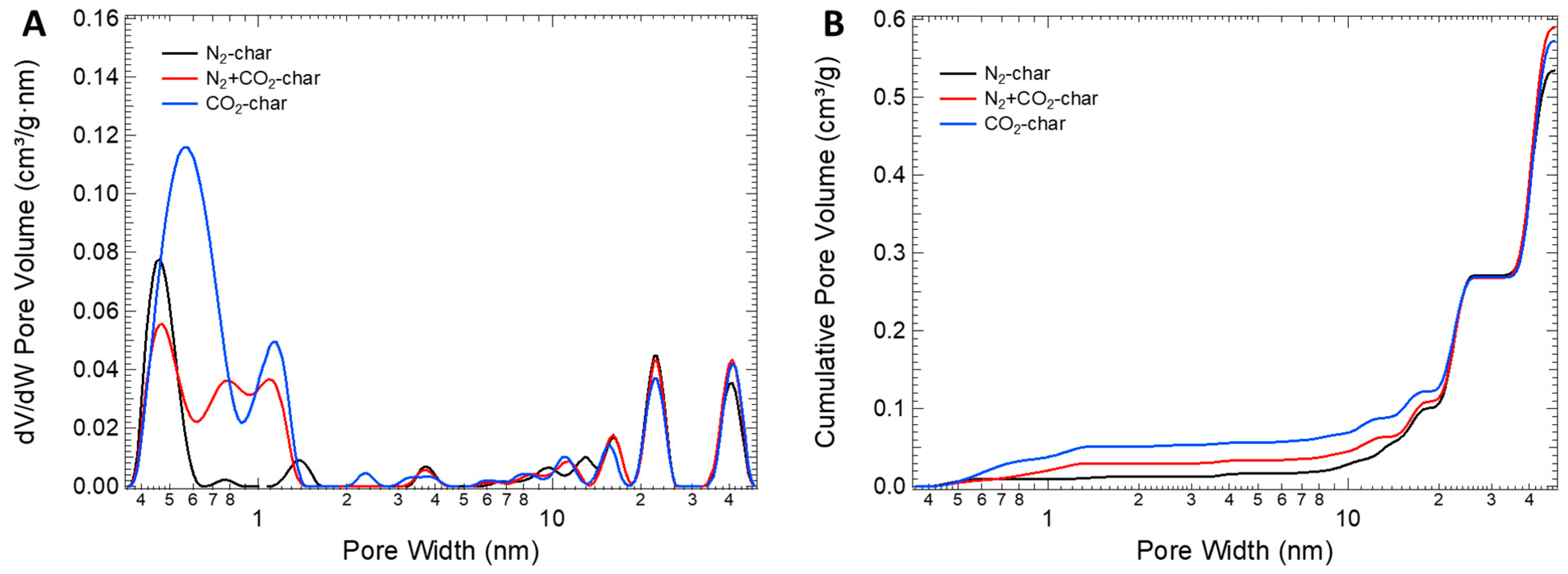

| C (% w/w) | 82.8 |
| H (% w/w) | 7.1 |
| N (% w/w) | 1.5 |
| S (% w/w) | 1.6 |
| O (% w/w) | 7 |
| Humidity (% w/w) | 0 |
| Volatiles (% w/w) | 66 |
| Ashes (% w/w) | 5 |
| Fixed carbon (% w/w) | 29 |
| Sample | N2-char | N2+CO2-char | CO2-char | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Time (min) | Temperature (°C) | Gas | Flow (Nl/min) | Rate (°C/min) | Time (min) | Temperature (°C) | Gas | Flow (Nl/min) | Rate (°C/min) | Time (min) | Temperature (°C) | Gas | Flow (Nl/min) | Rate (°C/min) |
| Value | - | 25 | - | - | - | - | 25 | - | - | - | - | 25 | - | - | - |
| 80 | 500 | N2 | 0.5 | 6.0 | 80 | 500 | N2 | 0.5 | 6.0 | 80 | 500 | CO2 | 0.5 | 6.0 | |
| 60 | 700 | N2 | 0.5 | 3.3 | 60 | 700 | N2 | 0.5 | 3.3 | 60 | 700 | CO2 | 0.5 | 3.3 | |
| 120 | 900 | N2 | 0.5 | 1.6 | 120 | 900 | N2 | 0.5 | 1.6 | 120 | 900 | CO2 | 0.5 | 1.6 | |
| 60 | 900 | N2 | 0.5 | - | 60 | 900 | CO2 | 0.5 | - | 60 | 900 | CO2 | 0.5 | - | |
| Sample | SBET (m2/g) | V < 0.7 nm (cm3/g) | 0.7 nm < V < 2 nm (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) | VT (cm3/g) | Vmic/VT (%) |
|---|---|---|---|---|---|---|---|
| “as is”-char | - | - | - | - | - | - | - |
| N2-char | 79 | 0.010 | 0.003 | 0.013 | 0.521 | 0.534 | 2 |
| N2+CO2-char | 123 | 0.011 | 0.019 | 0.030 | 0.559 | 0.589 | 5 |
| CO2-char | 174 | 0.027 | 0.024 | 0.051 | 0.521 | 0.572 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, P.; Calandra, P.; Policicchio, A.; Conte, G.; Agostino, R.G.; Pochylski, M.; Abe, A.; Oliviero Rossi, C. Char from Pyrolysis of Waste Tires to Increase Bitumen Performances. Appl. Sci. 2024, 14, 30. https://doi.org/10.3390/app14010030
Caputo P, Calandra P, Policicchio A, Conte G, Agostino RG, Pochylski M, Abe A, Oliviero Rossi C. Char from Pyrolysis of Waste Tires to Increase Bitumen Performances. Applied Sciences. 2024; 14(1):30. https://doi.org/10.3390/app14010030
Chicago/Turabian StyleCaputo, Paolino, Pietro Calandra, Alfonso Policicchio, Giuseppe Conte, Raffaele G. Agostino, Mikolaj Pochylski, Abraham Abe, and Cesare Oliviero Rossi. 2024. "Char from Pyrolysis of Waste Tires to Increase Bitumen Performances" Applied Sciences 14, no. 1: 30. https://doi.org/10.3390/app14010030












