Beyond Simple Tapping: Is Timed Body Movement Influenced When Balance Is Threatened?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
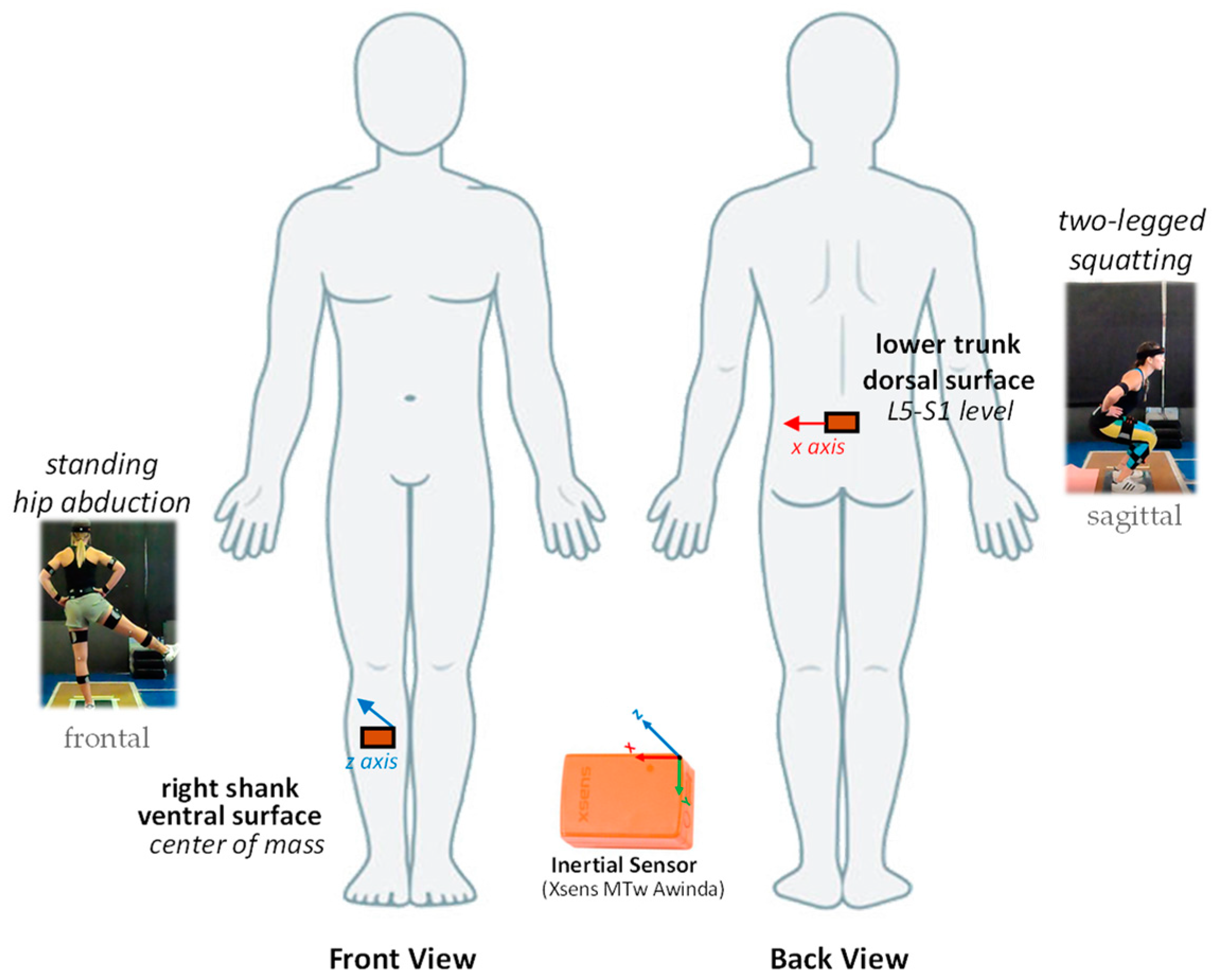
2.2. Experimental Procedure
2.3. Timing Data Recording—Instrumentation
2.4. Data Procession and Analysis
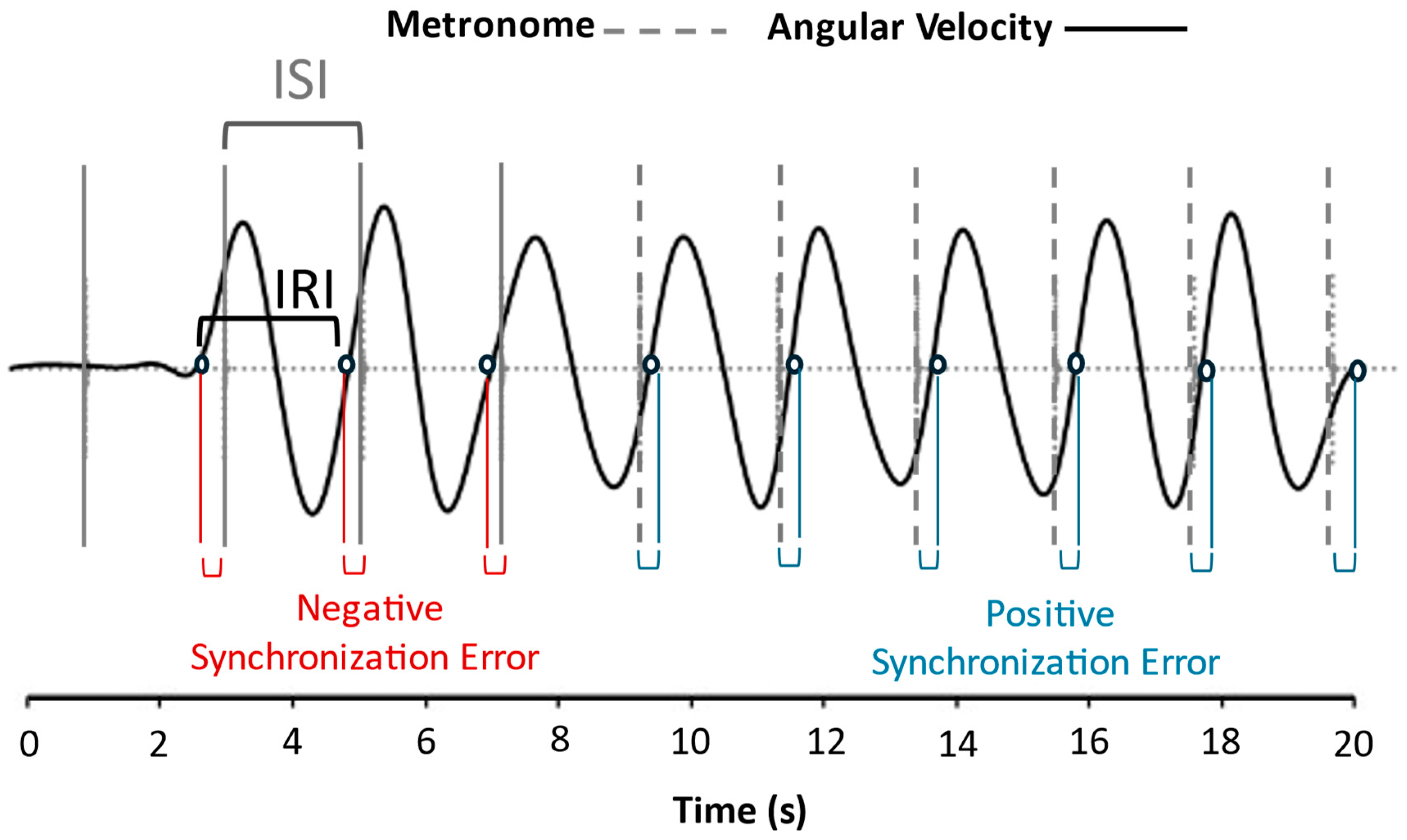
2.4.1. Inter-Response Interval (IRI) Estimation
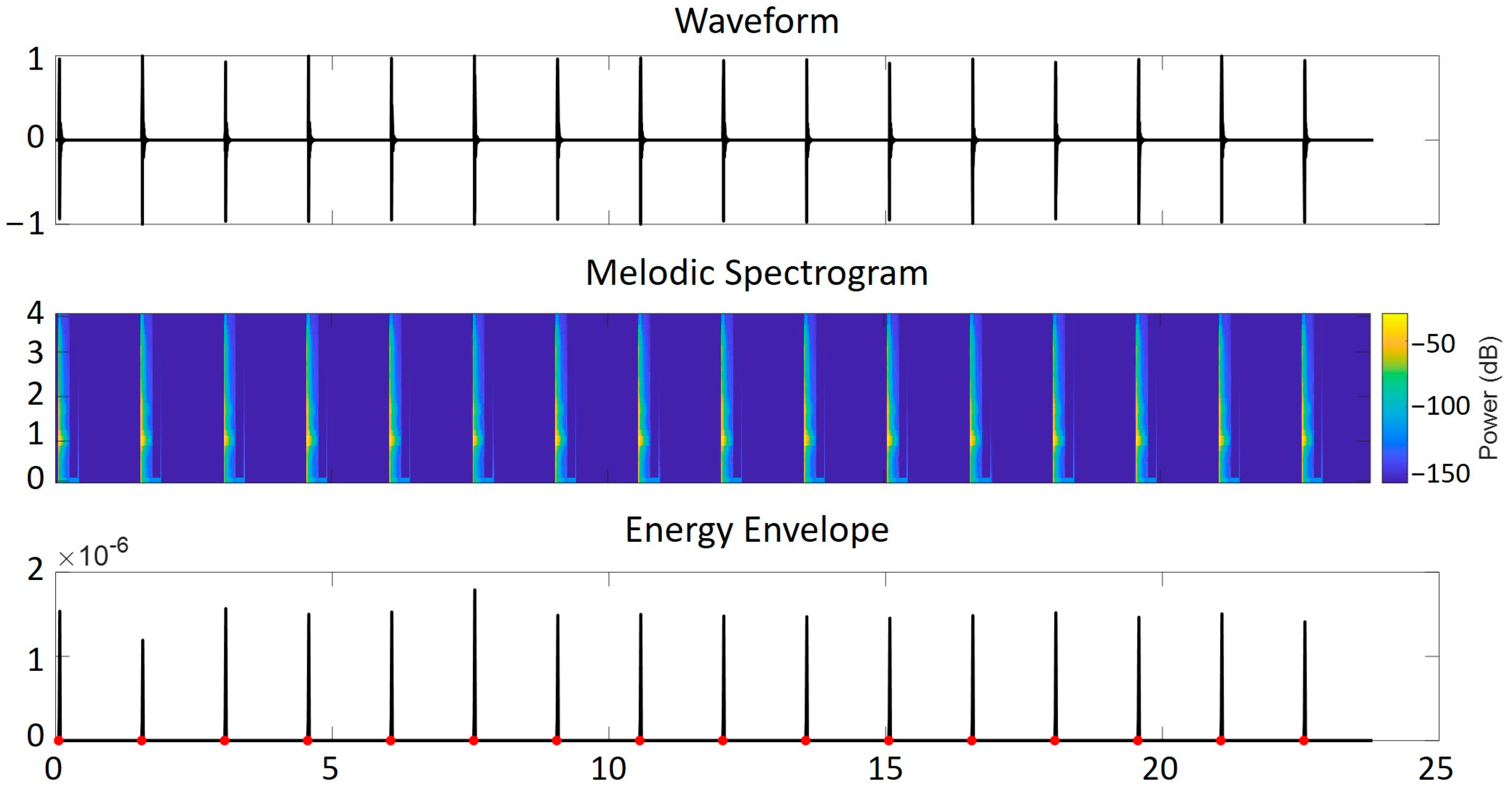
2.4.2. Metronome Beat Detection
2.4.3. Calculation of Timing Variables
Entrainment Variables
Synchronization Variables
Pace Stability
2.5. Statistical Analysis
3. Results
3.1. Inter-Response Interval (IRI)
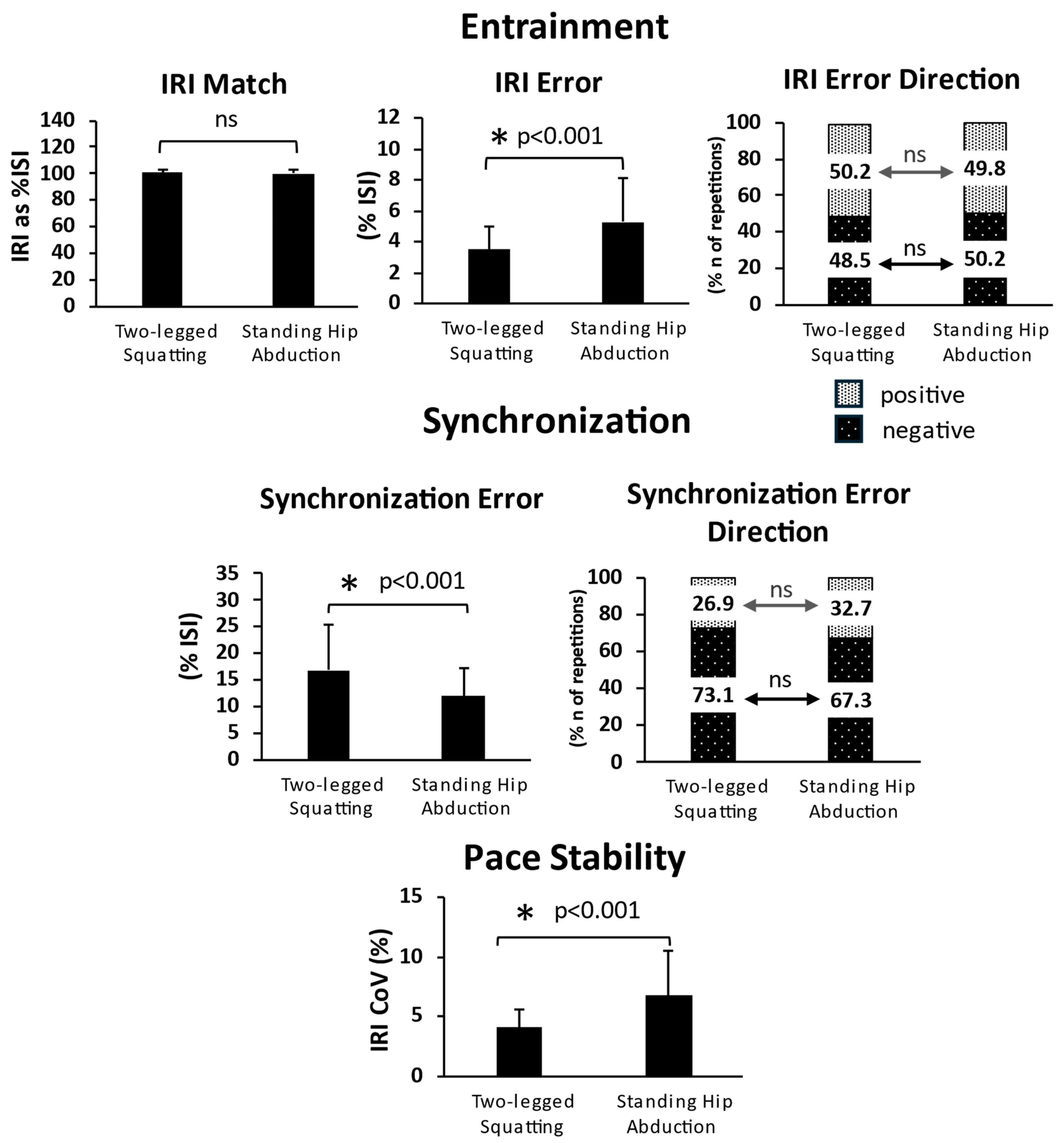
3.2. Entrainment
3.3. Synchronization
3.4. Pace Stability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Bood, R.J.; Nijssen, M.; Van Der Kamp, J.; Roerdink, M. The Power of Auditory-Motor Synchronization in Sports: Enhancing Running Performance by Coupling Cadence with the Right Beats. PLoS ONE 2013, 8, e70758. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.W.M.; Craig, C.M. Timing Movements to Interval Durations Specified by Discrete or Continuous Sounds. Exp. Brain Res. 2011, 214, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Schaffert, N.; Janzen, T.B.; Mattes, K.; Thaut, M.H. A Review on the Relationship between Sound and Movement in Sports and Rehabilitation. Front. Psychol. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H.; Miller, R.A.; Schauer, L.M. Multiple Synchronization Strategies in Rhythmic Sensorimotor Tasks: Phase vs Period Correction. Biol. Cybern. 1998, 79, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.; Ott, L.; Guérin, S.M.R.; Annett, L.E.; Lovatt, P.; Delevoye-Turrell, Y.N. A General Procedure to Measure the Pacing of Body Movements Timed to Music and Metronome in Younger and Older Adults. Sci. Rep. 2021, 11, 3264. [Google Scholar] [CrossRef]
- Rose, D.; Delevoye-Turrell, Y.; Ott, L.; Annett, L.E.; Lovatt, P.J. Music and Metronomes Differentially Impact Motor Timing in People with and without Parkinson’s Disease: Effects of Slow, Medium, and Fast Tempi on Entrainment and Synchronization Performances in Finger Tapping, Toe Tapping, and Stepping on the Spot Tasks. Parkinson’s Dis. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Merker, B.H.; Madison, G.S.; Eckerdal, P. On the Role and Origin of Isochrony in Human Rhythmic Entrainment. Cortex 2009, 45, 4–17. [Google Scholar] [CrossRef]
- Repp, B.H. Sensorimotor Synchronization: A Review of the Tapping Literature. Psychon. Bull. Rev. 2005, 12, 969–992. [Google Scholar] [CrossRef]
- Wilquin, H.; Delevoye-Turrell, Y.; Dione, M.; Giersch, A. Motor Synchronization in Patients with Schizophrenia: Preserved Time Representation with Abnormalities in Predictive Timing. Front. Hum. Neurosci. 2018, 12, 193. [Google Scholar] [CrossRef]
- Aschersleben, G. Temporal Control of Movements in Sensorimotor Synchronization. Brain Cogn. 2002, 48, 66–79. [Google Scholar] [CrossRef]
- Bieńkiewicz, M.M.N.; Rodger, M.W.M.; Craig, C.M. Timekeeping Strategies Operate Independently from Spatial and Accuracy Demands in Beat-Interception Movements. Exp. Brain Res. 2012, 222, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Semjen, A.; Schulze, H.H.; Vorberg, D. Timing Precision in Continuation and Synchronization Tapping. Psychol. Res. 2000, 63, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Zelaznik, H.N.; Spencer, R.M.C.; Ivry, R.B. Dissociation of Explicit and Implicit Timing in Repetitive Tapping and Drawing Movements. J. Exp. Psychol. Hum. Percept. Perform. 2002, 28, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.M.C.; Zelaznik, H.N.; Diedrichsen, J.; Ivry, R.B. Disrupted Timing of Discontinuous but Not Continuous Movements by Cerebellar Lesions. Science 2003, 300, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.L.; Spurgeon, L.C.; Elliott, M.T. Corrigendum: Stepping to phase-perturbed metronome cues: Multisensory advantage in movement synchrony but not Correction. Front. Hum. Neurosci. 2015, 9, 441. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wing, A.M.; Pratt, D. The Synchronisation of Lower Limb Responses with a Variable Metronome: The Effect of Biomechanical Constraints on Timing. Gait Posture 2006, 23, 307–314. [Google Scholar] [CrossRef]
- Gabbard, C.; Hart, S. Effects of Standing and Sitting on Finger-Tapping Speed in Healthy Adults. J. Orthop. Sports Phys. Ther. 2002, 32, 525–529. [Google Scholar] [CrossRef]
- Grgic, J.; Schoenfeld, B.J.; Skrepnik, M.; Davies, T.B.; Mikulic, P. Effects of Rest Interval Duration in Resistance Training on Measures of Muscular Strength: A Systematic Review. Sports Med. 2018, 48, 137–151. [Google Scholar] [CrossRef]
- Emmanouil, A.; Rousanoglou, E.; Boudolos, K. Two Repetitions May Be Enough! Reliability of Movement Timing in Physical Fitness Exercises Performed by Young, Trained Adults Using Inertial Sensors. Biomechanics 2024, 4, 84–108. [Google Scholar] [CrossRef]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments; Willey: New York, NY, USA, 1986. [Google Scholar]
- Soulard, J.; Vaillant, J.; Balaguier, R.; Vuillerme, N. Spatio-Temporal Gait Parameters Obtained from Foot-Worn Inertial Sensors Are Reliable in Healthy Adults in Single- and Dual-Task Conditions. Sci. Rep. 2021, 11, 10229. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Kristensen, M.T.; Josefsen, C.O.; Lykkegaard, K.L.; Jønsson, L.R.; Pedersen, M.M. Validation of Two Activity Monitors in Slow and Fast Walking Hospitalized Patients. Rehabil. Res. Pract. 2022, 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Nevill, A.M. Statistical Methods for Assessing Measurement Error (Reliability) in Variables Relevant to Sports Medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Kribus-Shmiel, L.; Zeilig, G.; Sokolovski, B.; Plotnik, M. How Many Strides Are Required for a Reliable Estimation of Temporal Gait Parameters? Implementation of a New Algorithm on the Phase Coordination Index. PLoS ONE 2018, 13, e0192049. [Google Scholar] [CrossRef] [PubMed]
- Yoke, M.M.; Armbruster, C. Applying Music Skills in Group Exercise: Beat-Based Techniques. In Methods of Group Exercise Instruction, 4th ed.; Human Kinetics: Champaign, IL, USA, 2019; pp. 51–75. [Google Scholar]
- Paulich, M.; Schepers, M.; Rudigkeit, N.; Bellusci, G. Xsens MTw Awinda: Miniature Wireless Inertial-Magnetic Motion Tracker for Highly Accurate 3D Kinematic Applications; MTw A Awinda Whitepaper—MW0404P.A; Xsens Technologies B.V.: Enschede, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Berglund, B.; Lindvall, T.; Shwela, D.H. Guidelines for Community Noise; World Health Organization: Geneva, Switzerland, 1999; Available online: https://www.who.int/publications/i/item/a68672 (accessed on 1 July 2024).
- Stevens, S.S.; Volkman, J.; Newman, E.B. A Scale for the Measurement of the Psychological Magnitude Pitch. J. Acoust. Soc. Am. 1937, 8, 185–190. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Fein, E.C.; Gilmour, J.; Machin, T.; Hendry, L. Statistics for Research Students: An Open Access Resource with Self-Tests and Illustrative Examples; University of Southern Queensland: Brisbane, Australia, 2022; Available online: https://open.umn.edu/opentextbooks/textbooks/1191 (accessed on 1 July 2024).
- Peckel, M.; Pozzo, T.; Bigand, E. The Impact of the Perception of Rhythmic Music on Self-Paced Oscillatory Movements. Front. Psychol. 2014, 5, 1037. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. The Role of Limb Movements in Maintaining Upright Stance: The “Change-in-Support” Strategy. Phys. Ther. 1997, 77, 488–507. [Google Scholar] [CrossRef]
- Varlet, M.; Williams, R.A.; Keller, P.E. Effects of Pitch and Tempo of Auditory Rhythms on Spontaneous Movement Entrainment and Stabilisation. Psychol. Res. 2020, 84, 568–584. [Google Scholar] [CrossRef]
- Bouvet, C.J.; Varlet, M.; Bella, S.D.; Keller, P.E.; Zelic, G.; Bardy, B.G. Preferred Frequency Ratios for Spontaneous Auditory-Motor Synchronization: Dynamical Stability and Hysteresis. Acta Psychol. 2019, 196, 33–41. [Google Scholar] [CrossRef]
- Burger, B.; Thompson, M.R.; Luck, G.; Saarikallio, S.H.; Toiviainen, P. Hunting for the Beat in the Body: On Period and Phase Locking in Music-Induced Movement. Front. Hum. Neurosci. 2014, 8, 903. [Google Scholar] [CrossRef]
- Roerdink, M.; Bank, P.J.M.; Peper, C.E.; Beek, P.J. Walking to the Beat of Different Drums: Practical Implications for the Use of Acoustic Rhythms in Gait Rehabilitation. Gait Posture 2011, 33, 690–694. [Google Scholar] [CrossRef]
- Hamacher, D.; Zech, A. Development of Functional Variability during the Motor Learning Process of a Complex Cyclic Movement. J. Biomech. 2018, 77, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Repp, B.H.; Keller, P.E. Adaptation to Tempo Changes in Sensorimotor Synchronization: Effects of Intention, Attention, and Awareness. Q. J. Exp. Psychol. Sect. A 2004, 57, 499–521. [Google Scholar] [CrossRef] [PubMed]
- Cowin, J.; Nimphius, S.; Fell, J.; Culhane, P.; Schmidt, M. A Proposed Framework to Describe Movement Variability within Sporting Tasks: A Scoping Review. Sports Med. 2022, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Angelidis, A.; Solis, E.; Lautenbach, F.; Van Der Does, W.; Putman, P. I’m Going to Fail! Acute Cognitive Performance Anxiety Increases Threat-Interference and Impairs WM Performance. PLoS ONE 2019, 14, e0210824. [Google Scholar] [CrossRef] [PubMed]
- Van Der Steen, M.C.; Keller, P.E. The ADaptation and Anticipation Model (ADAM) of Sensorimotor Synchronization. Front. Hum. Neurosci. 2013, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Onishi, Y.; Pöppel, E. Two Types of Anticipation in Synchronization Tapping. Acta Neurobiol. Exp. 2004, 64, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Repp, B.H.; Su, Y.-H. Sensorimotor Synchronization: A Review of Recent Research (2006–2012). Psychon. Bull. Rev. 2013, 20, 403–452. [Google Scholar] [CrossRef]
- Guérin, S.M.R.; Boitout, J.; Delevoye-Turrell, Y.N. Attention Guides the Motor-Timing Strategies in Finger-Tapping Tasks When Moving Fast and Slow. Front. Psychol. 2021, 11, 574396. [Google Scholar] [CrossRef]
- Gagnon, K.T.; Geuss, M.N.; Stefanucci, J.K. Fear Influences Perceived Reaching to Targets in Audition, but Not Vision. Evol. Hum. Behav. 2013, 34, 49–54. [Google Scholar] [CrossRef]
- Fan, H.; Kong, L.-W.; Lai, Y.-C.; Wang, X. Anticipating Synchronization with Machine Learning. Phys. Rev. Res. 2021, 3, 023237. [Google Scholar] [CrossRef]

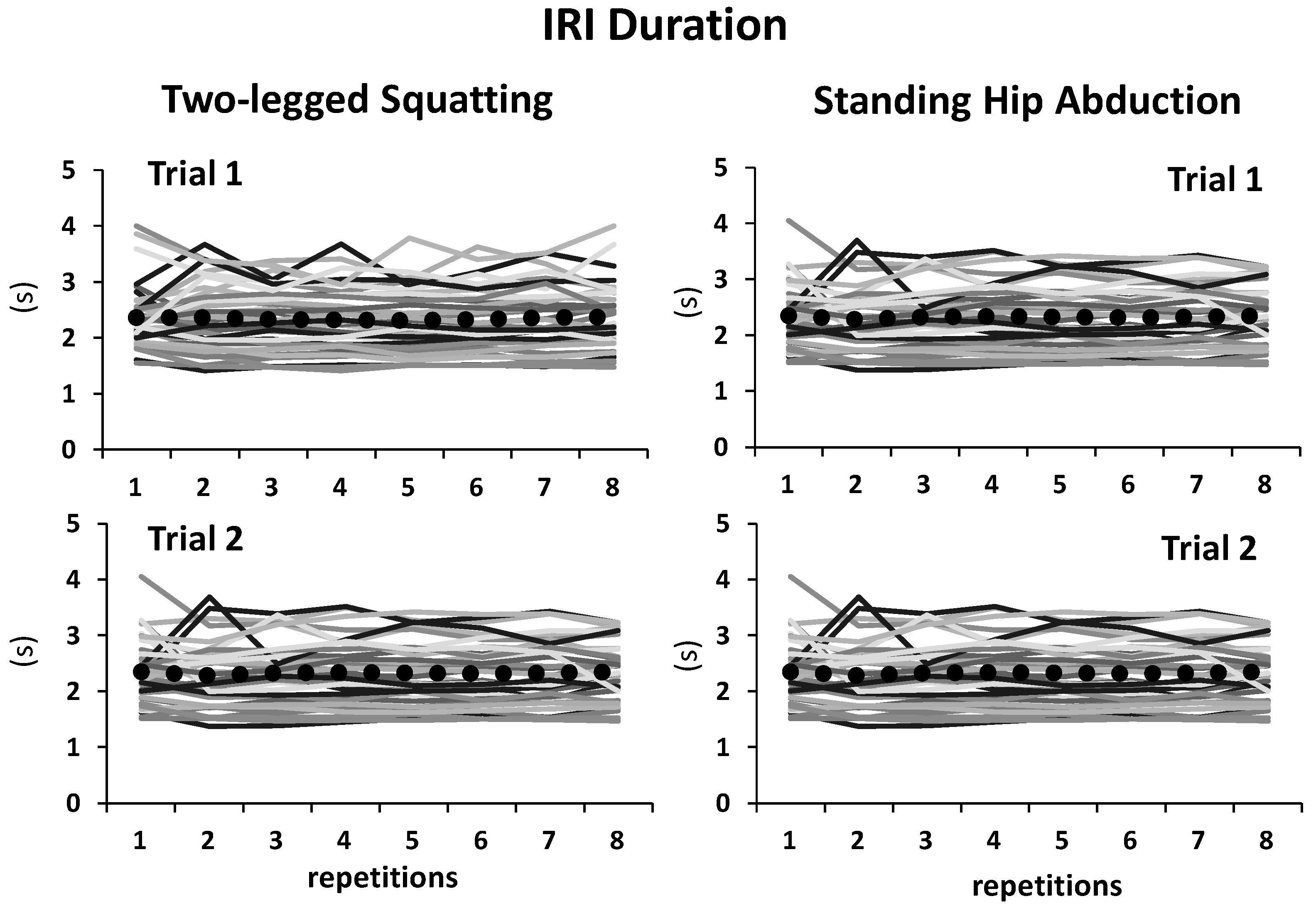
| Two-Legged Squatting | Standing Hip Abduction |
|---|---|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmanouil, A.; Boudolos, K.; Rousanoglou, E. Beyond Simple Tapping: Is Timed Body Movement Influenced When Balance Is Threatened? Appl. Sci. 2024, 14, 8541. https://doi.org/10.3390/app14188541
Emmanouil A, Boudolos K, Rousanoglou E. Beyond Simple Tapping: Is Timed Body Movement Influenced When Balance Is Threatened? Applied Sciences. 2024; 14(18):8541. https://doi.org/10.3390/app14188541
Chicago/Turabian StyleEmmanouil, Analina, Konstantinos Boudolos, and Elissavet Rousanoglou. 2024. "Beyond Simple Tapping: Is Timed Body Movement Influenced When Balance Is Threatened?" Applied Sciences 14, no. 18: 8541. https://doi.org/10.3390/app14188541







