Molecular and Enzymatic Responses of Chlorococcum dorsiventrale to Heavy Metal Exposure: Implications for Their Removal
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Isolation and Cultivation Conditions
2.2. Genomic DNA Extraction, PCR, Sequencing and Phylogenetic Analysis
2.3. Heavy Metal Exposure Conditions
2.4. Effect of Cd, Pb and Cr Ions on Growth and Biochemistry
2.4.1. Growth Measurement
2.4.2. Chlorophyll Content Measurement
2.4.3. Lipid Content Measurement
2.4.4. Protein Content Measurement
2.4.5. Carbohydrate Content Measurement
2.5. Determination of Metals Concentration
2.6. Gene Expression Analysis
2.6.1. RNA Extraction Method
2.6.2. RNA Reverse Transcription and cDNA Generation
2.6.3. Real-Time qPCR
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Statistical Analysis
3. Results
3.1. Isolation and Identification of the Microalga Strain
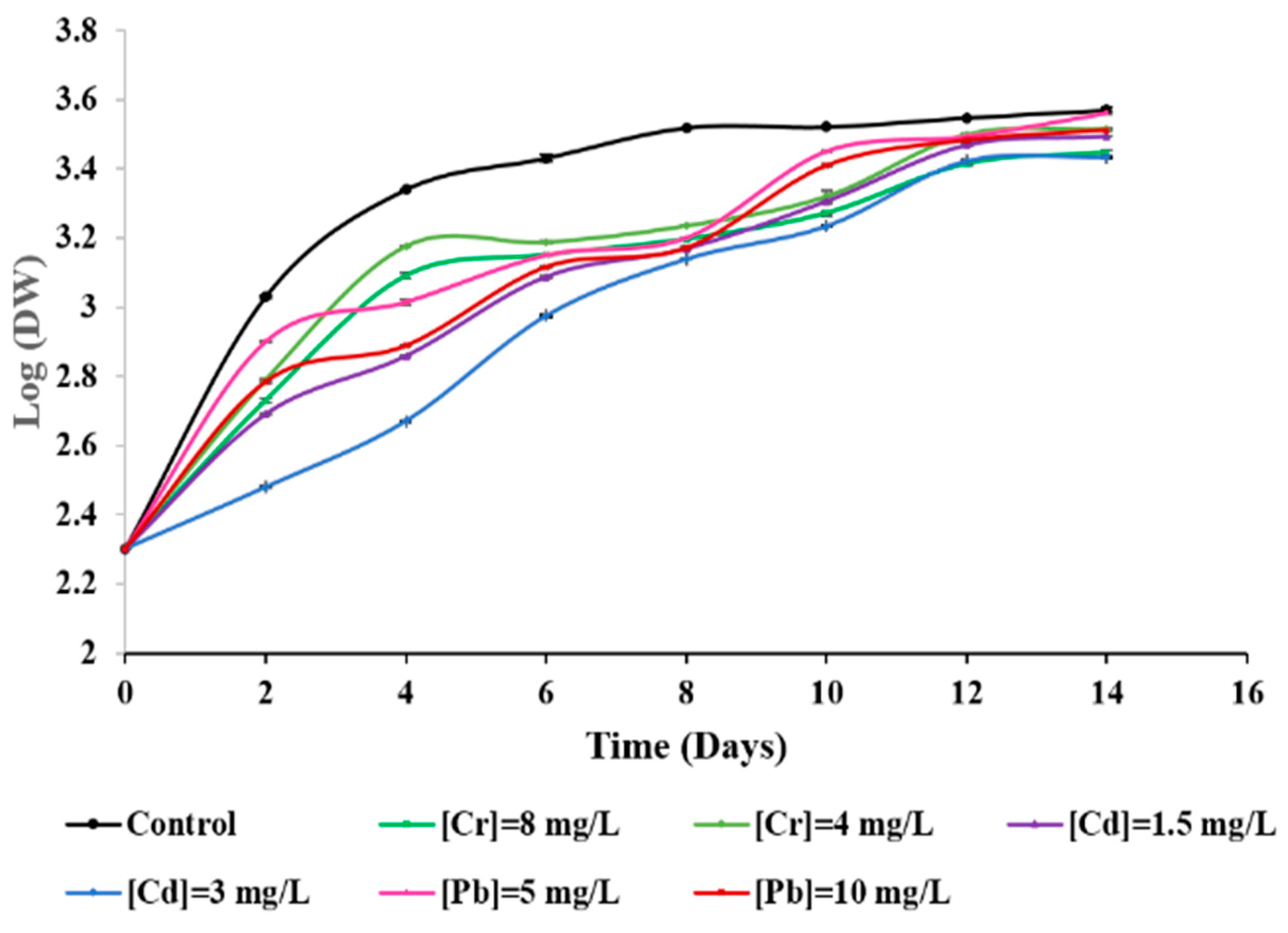
3.2. Effects of Metal Ions on the Microalga Growth
3.3. Effects of Metal Ions on Bioproducts Synthesis
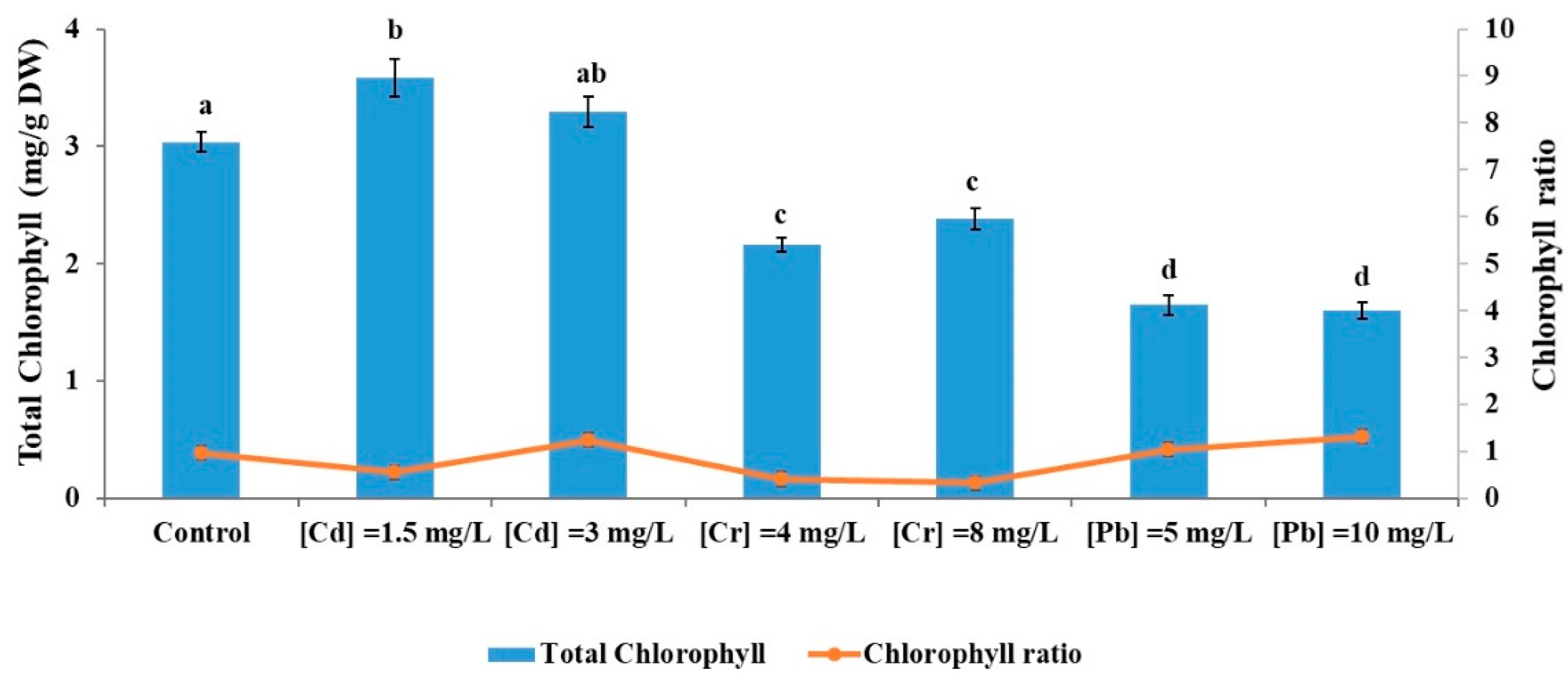
3.3.1. Impact of Metal Ions on Chlorophyll Content
3.3.2. Impact of Metal Ions on Soluble-Protein Content
3.3.3. Impact of Metal Ions on Lipid Content
3.3.4. Impact of Metal Ions on Polysaccharide Content
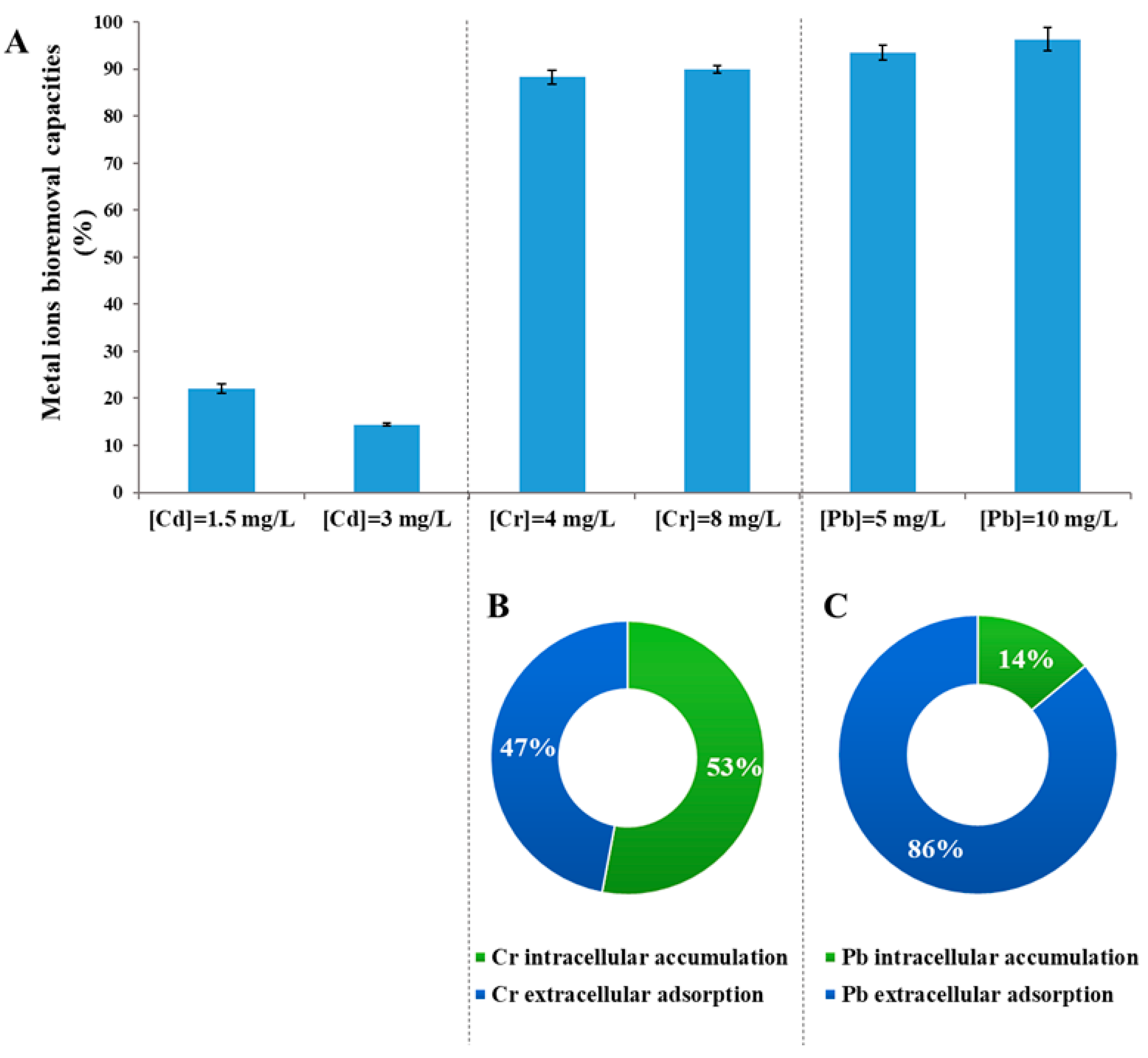
3.4. Metal Removal Rates
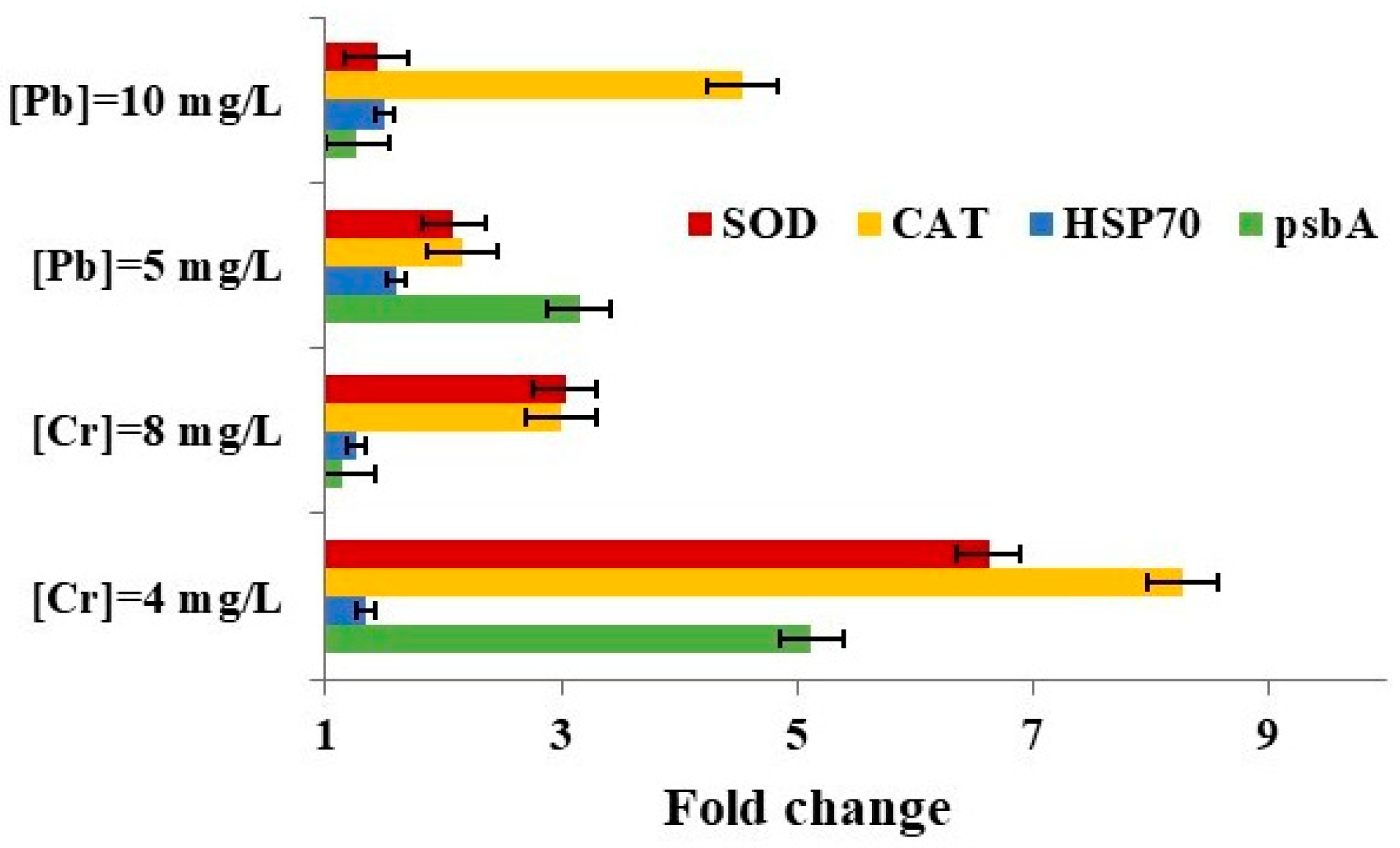
3.5. Impact of Metal Ions on Gene Expression
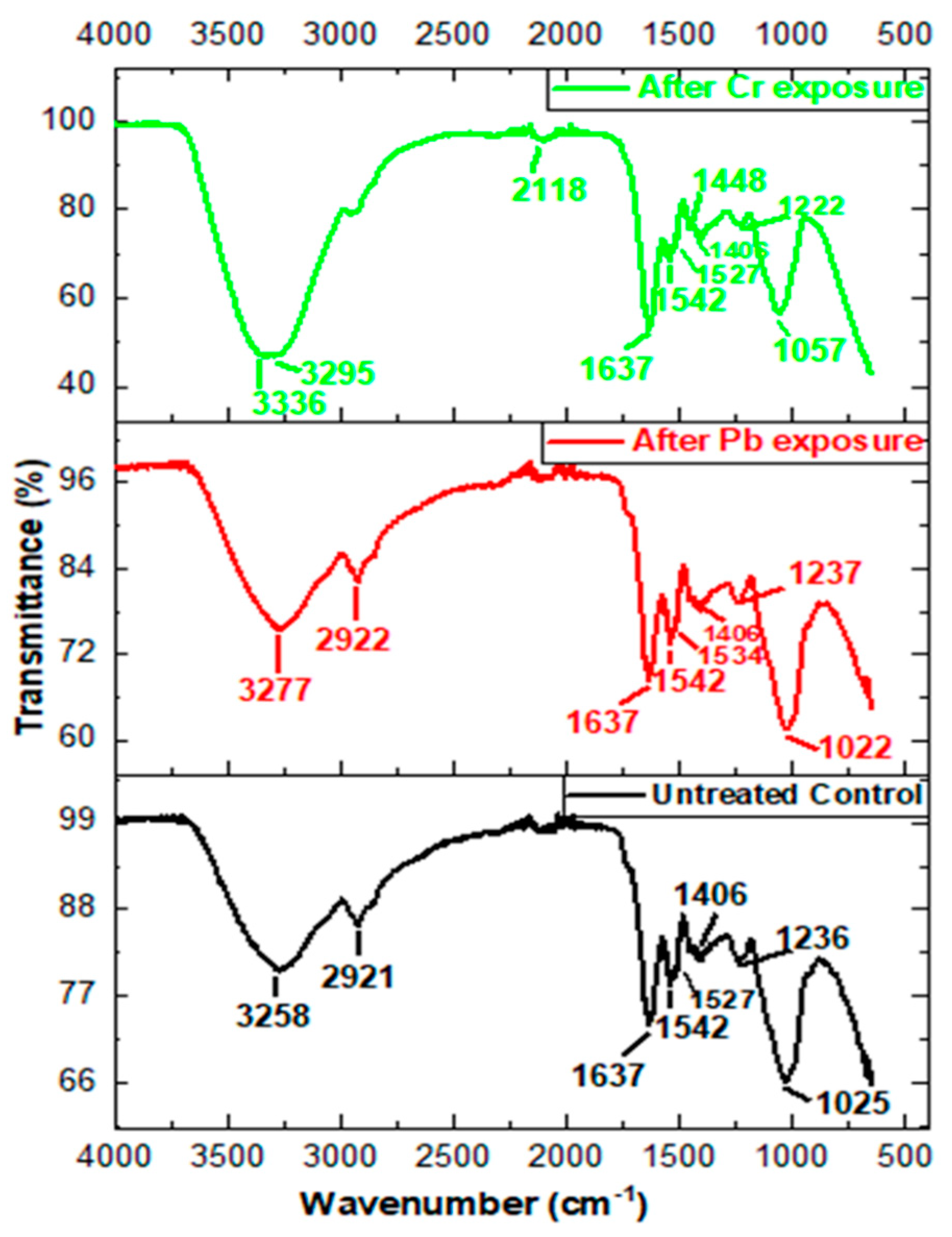
3.6. FTIR Band Assignment and Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal metallothioneins and phytochelatins and their potential use in bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, Y.; Fang, A.; Feng, D.; Du, M.; Tang, A.; Chen, S.; Lial, A. Insight into biosorption of heavy metals by extracellular polymer substances and the improvement of the efficacy: A review. Lett. Appl. Microbiol. 2022, 75, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, F.; Liang, Y.; Li, Y.; Liu, Q.; Liu, F. Diversity and functions of microbes in surface sediments under heavy metal pollution of western Chaohu Lake. Lett. Appl. Microbiol. 2022, 75, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Ali Khan, K.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Efremova, A.S.; Aaron, J.J. Toxic heavy metals: Impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ. Sci. Pollut. Res. 2020, 27, 29927–29942. [Google Scholar] [CrossRef]
- Aendo, P.; Netvichian, R.; Thiendedsakul, P.; Khaodhiar, S.; Tulayaku, P. Carcinogenic risk of Pb, Cd, Ni, and Cr and critical ecological risk of Cd and Cu in soil and groundwater around the municipal solid waste open dump in central Thailand. J. Environ. Public Health 2022, 2022, 3062215. [Google Scholar] [CrossRef]
- Ayele, A.; Haile, M.; Setegn, S.; Alemu, H.; Digafe, S.; Kamaraj, M. Comparative utilization of dead and live fungal biomass for the removal of heavy metal: A concise review. Sci. World J. 2021, 2021, 5588111. [Google Scholar] [CrossRef]
- Cao, J.; Xie, C.; Hou, Z. Ecological evaluation of heavy metal pollution in the soil of Pb-Zn mines. Ecotoxicology 2022, 31, 259–270. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, M.; Liu, Q.; Zhang, Z.; Fei, X.; Xiao, R.; Lv, X. Contamination assessment, health risk evaluation, and source identification of heavy metals in the soil-rice system of typical agricultural regions on the Southeast Coast of China. Environ. Sci. Pollut. Res. 2021, 28, 12870–12880. [Google Scholar] [CrossRef]
- Carmona, B.; Abejón, R. Innovative membrane technologies for the treatment of wastewater polluted with heavy metals: Perspective of the potential of electrodialysis, membrane distillation, and forward osmosis from a bibliometric analysis. Membranes 2023, 13, 385. [Google Scholar] [CrossRef]
- Chaemiso, T.D.; Nefo, T. Removal methods of heavy metals from laboratory wastewater. J. Nat. Sci. Res. 2019, 9, 36–42. [Google Scholar] [CrossRef]
- Sarma, U.; Hoque, M.E.; Thekkangil, A.; Venkatarayappa, N.; Rajagopal, S. Microalgae in removing heavy metals from wastewater—An advanced green technology for urban wastewater treatment. J. Hazard. Mater. Adv. 2024, 15, 100444. [Google Scholar] [CrossRef]
- Chen, Z.; Osman, A.I.; Rooney, D.W.; Oh, W.-D.; Yap, P.-S. Remediation of heavy metals in polluted water by immobilized algae: Current applications and future perspectives. Sustainability 2023, 15, 5128. [Google Scholar] [CrossRef]
- Chugh, M.; Kumar, L.; Shah, M.P.; Bharadvaja, N. Algal bioremediation of heavy metals: An insight into removal mechanisms, recovery of by-products, challenges, and future opportunities. Energy Nexus 2022, 7, 100129. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef]
- Elleuch, J.; Hmani, R.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci. Total Environ. 2021, 799, 149464. [Google Scholar] [CrossRef]
- Ben Mohamed, J.; Elleuch, J.; Drira, M.; Esteban, M.Á.; Michaud, P.; Abdelkafi, S.; Fendri, I. Characterization and biotechnological potential of two native marine microalgae isolated from the Tunisian coast. Appl. Sci. 2021, 11, 5295. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef]
- Vidya, D.; Nayana, K.; Sreelakshmi, M.; Keerthi, K.V.; Sneha Mohan, K.; Sudhakar, M.P.; Arunkumar, K. A sustainable cultivation of microalgae using dairy and fish wastes for enhanced biomass and bio-product production. Biomass Convers. Biorefinery 2023, 13, 6859–6873. [Google Scholar] [CrossRef]
- Thabet, J.; Elleuch, J.; Martínez, F.; Abdelkafi, S.; Hernández, L.E.; Fendri, I. Characterization of cellular toxicity induced by sub-lethal inorganic mercury in the marine microalgae Chlorococcum dorsiventrale isolated from a metal-polluted coastal site. Chemosphere 2023, 338, 139391. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Interaction effects of polycyclic aromatic hydrocarbons and heavy metals on a soil microalga, Chlorococcum sp. MM11. Environ. Sci. Pollut. Res. 2015, 22, 8876–8889. [Google Scholar] [CrossRef]
- Nam, S.-H.; Kim, D.; An, S.; An, Y.-J. Validation of the paper-disc soil method using soil alga Chlorococcum infusionum to quantitatively determine the toxicity of heavy metals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 258, 109380. [Google Scholar] [CrossRef]
- Liyanage, L.M.M.; Lakmali, W.G.M.; Athukorala, S.N.P.; Jayasundera, K.B. Application of live Chlorococcum aquaticum biomass for the removal of Pb(II) from aqueous solutions. J. Appl. Phycol. 2020, 32, 4069–4080. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Nasopoulou, C.; Zabetakis, I.; Murray, P.; Saha, S.K. Bioactive lipids of marine microalga Chlorococcum sp. SABC 012504 with anti-inflammatory and anti-thrombotic activities. Mar. Drugs 2021, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, J.; Ben Amor, F.; Chaaben, Z.; Frikha, F.; Michaud, P.; Fendri, I.; Abdelkafi, S. Zinc biosorption by Dunaliella sp. AL-1: Mechanism and effects on cell metabolism. Sci. Total Environ. 2021, 773, 145024. [Google Scholar] [CrossRef]
- Mehra, A.; Zafar, S.U.; Jutur, P.P. Optimization of biomass production by Chlorella saccharophila UTEX 247 employing response surface methodology. Biomass Convers. Biorefinery 2024, 14, 8549–8561. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Manzi, H.P.; Yang, Q.; Guo, Z.; Zheng, Y.; Liu, X.; Salama, E.S. Isolation and screening of Tetradesmus dimorphus and Desmodesmus asymmetricus from natural habitats in Northwestern China for clean fuel production and N, P removal. Biomass Convers. Biorefinery 2022, 12, 1503–1512. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer US: New York City, NY, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Baccari, O.; Elleuch, J.; Barkallah, M.; Boukedi, H.; Ayed, N.B.; Hammami, A.; Fendri, I.; Abdelkafi, S. Development of a new TaqMan-based PCR assay for the specific detection and quantification of Simkania negevensis. Mol. Cell. Probes 2020, 53, 101645. [Google Scholar] [CrossRef]
- Ben Amor, F.; Elleuch, F.; Ben Hlima, H.; Garnier, M.; Saint-Jean, B.; Barkallah, M.; Pichon, C.; Abdelkafi, S.; Fendri, I. Proteomic analysis of the chlorophyta Dunaliella new strain AL-1 revealed global changes of metabolism during high carotenoid production. Mar. Drugs 2017, 15, 293. [Google Scholar] [CrossRef]
- Elleuch, J.; Tounsi, S.; Belguith Ben Hassen, N.; Noël Lacoix, M.; Chandre, F.; Jaoua, S.; Zribi Zghal, R. Characterisation of novel Bacillus thuringiensis isolates against Aedes aegypti (Diptera: Culicidae) and Ceratitis capitata (Diptera: Tephridae). J. Invertebr. Pathol. 2015, 124, 90–97. [Google Scholar] [CrossRef]
- Ahmad, S.; Pandey, A.; Pathak, V.V.; Tyagi, V.V.; Kothari, R. Phycoremediation: Algae as eco-friendly tools for the removal of heavy metals from wastewaters. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Springer: Singapore, 2020; pp. 53–76. [Google Scholar] [CrossRef]
- Cameron, H.; Mata, M.T.; Riquelme, C. The effect of heavy metals on the viability of Tetraselmis marina AC16-MESO and an evaluation of the potential use of this microalga in bioremediation. PeerJ 2018, 6, e5295. [Google Scholar] [CrossRef] [PubMed]
- Duque, D.; Montoya, C.; Botero, L.R. Cadmium (Cd) tolerance evaluation of three strains of microalgae of the genus Ankistrodesmus, Chlorella, and Scenedesmus. Rev. Fac. Ing. Univ. Antioq. 2019, 92, 60–69. [Google Scholar] [CrossRef]
- Elleuch, J.; HadjKacem, F.; Ben Amor, F.; Hadrich, B.; Michaud, P.; Fendri, I.; Abdelkafi, S. Extracellular neutral protease from Arthrospira platensis: Production, optimization and partial characterization. Int. J. Biol. Macromol. 2021, 167, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Luo, S.; Fan, X.; Yang, Z.; Guo, R. Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2. Appl. Energy 2011, 88, 3336–3341. [Google Scholar] [CrossRef]
- Rugnini, L.; Costa, G.; Congestri, R.; Bruno, L. Testing of two different strains of green microalgae for Cu and Ni removal from aqueous media. Sci. Total Environ. 2017, 601–602, 959–967. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls a and B of leaf extracts in different solvents. In Advances in Photosynthesis Research; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 2, pp. 1–12. [Google Scholar] [CrossRef]
- Kumar, P.; Ramakritinan, C.M.; Kumaraguru, A.K. Solvent extraction and spectrophotometric determination of pigments of some algal species from the shore of Puthumadam, Southeast Coast of India. Int. J. Ocean. Oceanogr. 2010, 4, 29–34. Available online: https://www.researchgate.net/publication/229148336 (accessed on 5 August 2024).
- Folch, J.; Lees, M.; Sloan-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fendri, I.; Chaari, A.; Dhouib, A.; Jlassi, B.; Abousalham, A.; Carrière, F.; Sayadi, S.; Abdelkafi, S. Isolation, identification and characterization of a new lipolytic Pseudomonas sp., strain AHD-1, from Tunisian soil. Environ. Technol. 2010, 31, 87–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Elleuch, J.; Zghal, R.Z.; Jemaà, M.; Azzouz, H.; Tounsi, S.; Jaoua, S. New Bacillus thuringiensis toxin combinations for biological control of lepidopteran larvae. Int. J. Biol. Macromol. 2014, 65, 148–154. [Google Scholar] [CrossRef]
- Elleuch, J.; Drira, M.; Ghribi, I.; Hadjkacem, F.; Pierre, G.; Khemakhem, H.; Abdelkafi, S. Lead removal from the aqueous solution by extracellular polymeric substances produced by the marine diatom Navicula salinicola. Environ. Technol. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Toni, L.S.; Garcia, A.M.; Jeffrey, D.A.; Jiang, X.; Stauffer, B.L.; Miyamoto, S.D.; Sucharov, C.C. Optimization of phenol-chloroform RNA extraction. Methods 2018, 5, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Derbel, H.; Elleuch, J.; Tounsi, L.; Nicolo, M.S.; Rizzo, M.G.; Michaud, P.; Fendri, I.; Abdelkafi, S. Improvement of biomass and phycoerythrin production by a strain of Rhodomonas sp. isolated from the Tunisian coast of Sidi Mansour. Biomolecules 2022, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Drira, M.; Elleuch, J.; Hadjkacem, F.; Hentati, F.; Drira, R.; Pierre, G.; Gardarin, C.; Delattre, C.; El Alaoui-Talibi, Z.; El Modafar, C.; et al. Influence of the sulfate content of the exopolysaccharides from Porphyridium sordidum on their elicitor activities on date palm vitroplants. Plant Physiol. Biochem. 2022, 186, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, F.; Ben Hlima, H.; Barkallah, M.; Baril, P.; Abdelkafi, S.; Pichon, C.; Fendri, I. Carotenoids overproduction in Dunaliella sp.: Transcriptional changes and new insights through lycopene β cyclase regulation. Appl. Sci. 2019, 9, 5389. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.H.; Hu, L.D.; Zhang, J.Q.; Jiang, Y.; Yang, Y.; Yan, Y.B. DsCaf1 is involved in environmental stress response of Dunaliella salina. Int. J. Biol. Macromol. 2016, 82, 369–374. [Google Scholar] [CrossRef]
- Li, M.; Hu, C.; Zhu, Q.; Chen, L.; Kong, Z.; Liu, Z. Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere 2006, 62, 565–572. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef]
- Vo, M.T.; Nguyen, V.T.; Dao, T.S. Responses of green algae and diatom upon exposure to chromium and cadmium. Vietnam J. Sci. Technol. Eng. 2020, 62, 69–73. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, H.; Guo, Q.; Zhang, Z.; Zhao, J.; Yang, D. Effects of cadmium stress on growth and amino acid metabolism in two compositae plants. Ecotoxicol. Environ. Saf. 2018, 158, 300–308. [Google Scholar] [CrossRef]
- Soeprobowati, T.R.; Hariyati, R. Phycoremediation of Pb, Cd, Cu, and Cr by Chaetoceros calcitrans (Paulsen) Takano. Int. J. Adv. Chem. Eng. Biol. Sci. 2014, 1, 37–40. [Google Scholar] [CrossRef]
- Lamai, C.; Kruatrachue, M.; Pokethitiyook, P.; Upatham, E.S.; Soonthornsarathool, V. Toxicity and accumulation of lead and cadmium in the filamentous green alga Cladophora fracta (O.F. Muller ex Vahl) Kutzing: A laboratory study. Sci. Asia 2005, 31, 121–127. [Google Scholar] [CrossRef]
- Elleuch, J.; Thabet, J.; Ghribi, I.; Jabeur, H.; Hernández, L.E.; Fendri, I.; Abdelkafi, S. Responses of Dunaliella sp. AL-1 to chromium and copper: Biochemical and physiological studies. Chemosphere 2024, 364, 143133. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.K.; Singh, L.; Singh, R.; Singh, D.P.; Saxena, G. Bioaccumulation and toxicity of As in the alga Chlorococcum sp.: Prospects for As bioremediation. Bull. Environ. Contam. Toxicol. 2022, 108, 500–506. [Google Scholar] [CrossRef]
- Ajitha, V.; Sreevidya, C.P.; Sarasan, M.; Park, J.C.; Mohandas, A.; Singh, I.S.; Puthumana, J.; Lee, J.S. Effects of zinc and mercury on ROS-mediated oxidative stress-induced physiological impairments and antioxidant responses in the microalga Chlorella vulgaris. Environ. Sci. Pollut. Res. 2021, 28, 32475–32492. [Google Scholar] [CrossRef]
- Čypaitė, A.; Žaltauskaitė, J.; Venclovienė, J. Assessment of chlorophyll-a, chlorophyll-b and growth rate in freshwater green algae Pseudokirchneriella subcapitata exposed to cadmium and copper. In Proceedings of the 9th International Conference Environmental Engineering (9th ICEE)—Selected Papers, Vilnius, Lithuania, 22–23 May 2014. [Google Scholar] [CrossRef]
- Zamani-Ahmadmahmoodi, R.; Malekabadi, M.B.; Rahimi, R.; Johari, S.A. Aquatic pollution caused by mercury, lead, and cadmium affects cell growth and pigment content of marine microalga Nannochloropsis oculata. Environ. Monit. Assess. 2020, 192, 330. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Cao, F.; Zhang, J.; Zhao, F.; Li, C.; Wang, C. Toxic effects of boscalid on the growth, photosynthesis, antioxidant system and metabolism of Chlorella vulgaris. Environ. Pollut. 2018, 242, 171–181. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Yu, L.; Han, B.; Li, T.; Yu, X. Influence of cadmium stress on the lipid production and cadmium bioresorption by Monoraphidium sp. QLY-1. Energy Convers. Manag. 2019, 188, 76–85. [Google Scholar] [CrossRef]
- Dao, L.H.T.; Beardall, J. Effects of lead on two green microalgae Chlorella and Scenedesmus: Photosystem II activity and heterogeneity. Algal Res. 2016, 16, 150–159. [Google Scholar] [CrossRef]
- Chia, M.A.; Chimdirim, P.K.; Japhet, W.S. Lead induced antioxidant response and phenotypic plasticity of Scenedesmus quadricauda (Turp.) de Brébisson under different nitrogen concentrations. J. Appl. Phycol. 2015, 27, 293–302. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Baouchi, A.; El Arroussi, H. Heavy metals phycoremediation using tolerant green microalgae: Enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J. Environ. Chem. Eng. 2020, 8, 104460. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jayaraj, M.; Manikandan, R.; Geetha, N.; Rene, E.R.; Sharma, N.C.; Sahi, S.V. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: A physiochemical analysis. Plant Physiol. Biochem. 2017, 110, 59–69. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants—Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: A review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef] [PubMed]
- Kós, P.B.; Deák, Z.; Cheregi, O.; Vass, I. Differential regulation of psbA and psbD gene expression, and the role of the different D1 protein copies in the cyanobacterium Thermosynechococcus elongatus BP-1. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 74–83. [Google Scholar] [CrossRef]
- Mu, W.; Chen, Y.; Liu, Y.; Pan, X.; Fan, Y. Toxicological effects of cadmium and lead on two freshwater diatoms. Environ. Toxicol. Pharmacol. 2018, 59, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.S. Heat shock protein genes in the green alga Tetraselmis suecica and their role against redox and non-redox active metals. Eur. J. Protistol. 2019, 69, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Bierkens, J.G. Applications and pitfalls of stress-proteins in biomonitoring. Toxicology 2000, 153, 61–72. [Google Scholar] [CrossRef]
- Köhler, H.R.; Eckwert, H.; Triebskorn, R.; Bengtsson, G. Interaction between tolerance and 70 kDa stress protein (hsp70) induction in collembolan populations exposed to long-term metal pollution. Appl. Soil Ecol. 1999, 11, 43–52. [Google Scholar] [CrossRef]
- Rößner, P.; Binková, B.; Šrám, R.J. Heat shock proteins hsp32 and hsp70 as biomarkers of an early response? In vitro induction of heat shock proteins after exposure of cell culture to carcinogenic compounds and their real mixtures. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2003, 542, 105–116. [Google Scholar] [CrossRef]
- Al-Enazi, N.M. Biosorption of Pb2+ from water solution by using Chroococcus minutus and Chlorococcum aegyptiacum as biosorbents. Int. J. Pharm. Res. Allied Sci. 2017, 6, 247–254. [Google Scholar]
- Alharbi, R.M. Toxicity and bioaccumulation of lead, cadmium and zinc in Chroococcus minutus and Chlorococcum aegyptiacum. Int. J. Pharm. Res. Allied Sci. 2017, 6, 290–300. [Google Scholar]
- Shaker, I.M.; Islam, M.; Ahmed, M.H.; Salama, M.M. Potentiality of Chroococcus minutus (Cyanophyta) and Chlorella vulgaris (Chlorophyta) isolated from Manzala Lake for bioremediation of cadmium and lead. Int. J. Pharm. Res. Allied Sci. 2019, 12, 207–230. [Google Scholar]

| Gene Abbreviation | Description | Primer Sequence (F = Forward; R = Reverse) | References |
|---|---|---|---|
| SOD | Superoxide dismutase | F: 5′ CGCATGATCCTTTGGCTTCG 3′ R: 5′ TCCTGGTTGGCTGTGGTTTC 3′ | [16] |
| CAT | Catalase | F: 5′ TCCTGTTATCGTTCGTTTCTCA 3′ R: 5′ CAAAGTTCCCCTCTCTGGTGTA 3′ | [16] |
| psbA | Photosystem II protein D1 | F: 5′ ACTTCTTCTTAGCTGCTTGGC 3′ R: 5′ GACCTTGTGAGTCTACTACTG 3′ | This study |
| HSP70 | Heat shock protein 70 | F: 5′ TGCAGGCTGGTGTGCTGTCT 3′ R: 5′ AGGGTGGTGTTGCGGGTGAT 3′ | [48] |
| β-TUB | Beta tubulin | F: 5′ ATCTGCTTCCGCACCCTGAA 3′ R: 5′ AGCCGACCATGAAGAAGTGC 3′ | This study |
| 18S | 18S RNA | F: 5′ TTGGGTAGTCGGGCTGGTC 3′ R: 5′ CGCTGCGTTCTTCATCGTT 3′ | [49] |
| Metal Ion | Concentration (mg/L) | Growth Rates μ (Day−1) |
|---|---|---|
| Control | 0 | 0.2090 ±0.0033 a* |
| Cd | 1.5 | 0.1962 ±0.0033 c |
| 3 | 0.1862 ±0.0006 d | |
| Cr | 4 | 0.1999 ±0.0004 b |
| 8 | 0.1883 ±0.0011 d | |
| Pb | 5 | 0.2073 ±0.0011 a |
| 10 | 0.1992 ±0.0002 b |
| Untreated Control (cm−1) | After Pb Exposure (cm−1) | After Cr Exposure (cm−1) | Wavenumber Range (cm−1) | Functional Groups a |
|---|---|---|---|---|
| 3258 | 3266 | 3295 | 3639–3029 | υO-H/υ N-H |
| 2921 | 2922 | − | 3012–2809 | υas CH2 |
| − | − | 2118 | 2270–1950 | X=C=Y |
| 1637 | 1637 | 1637 | 1680–1600 | υC=O |
| 1542 | 1542 | 1542 | 1545–1540 | δ N-H, υC-N |
| − | − | 1448 | 1460–1420 | CH, C=O |
| 1406 | 1406 | 1406 | 1425–1330 | υ COO |
| 1236 | 1237 | 1222 | 1356–1191 | υas P=O, S=O |
| 1025 | 1022 | 1057 | 1072–980 | υ C-O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hmani, R.; Elleuch, J.; Elleuch, F.; Drira, M.; Michaud, P.; Aleya, L.; Abdelkafi, S.; Fendri, I. Molecular and Enzymatic Responses of Chlorococcum dorsiventrale to Heavy Metal Exposure: Implications for Their Removal. Appl. Sci. 2024, 14, 8551. https://doi.org/10.3390/app14188551
Hmani R, Elleuch J, Elleuch F, Drira M, Michaud P, Aleya L, Abdelkafi S, Fendri I. Molecular and Enzymatic Responses of Chlorococcum dorsiventrale to Heavy Metal Exposure: Implications for Their Removal. Applied Sciences. 2024; 14(18):8551. https://doi.org/10.3390/app14188551
Chicago/Turabian StyleHmani, Rihab, Jihen Elleuch, Fatma Elleuch, Marwa Drira, Philippe Michaud, Lotfi Aleya, Slim Abdelkafi, and Imen Fendri. 2024. "Molecular and Enzymatic Responses of Chlorococcum dorsiventrale to Heavy Metal Exposure: Implications for Their Removal" Applied Sciences 14, no. 18: 8551. https://doi.org/10.3390/app14188551









