Safety of 3D-Printed Acrylic Resins for Prosthodontic Appliances: A Comprehensive Cytotoxicity Review
Abstract
:1. Introduction
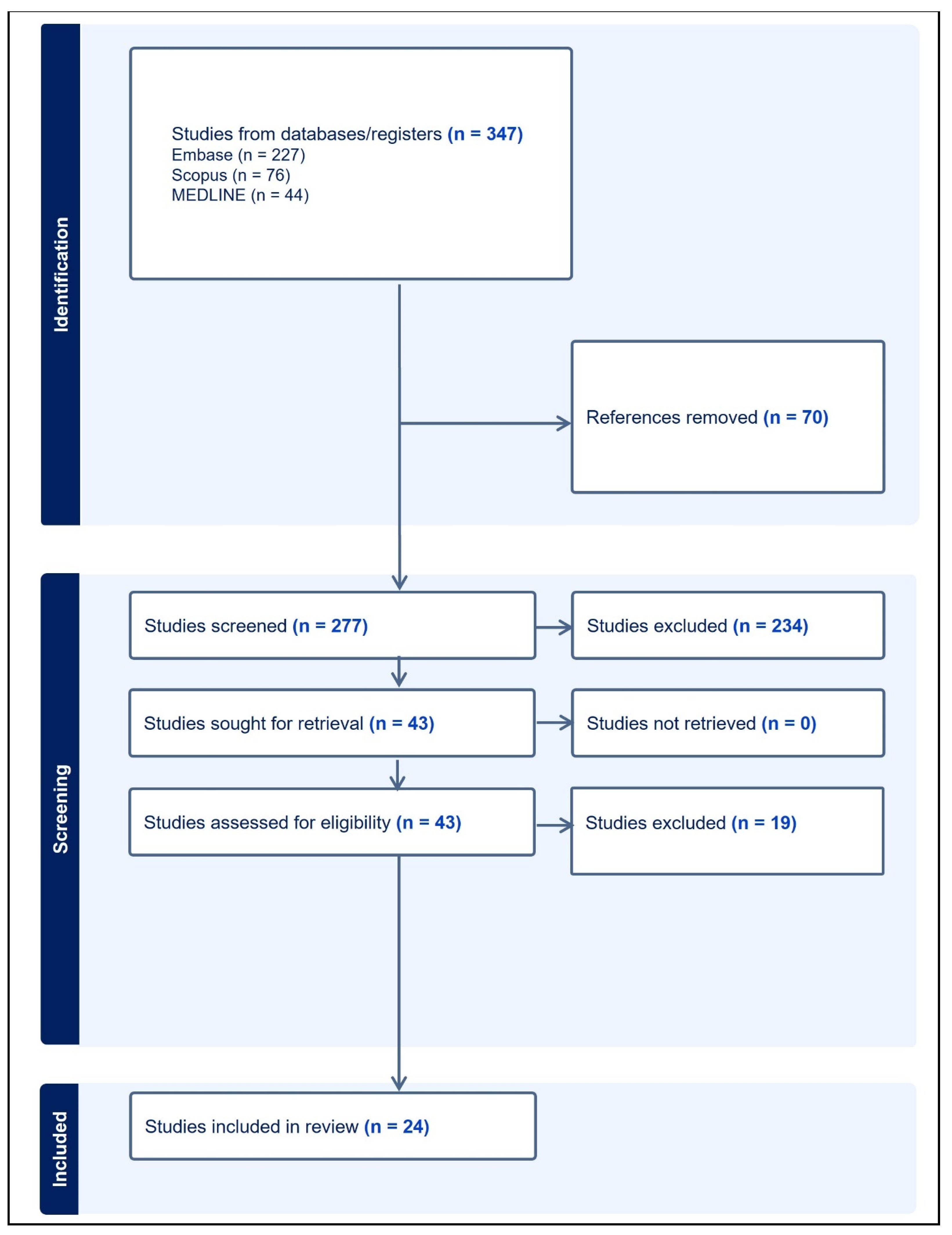
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, G.; Wu, L.; Hu, J.; Zhou, X.; He, F.; Wan, L.; Pan, S.-T. Main Applications and Recent Research Progresses of Additive Manufacturing in Dentistry. BioMed Res. Int. 2022, 2022, e5530188. [Google Scholar] [CrossRef]
- Rekow, E.D. Digital dentistry: The new state of the art—Is it disruptive or destructive? Dent. Mater. 2020, 36, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Conejo, J. The Current State of Chairside Digital Dentistry and Materials. Dent. Clin. N. Am. 2019, 63, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, L.; Güth, J.-F.; Schweiger, J.; Kollmuss, M.; Reichl, F.-X.; Edelhoff, D.; Högg, C. Elution behavior of a 3D-printed, milled and conventional resin-based occlusal splint material. Dent. Mater. 2021, 37, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.M.; Immich, F.; De Araujo, T.S.; Lund, R.G.; Da Silva, A.F.; Piva, E.; Da Rosa, W.L.D.O. Photosensitive resins used in additive manufacturing for oral application in dentistry: A scoping review from lab to clinic. J. Mech. Behav. Biomed. Mater. 2023, 141, 105732. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sayed, M.E.; Shetty, M.; Alqahtani, S.M.; Al Wadei, M.H.D.; Gupta, S.G.; Othman, A.A.A.; Alshehri, A.H.; Alqarni, H.; Mobarki, A.H.; et al. Physical and Mechanical Properties of 3D-Printed Provisional Crowns and Fixed Dental Prosthesis Resins Compared to CAD/CAM Milled and Conventional Provisional Resins: A Systematic Review and Meta-Analysis. Polymers 2022, 14, 2691. [Google Scholar] [CrossRef] [PubMed]
- Wuersching, S.N.; Hickel, R.; Edelhoff, D.; Kollmuss, M. Initial biocompatibility of novel resins for 3D printed fixed dental prostheses. Dent. Mater. 2022, 38, 1587–1597. [Google Scholar] [CrossRef]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.-I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Shrestha, B.; Patel, J.; Shih, B.; Shearston, K.; Ngo, H.; Fawzy, A. Effect of post-curing light exposure time on the physico–mechanical properties and cytotoxicity of 3D-printed denture base material. Dent. Mater. 2022, 38, 57–67. [Google Scholar] [CrossRef]
- Alamo, L.; Cassiano, F.B.; Bordini, E.A.F.; Stuani, V.T.; Pacheco, L.E.; Gallinari, M.D.O.; Souza Costa, C.A.; Mondelli, R.F.L.; Soares, D.G. An organotypic model of oral mucosa cells for the biological assessment of 3D-printed resins for interim restorations. J. Prosthet. Dent. 2022, 132, 251–259. [Google Scholar] [CrossRef]
- Atria, P.J.; Bordin, D.; Marti, F.; Nayak, V.V.; Conejo, J.; Benalcázar Jalkh, E.; Witek, L.; Sampaio, C.S. 3D-printed resins for provisional dental restorations: Comparison of mechanical and biological properties. J. Esthet. Restor. Dent. 2022, 34, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaikhan, E.; Gu, H.; Hwangbo, N.-K.; Lim, J.-H.; Shim, J.-S.; Lee, K.-W.; Kim, J.-E. Influence of different postcuring parameters on mechanical properties and biocompatibility of 3D printed crown and bridge resin for temporary restorations. J. Mech. Behav. Biomed. Mater. 2022, 128, 105127. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaikhan, E.; Lim, J.-H.; Shin, S.-H.; Park, K.-H.; Park, Y.-B.; Lee, J.-H.; Kim, J.-E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Bieger, V.; Thieringer, F.M.; Fischer, J.; Rohr, N. Fibroblast behavior on conventionally processed, milled, and printed occlusal device materials with different surface treatments. J. Prosthet. Dent. 2023, 129, 939–945. [Google Scholar] [CrossRef]
- Britto, V.T.; Cantelli, V.; Collares, F.M.; Bertol, C.D.; Della Bona, Á. Biomechanical properties of a 3D printing polymer for provisional restorations and artificial teeth. Dent. Mater. 2022, 38, 1956–1962. [Google Scholar] [CrossRef]
- Burgers, R.; Schubert, A.; Muller, J.; Krohn, S.; Rodiger, M.; Leha, A.; Wassmann, T. Cytotoxicity of 3D-printed, milled, and conventional oral splint resins to L929 cells and human gingival fibroblasts. Clin. Exp. Dent. Res. 2022, 8, 650–657. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, D.-H.; Huang, S.-C.; Lin, Y.-M. Comparison of flexural properties and cytotoxicity of interim materials printed from mono-LCD and DLP 3D printers. J. Prosthet. Dent. 2021, 126, 703–708. [Google Scholar] [CrossRef]
- Dai, J.; Luo, K.; Spintzyk, S.; Unkovskiy, A.; Li, P.; Xu, S.; Fernandez, P. Post-processing of DLP-printed denture base polymer: Impact of a protective coating on the surface characteristics, flexural properties, cytotoxicity, and microbial adhesion. Dent. Mater. 2022, 38, 2062–2072. Available online: https://www.sciencedirect.com/science/article/pii/S0109564122003128 (accessed on 19 September 2023). [CrossRef]
- Guerrero-Gironés, J.; López-García, S.; Pecci-Lloret, M.R.; Pecci-Lloret, M.P.; Rodríguez Lozano, F.J.; García-Bernal, D. In vitro biocompatibility testing of 3D printing and conventional resins for occlusal devices. J. Dent. 2022, 123, 104163. [Google Scholar] [CrossRef]
- Hwangbo, N.-K.; Nam, N.-E.; Choi, J.-H.; Kim, J.-E. Effects of the Washing Time and Washing Solution on the Biocompatibility and Mechanical Properties of 3D Printed Dental Resin Materials. Polymers 2021, 13, 4410. [Google Scholar] [CrossRef]
- Jin, G.; Gu, H.; Jang, M.; Bayarsaikhan, E.; Lim, J.-H.; Shim, J.-S.; Lee, K.-W.; Kim, J.-E. Influence of postwashing process on the elution of residual monomers, degree of conversion, and mechanical properties of a 3D printed crown and bridge materials. Dent. Mater. 2022, 38, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kwon, J.-S.; Park, J.-M.; Lo Russo, L.; Shim, J.-S. Effects of postpolymerization conditions on the physical properties, cytotoxicity, and dimensional accuracy of a 3D-printed dental restorative material. J. Prosthet. Dent. 2022, 132, 241–250. [Google Scholar] [CrossRef]
- Li, P.; Lambart, A.-L.; Stawarczyk, B.; Reymus, M.; Spintzyk, S. Postpolymerization of a 3D-printed denture base polymer: Impact of post-curing methods on surface characteristics, flexural strength, and cytotoxicity. J. Dent. 2021, 115, 103856. [Google Scholar] [CrossRef]
- Nam, N.-E.; Hwangbo, N.-K.; Jin, G.; Shim, J.-S.; Kim, J.-E. Effects of heat-treatment methods on cytocompatibility and mechanical properties of dental products 3D-printed using photopolymerized resin. J. Prosthodont. Res. 2023, 67, 121–131. [Google Scholar] [CrossRef]
- Oh, R.; Lim, J.-H.; Lee, C.-G.; Lee, K.-W.; Kim, S.-Y.; Kim, J.-E. Effects of washing solution temperature on the biocompatibility and mechanical properties of 3D-Printed dental resin material. J. Mech. Behav. Biomed. Mater. 2023, 143, 105906. [Google Scholar] [CrossRef]
- Srinivasan, M.; Kalberer, N.; Kamnoedboon, P.; Mekki, M.; Durual, S.; Özcan, M.; Müller, F. CAD-CAM complete denture resins: An evaluation of biocompatibility, mechanical properties, and surface characteristics. J. Dent. 2021, 114, 103785. [Google Scholar] [CrossRef] [PubMed]
- Wulff, J.; Schweikl, H.; Rosentritt, M. Cytotoxicity of printed resin-based splint materials. J. Dent. 2022, 120, 104097. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xepapadeas, A.B.; Koos, B.; Geis-Gerstorfer, J.; Li, P.; Spintzyk, S. Effect of post-rinsing time on the mechanical strength and cytotoxicity of a 3D printed orthodontic splint material. Dent. Mater. 2021, 37, e314–e327. [Google Scholar] [CrossRef]
- Jorge, J.H.; Giampaolo, E.T.; Machado, A.L.; Vergani, C.E. Cytotoxicity of denture base acrylic resins: A literature review. J. Prosthet. Dent. 2003, 90, 190–193. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 9 September 2024).
- Bhargav, A.; Sanjairaj, V.; Rosa, V.; Feng, L.W.; Fuh YH, J. Applications of additive manufacturing in dentistry: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2058–2064. [Google Scholar] [CrossRef]
- Huang, F.M.; Tai, K.W.; Hu, C.C.; Chang, Y.C. Cytotoxic effects of denture base materials on a permanent human oral epithelial cell line and on primary human oral fibroblasts in vitro. Int. J. Prosthodont. 2001, 14, 439–443. [Google Scholar] [PubMed]
- Raszewski, Z. Influence of polymerization method on the cytotoxicity of three different denture base acrylic resins polymerized in different methods. Saudi J. Biol. Sci. 2020, 27, 2612–2616. [Google Scholar] [CrossRef] [PubMed]
- Maktabi, H.; Ibrahim, M.S.; Balhaddad, A.A.; Alkhubaizi, Q.; Garcia, I.M.; Collares, F.M.; Strassler, H.; Fugolin, A.P.P.; Pfeifer, C.S.; Melo, M.A.S. Improper Light Curing of Bulkfill Composite Drives Surface Changes and Increases S. mutans Biofilm Growth as a Pathway for Higher Risk of Recurrent Caries around Restorations. Dent. J. 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]


| PICO | Keywords |
|---|---|
| Population | 3D print$4 OR 3-D print$4 OR 3-dimensional print$4 OR three-dimensional print$4 |
| Intervention | “dental night guard *” OR “occlusal splint *” OR “night guard *” OR bruxism OR prosthodontic * OR “dental restoration *” OR denture * OR “artificial tooth” OR “artificial teeth” |
| Comparison | “three dimensional print *” OR “three-dimensional print *” |
| Outcome | Biocompatib * OR cytotoxic * OR genotoxic * |
| Author/Year | Materials/n | Printer | Processing Method | Variables | Bio Method/Cell | Main Results/Conclusion |
|---|---|---|---|---|---|---|
| Aati S, 2022 [9] | PMMA-based 3D-print (Dentca Denture Base II) heat-cured (Vertex Rapid Simplified) n = ? | DLP Kulzer 3D Printer System | Cleaned sonicated with isopropanol. Soaked in a glycerol bath and post-cured. Curing unit at 200 W wavelength 390–540 nm. Finished with ascending grit size: 600, 800, and 1200. Polishing with 1, 0.25, and 0.05 µm paste. | Post-curing for 0, 5, 10, or 20 min | (APH) assay extract method: 24 and 72 h; direct contact method: 24 h Human periodontal ligament fibroblasts (HPLFs) | Extracts of uncured resin reduced cell viability by 50% (p > 0.001). Direct contact: uncured resins reduced cell viability by approximately 60–65% (p < 0.0001). This effect was significantly (p < 0.0001) reduced when resins were cured for at least 5 min. An increase in post-curing time enhanced cell viability and biocompatibility. |
| Alamo A, 2022 [10] | Prizma 3D Smart Print Bio; Cosmos DLP Temp n = 6 | DLP FlashForge Hunter | Specimens were immersed in isopropyl alcohol for 10 min under agitation; finished using 600- and 1200-grit abrasive paper; post-polymerization in an ultraviolet (UV) light chamber (90 W, 405 nm). | Post-curing for 1, 10, or 20 min | Cell metabolism (Alamar Blue; n = 6); cell viability (Live/Dead assay; n = 2)/1, 3, and 7 days. Immortalized normal oral keratinocytes (NOK-Si; ID:CVCL_BW57) 3D culture of human gingival fibroblasts (HGF) | Severe reduction in metabolism (>70%) and viability of keratinocytes occurred for post-curing 1 min. 10 and 20 min promoted a mild–moderate cytotoxic effect. The 3DP resins submitted to post-polymerization for 20 min showed a pattern similar to that of resin composite. The biological compatibility of 3D printing resins for interim restorations can be negatively influenced by inadequate post-polymerization processing. |
| Atria PJ, 2022 [11] | Temporary Crown and Bridge (Formlabs); Crowntec C&B Nextdent, Permanent Bridge resin n = 30 | SLA-printer Formlabs A2; DLP Nextdent 5100 | Formlabs: 3 min washing machine (99% IPA). Post-curing time was set to 60 °C for 20 min; Crowntec: alcohol-soaked (96%) cloth. Final curing UV-light box 320–500 nm, twice, for 180 s each. Nextdent: specimens cleaned in 91%IPA (ultrasonic unit) for 5 min, post-curing for 30 min. | Three commercially available resins and the experimental resin | Cell viability PrestoBlue assay; /24, 48, 72 h, 5 days, and 7 days human periodontal ligament fibroblasts (hPDLF) | There was no significant difference in cell proliferation of hPDLF cells in contact with different 3D-printed materials. Evaluation of cytotoxicity through the LDH assay yielded no cytotoxicity of the materials. There were no differences in cell viability, cell proliferation, and cell toxicity among different evaluated resin materials. |
| Bayarsaikhan E, 2022 [12] | crown and bridge resin (C&B resin, NextDent, Soesterburg, Netherlands) n = 5 | DLP 3D printer | 405 nm UV LED light (1.4 mW/cm3) Washed (ethanol) for 10 min. Four different UV-light polymerization chambers and a handheld curing device (5, 15, and 30 min). Post-curing chambers [LC-3D Print Box (LC), Form Cure (FC), Veltz 3D (VE), and Cure M (CM)]. Post-cured for 20, 40, and 60 s using VALO. | Post-curing for 5, 15, and 30 min four different chambers VALO | CELLOMAX™ viability kit /24 h Primary human gingival fibroblasts | The cell viability of the post-cured resin specimens ranged from 56.46% to 92.29%. The cell viability did not differ significantly with the post-curing time in the LC, FC, and VA groups, whereas it did in the CM and VE groups (p < 0.05). The cell viability varied significantly in all 30 min PCE groups and in the 20, 40, and 60 s VA groups (p < 0.05). Post-curing with different equipment showed significant changes in cell viability of the 3D-printed crown and bridge resin. |
| Bayarsaikhan E, 2021 [13] | PMMA resin (Denture teeth resin A2, Formlabs) n = ? | SLA 3D printer (Form 3, Formlabs) | Cleaned with 90% isopropanol for 10 min. Post-cured in a UV-light chamber. 405 nm light source heating up to 80 °C. Post-cured at temperatures of 40, 60, and 80 °C for 15, 30, 60, 90, and 120 min. | Post-cured at 40, 60, and 80 °C for 15, 30, 60, 90, and 120 min | CELLOMAX™ viability; a lactate dehydrogenase (LDH) release assay/24, 48, and 72 h Primary human gingival fibroblasts (HGFs) | There was a significant interaction effect between cultivation time, post-curing temperature, and post-curing time. Increasing the post-curing temperature and time results in significant enhancements in biocompatibility. |
| Bieger V, 2023 [14] | Dental LT Clear Resin (Formlabs Inc); FREE-PRINT splint (Detax GmbH & Co KG); n = 9 | SLA-printer (Form 2; Formlabs) | Rinsed (IPA 90%) for 5 min. Post-cured (2000 flashes—UV-light). Unpolished specimens were washed in the Millipore water (2 × 4 min). Polished specimens were cleaned ultrasonic bath of 96% ethanol (2 × 4 min) and washed in Millipore water (4 min). | Unpolished and polished | A WST-1 cell viability assay (Cell Proliferation Reagent WST-1; F. Hoffmann-La Roche)/24 h Human gingival fibroblast (HGF-1) cells | Overall, material (p < 0.001) and surface treatment (p < 0.001) significantly influenced the viability of HGF-1 cells. Similar behavior on conventionally processed, milled, and printed resin materials for occlusal devices when polished. On unpolished surfaces, cell viability on printed materials was reduced compared with conventionally processed and milled materials. Polishing of printed materials is essential to reduce cytotoxic effects. |
| Britto VT, 2022 [15] | Cosmos Temp, n = 3 | DLP Bego, Varseo 3D printer, | Wash specimens using 96% ethyl alcohol sonic bath for 10 min. Post-polymerization in a lighting chamber 400–450 nm; for 13 min. | Comparing a bis-acryl resin (BA) and an acrylic resin (AR) | Sulforhodamine B; MTT/72; 24 h primary gingival fibroblasts | AR (71.94 ± 11.82%) showed a lower cell viability (p < 0.05) than 3D (92.88 ± 11.36%) and bis-acryl composite resin (90.85 ± 11.60%) for sulforhodamine B analysis. No significant difference among materials for MTT analysis (p > 0.05). 3D-printed polymer fulfilled the material’s requirements for clinical use. |
| Bürgers R, 2022 [16] | Med610 (Stratasys), V-Print splint (FREEPRINT) ortho 385; Dental LT Clear n = 25 | Not reported | Not described | 3D resins compared to conventional resins | WST-8-based-assays; 24 h explosion L929 cells | No significant differences between the 3D-printed or milled resins. No significant differences between 3D printing, milling, thermoforming, and pressing. 3D printing and milling showed no significant differences compared with conventional methods. In human gingival fibroblasts, cytotoxicity of all resins was below a critical threshold. |
| Chen H, 2021 [17] | AA temp; C&B MFH n = 6 | DLP MiiCraft Ultra 125; Phrozen Sonic mono LCD | All specimens were washed with 95% alcohol and subjected to post-polymerization treatment by using the FormCure or PhrozenCure units for different times to enhance the polymerization. | 3D printing resins or DLP 3D printers can be used in a mono-LCD 3D printer | MTT/24 h Mouse fibroblasts (L929) | Without post-polymerization, both AA and CB had viability < 70% (cytotoxic potential). Post-polymerization (FormCure—15 min; PhrozenCure—1 min), all material-printer combinations reached near 100% cell viability. Resins designed for DLP 3D printers can be used in a mono-LCD 3D printer post-polymerized. |
| Dai J, 2022 [18] | Freeprint Denture, Detax GmbH, Ettlingen, Germany n = 4 | DLP printer UltraCraft DS | Ultrasonically cleaned in 99% isopropanol for 3 min twice and post-cured for 15 min in a light chamber (UltraCraft PCU mini, with an ultraviolet light 360 to 440 nm. | Not described | (LDH) release (LDH cytotoxicity assay)/24 h Mouse fibroblasts (L929) | L929 fibroblasts cultured in specimen extracts exhibited no compromised membrane integrity. No differences between extracts (p > 0.05). Relative LDH release of all the groups <30% of negative control. No differences between groups (p > 0.05). The coating treatment did not cause cytotoxic effects. |
| Guerrero-Girones J, 2022 [19] | Keysplint Soft NextDent Ortho Freeprint Splint (Detax) traditional resin Orthocryl (Dentaurum) n = 40 | SLA 3D printer (Phrozen Sonic Mini 4k) | IPA 99% for three minutes. Second bath for two minutes. Left 30 min in a dark room. Post-cured using a UV-polymerization Box (405 nm, 40 mW/cm2). Orthocryl mixture at a ratio of 2.5:1 (powder: fluid) for 5 min. The final polymerization in a pressure vessel (2.2 bar) at a temperature between 40 °C and 46 °C for 20 min. | 3D print resin and traditional resins used for dental splints | MTT; Cell migration assays; Cell cytoskeleton staining assays; Human gingival fibroblasts | At 1:4 dilution, no resin affected cell metabolic activity; at a 1:2 dilution, Freeprint Splint had the highest cytotoxicity at 24 and 72 h (p < 0.001), while other resins did not affect cell biocompatibility; undiluted Freeprint Splint-treated cells exhibited a significantly lower viability. The new dental resins for 3D printing and the conventional dental resins assessed in this study showed similar biocompatibility, except for Freeprint Splint, which was the most cytotoxic of the four dental resins studied on hGFs. |
| Hwangbo NK, 2021 [20] | NextDent C&B; Formlabs Denture Teeth resin n = 15 | DLP 3D printer (NextDent) SLA 3D printer (Form 3, Formlabs) | Washed with a 90% IPA or TPM solution for 3, 5, 10, 15, 30, 60, or 90 min. A non-washing group was the control group. The specimen was then post-cured for 30 min at 60 °C in a UV-light polymerization chamber using a 405 nm light source. | Washing solutions (IPA or TPM); Washing times | CELLOMAX™ viability assay; lactate dehydrogenase (LDH);/24, 48, and 72 h Primary human gingival fibroblasts (HGFs) | The cell viability varied significantly with the washing time (F = 216.669, p < 0.001) and material used (F = 79.899, p < 0.001), but not with the washing solution used (F = 1.298, p = 0.255). Cell viability increased with the higher washing time. Cytotoxicity decreased with an increase in washing time. Biocompatibility increased with the washing time for both solutions. |
| Jin G, 2022 [21] | Temporary crown and bridge resin (Nextdent C&B resin, 3D Systems n = ? | DLP printer (Nextdent 5100 3D printer) | Washing by a rotary washer (3, 6, 10, 20, and 60 min); soaking in a tank and washing by an ultrasonic bath (3, 6, 10, 20, and 60 min); specimens manually dunked and rinsed for 10 s without a post-washing process, with the residual liquid resin left on the surface (control group). | Different washing protocols | Cell viability assay: CELLOMAX viability kit; cell cytotoxicity assay: lactate dehydrogenase (LDH) release assay/24 h; 24 and 48 h. Primary human gingival fibroblasts (HGFs) | Cell viability rises with the prolonged post-washing time. Cytotoxicity decreases with the prolonged post-washing time. Ultrasonic bath provided more powerful detoxification effects to the Nextdent C&B resin than the rotary washer. The ultrasonic bath had a superior washing outcome in eluting residual monomers compared with using the rotary washer. Prolonged post-washing can efficiently reduce the quantity of the residual monomers |
| Kim JH, 2022 [22] | C&B MFH (NextDent) n = 3 | DLP 3D printer (ND5100; NextDent) | The printed specimens were washed (10 min) in a washer with IPA 99%. Specimens were kept in aluminum zipper bags to shield them from light. | Post-polymerization devices: D1; LC; FO; ME and MP | Water-soluble tetrazolium (WST)-1 assay/24 h L929 mouse fibroblast cells | No post-polymerization group showed a cell viability of 16.41%, whereas all other experimental groups showed results from 66.79% to 96.53%. D1 group had the highest level of cell viability (96.53%), MP group showed the lowest level (66.79%). Light intensity and time management improve the post-cure process. |
| Ping L, 2021 [23] | V-Print Denture & Solflex 650, n = 15 | Not reported | Rinsed in an ultrasonic bath 96% ethanol post-cured with different post-polymerization devices: Otoflash G171 (OF), Labolight DUO (LL), PCU LED (PCU), and LC-3DPrintbox (PB). | Different post-curing devices | Cell membrane damage: live/dead staining; cell morphologies: fluorescence microscope; metabolic activity: CCK-8 assay;/24 h; mouse fibroblasts (L929) | L929 fibroblasts cultured in the extracts of 3D-printed specimens displayed metabolic activities > 70% of the negative control. No differences in cell metabolic activities between different 3D-printed surfaces. Cytotoxic effect of the 3D-printed material can be decreased by any different post-curing methods. |
| Nam NE, 2023 [24] | NextDent C&B (NextDent) n = 5 | DLP 3D printer (NextDent 5100) | Specimen surface was washed (IPA 90%—10 min). Post-curing for 30 min (UV—220 µW/cm2—60 °C). The completed specimens were kept in darkness and divided into groups based on their heat-treatment method. | Storage at room temp. (25 °C); RT water; 80 °C and 100 °C water; autoclaving | Cell viability: CELLOMAX™ viability kit; cytotoxicity: (LDH) release assay; Primary human gingival fibroblasts | RT = cell malformation was observed. Water storage = better cytotoxicity; Autoclave = not significant; Greater time of immersion = better cell viability. Cell viability increases and cytotoxicity decreases by immersing a printed resin in 100 °C water for 1 or 5 min. |
| Oh R, 2023 [25] | Next Dent C&B, Next Dent BV, Netherlands n = 5 | DLP method (ASIGA MAX UV) | Washed for 5, 10, 15, 30, and 60 min at various temperatures with 95% ethyl alcohol, temperature modification (N/T, 18–20 °C), 30 °C, 40 °C, and 50 °C. | Washing and time | CELLOMAXTM viability/24 h Primary human gingival fibroblasts (HGFs) | Temperature-treated groups had significantly improved cell viability. The washing solution temperature and time significantly affected biological properties. Washing of 3D-printed samples at 30 ◦C for 30 min presented a significantly improved cell viability. |
| Srinivasan M, 2021 [26] | AvaDent Denture base puck, Avadent Extreme CAD CAM shaded NextDent Base, NextDent C&B, n = 9 | DLP Rapid Shape D30 | Rinsed twice (3 min) in a 96% ethanol solution in an ultrasonic bath to remove excess material. Placed in an ultraviolet light box for 10 min for additional polymerization, a wavelength of blue UV-A 315 to 400 nm, and an output of 43.2 kJ. | Milled or; 3D-printed | Resazurin assays/4, 7, 14, and 21 days human epithelial cells (A-431); human gingival cells (HGF-1) | Epithelial cells (A-431) and gingival cells (HGF-1) grew gradually around a 3- to 4-fold increase from day 4 to day 21, (same trend as the control group). No statistical difference among different resin groups (MB, MT, PT, and PB1) from day 4 to day 21 for either A-431 or HGF-1. CAD-CAM milled and complete denture resins had similar biocompatibility. |
| Wuersching SN, 2022 [7] | Tetric EvoCeram (TEC); Tetric CAD (TC); VarseoSmile (VSC); NextDent (ND); Protemp 4 (PT); Telio CAD (TEL); VarseoSmile Temp (VST); Temp PRINT (TP) P Pro Crown & Bridge (P) | DLP printer P30 (RapidShape); DLP printer DLP NextDent 5100 (NextDent); DLP printer Varseo XS (BEGO). | Managing different materials following the manufacturer’s instructions. | Different materials | Cell viability: RealTimeGlo® MT Cell Viability Assay; Induction of apoptosis: e RealTimeGlo™ Annexin V Apoptosis and Necrosis Assay/over 72 h; 24 h; 24 h; 24 h. human gingival fibroblasts (hGF-1) | Among the resins for permanent FDP, TEC, VSC, and ND were severely toxic. Four of the five resins intended for temporary FDP, namely, PT, VST, TP, and P, were also severely toxic. TC and TEL were the only resins that exhibited slight toxicity to hGF-1. In the presence of most resins, RLUs after 24 h were slightly higher compared to the basal level with DMEM. However, when incubated with TEC or PT, the RLUs were significantly lower than the negative control. TC and TEL showed more favorable cytotoxicity results. We suggest that further post-processing steps, such as additional light curing and washing, may improve the biocompatibility of printable materials. |
| Wullf J, 2022 [27] | Luxaprint OrthoPlus, DMG; V-Print Splint, Voco n = 25 | DLP (P30 printer, Straumann) | Alignment: Printing was performed under 90° (A1), 45° (A2), or 0° (A3) alignment to the building platform in a standard of 100 µm layers. Post-processing: Specimens were either automatically washed or manually cleaned with Isopropanol. Post-polymerization was performed with LED or Xenon light. | Printer alignment; wash system; post cure; materials | Crystal violet assay The murine RAW264.7 mouse macrophage cell line | Materials influenced cell survival in most cases (p ≤ 0.029). The wash system showed differences for most combinations (p ≤ 0.032). The type of post-cure provided significant differences for most systems (p ≤ 0.022). The results for the criterion alignment were not consistent. The individual parameters of material selection as well as the postprocessing (post-polymerization, washing procedure) affect in vitro cytotoxicity. Alignment during manufacturing does not have any effect on in vitro cytotoxicity. |
| Xu Y, 2021 [28] | Dental LT Clear Resin, Formlabs; Orthocryl Clear, Dentaurum GmbH & Co. KG, Ispringen, Germany (control); n = 4 | SLA 3D printer (Form 3B,) | Ultrasonically rinsed with IPA for 5 min, 12 min, 20 min, 30 min, 1 h, and 12 h, post-curing at 80 °C for 20 min. | Washing time | Cell morphology; cell viability (live/dead fluorescence staining) Direct contact test: Metabolic activity (CCK-8 assay); L929 mouse fibroblasts | Cell viability of groups from 5 min to 12 h did not differ from control. All post-processed samples with different post-rinsing times showed no cytotoxic effect (L929 fibroblasts). The removal of cytotoxic methacrylate monomers by post-rinsing could be achieved in 5 min. Extending the post-rinsing time did not improve the cytocompatibility. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arossi, G.A.; Abdou, N.A.; Hung, B.; Garcia, I.M.; Zimmer, R.; Melo, M.A. Safety of 3D-Printed Acrylic Resins for Prosthodontic Appliances: A Comprehensive Cytotoxicity Review. Appl. Sci. 2024, 14, 8322. https://doi.org/10.3390/app14188322
Arossi GA, Abdou NA, Hung B, Garcia IM, Zimmer R, Melo MA. Safety of 3D-Printed Acrylic Resins for Prosthodontic Appliances: A Comprehensive Cytotoxicity Review. Applied Sciences. 2024; 14(18):8322. https://doi.org/10.3390/app14188322
Chicago/Turabian StyleArossi, Guilherme Anziliero, Nauera Abou Abdou, Benjamin Hung, Isadora Martini Garcia, Roberto Zimmer, and Mary Anne Melo. 2024. "Safety of 3D-Printed Acrylic Resins for Prosthodontic Appliances: A Comprehensive Cytotoxicity Review" Applied Sciences 14, no. 18: 8322. https://doi.org/10.3390/app14188322







