Rock Wettability Alteration Induced by the Injection of Various Fluids: A Review
Abstract
:1. Introduction
2. Wettability Measurement Methods
2.1. Methods Based on Spontaneous Absorption of Liquid by Rock and Capillary Pressure
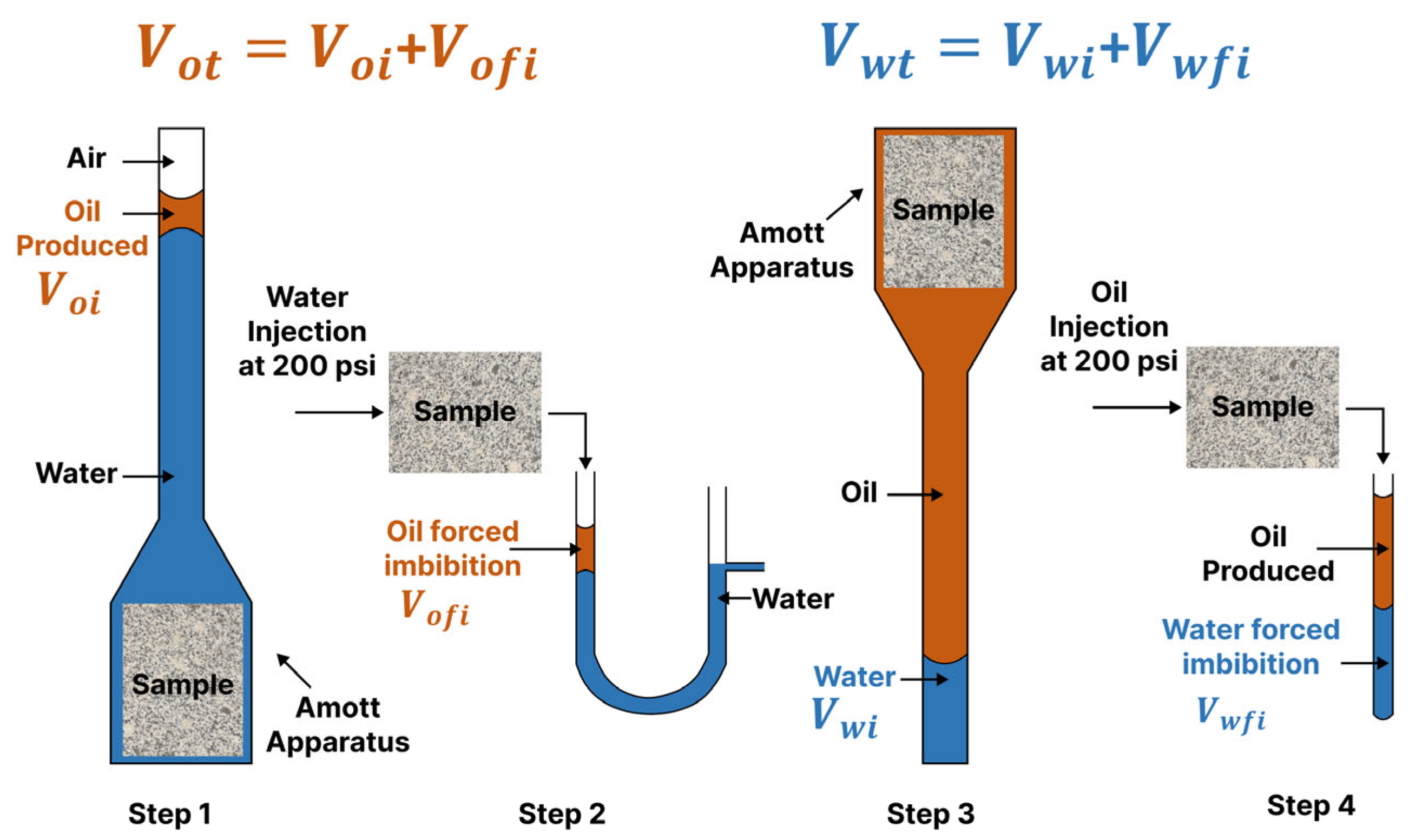
2.1.1. Amott–Harvey Test
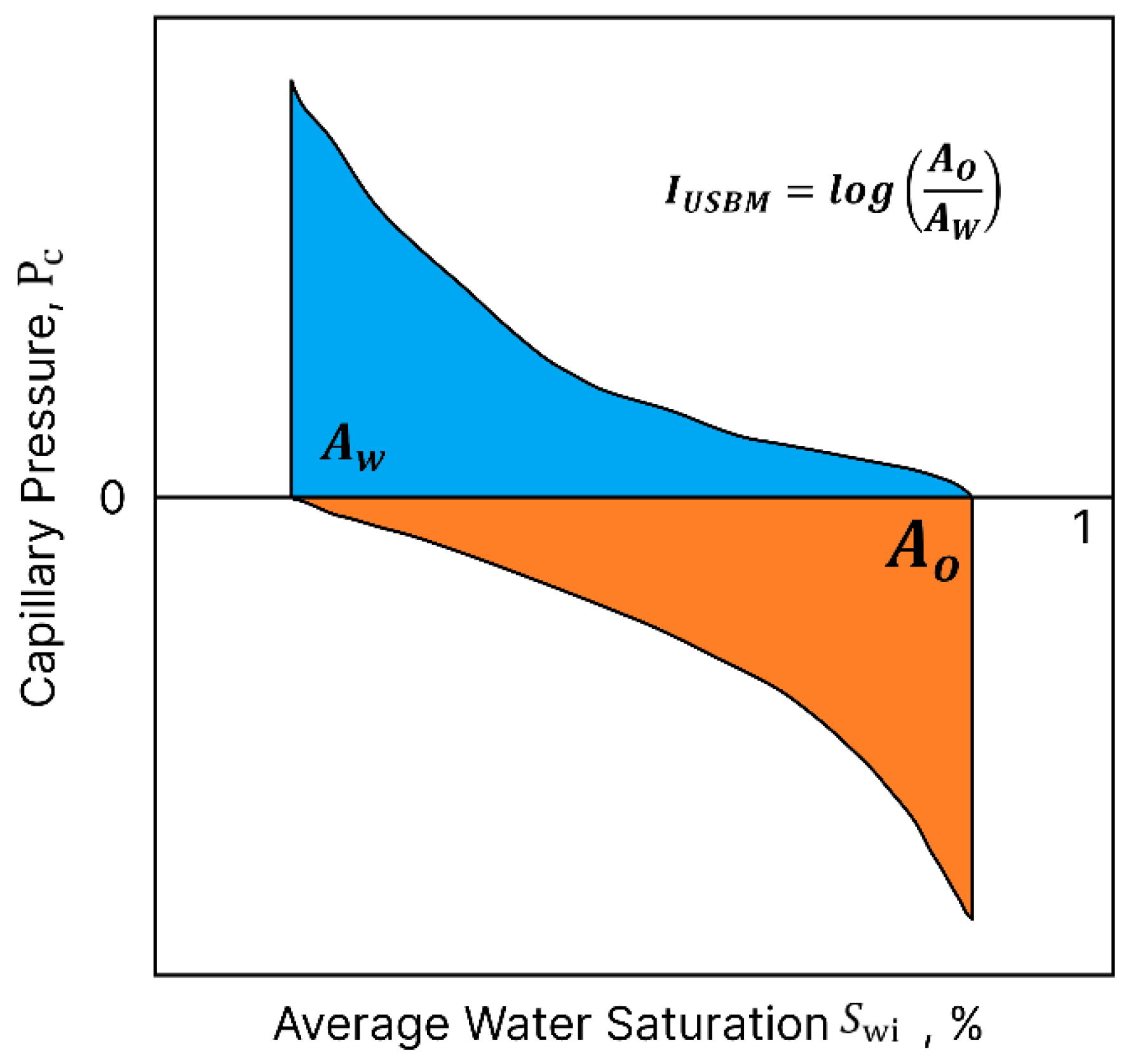
2.1.2. United States Bureau of Mines (USBM) Method
2.1.3. Flotation Test
2.2. Methods Based on Contact Angle Measurement
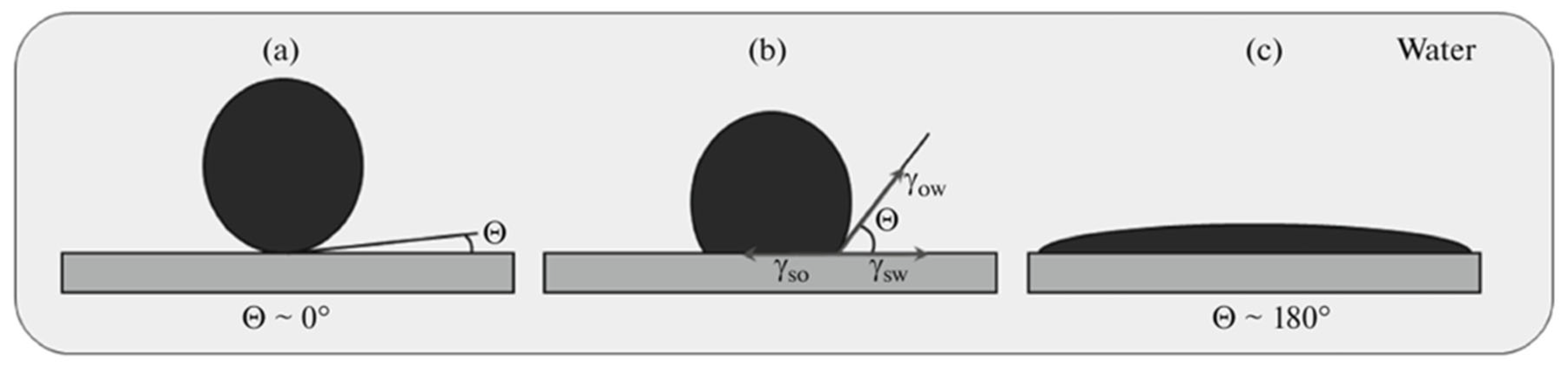
2.2.1. Visual Contact Angle Measurement Method
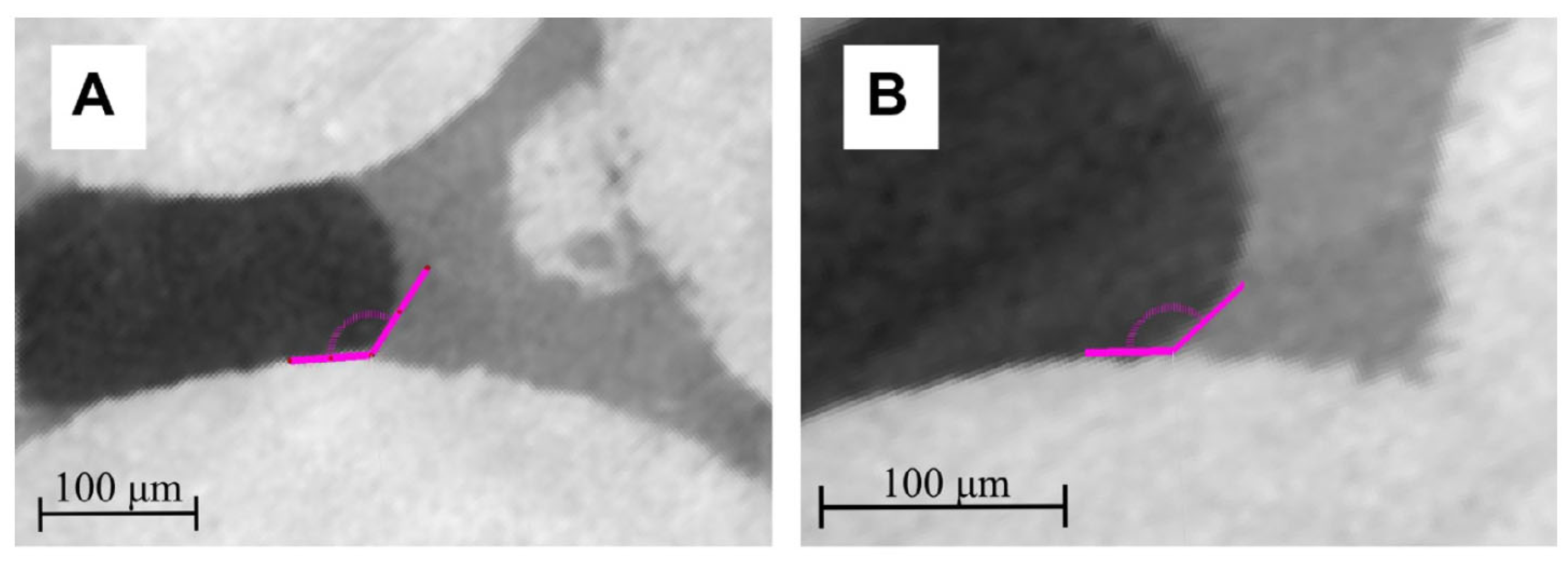
2.2.2. Contact Angle Measurement Using micro-CT
2.2.3. Contact Angle Measurement Using SEM
2.3. Methods That Consider the Characteristics of Liquid Absorption
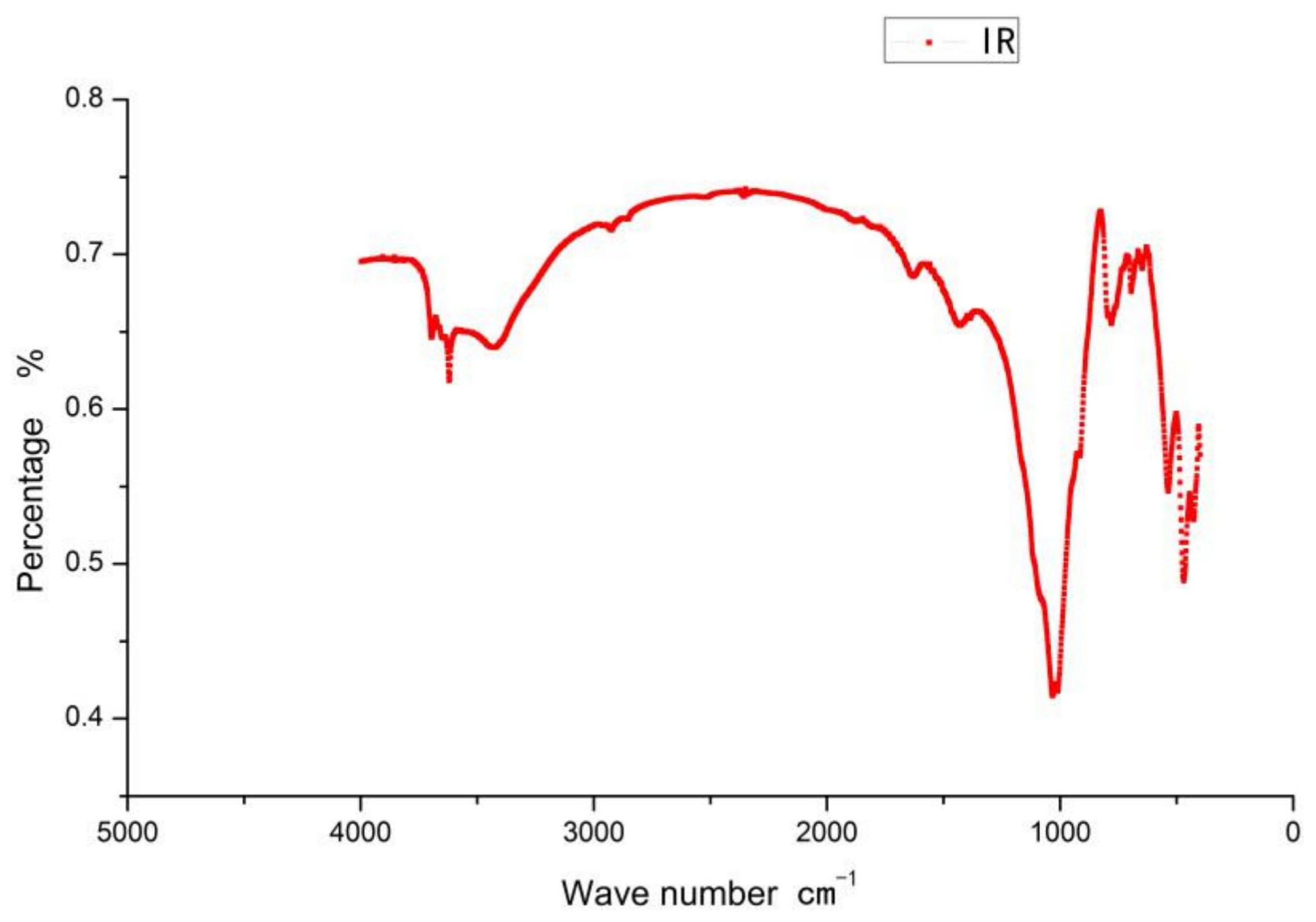
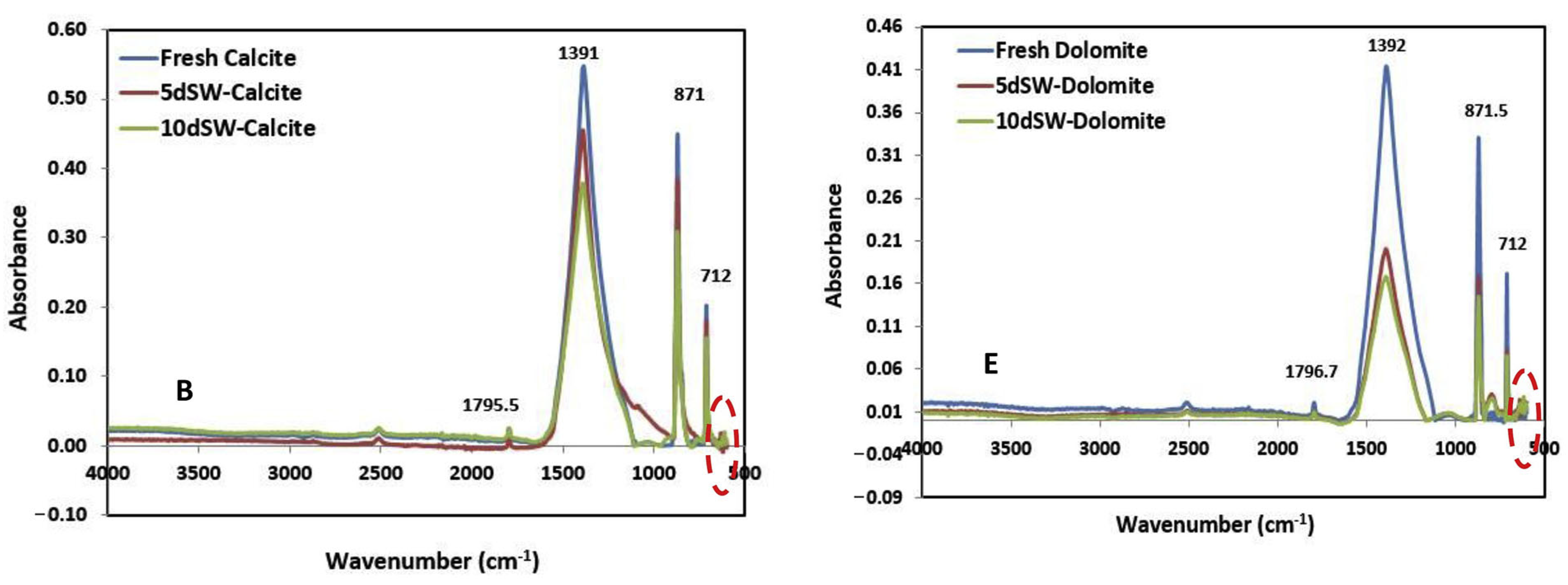
2.3.1. Infrared Spectroscopy (IR)
2.3.2. Nuclear Magnetic Resonance (NMR)
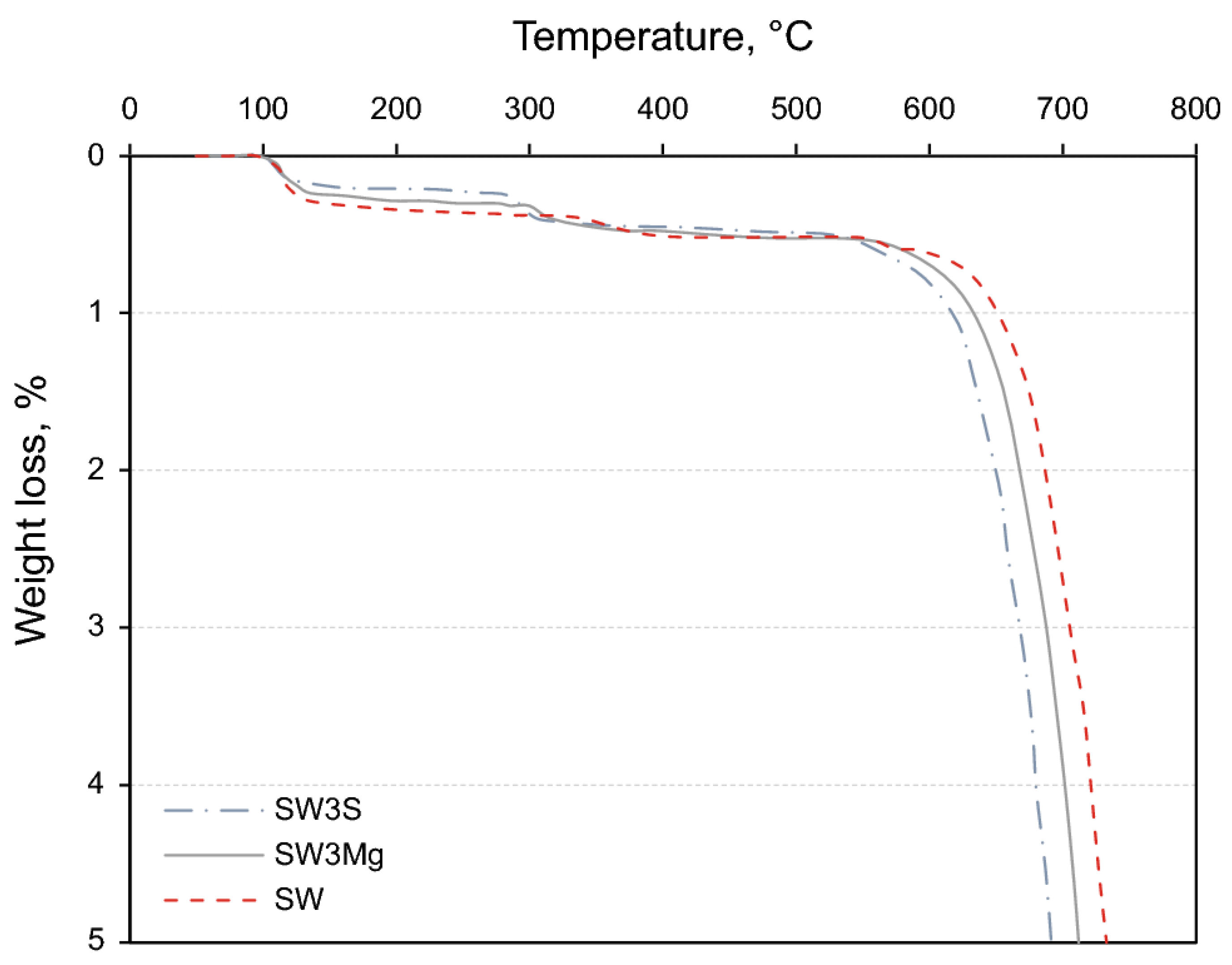
2.3.3. Thermogravimetric Analysis (TGA)
2.4. Analytical Methods
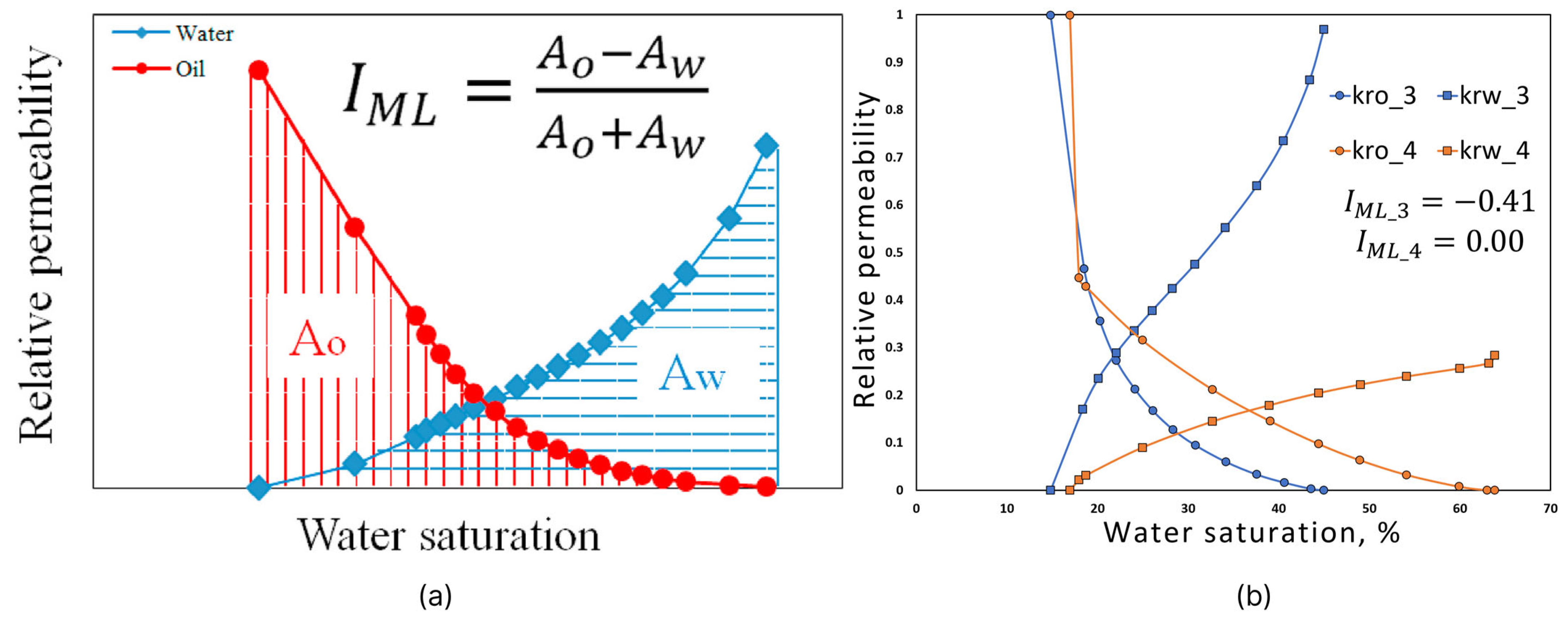
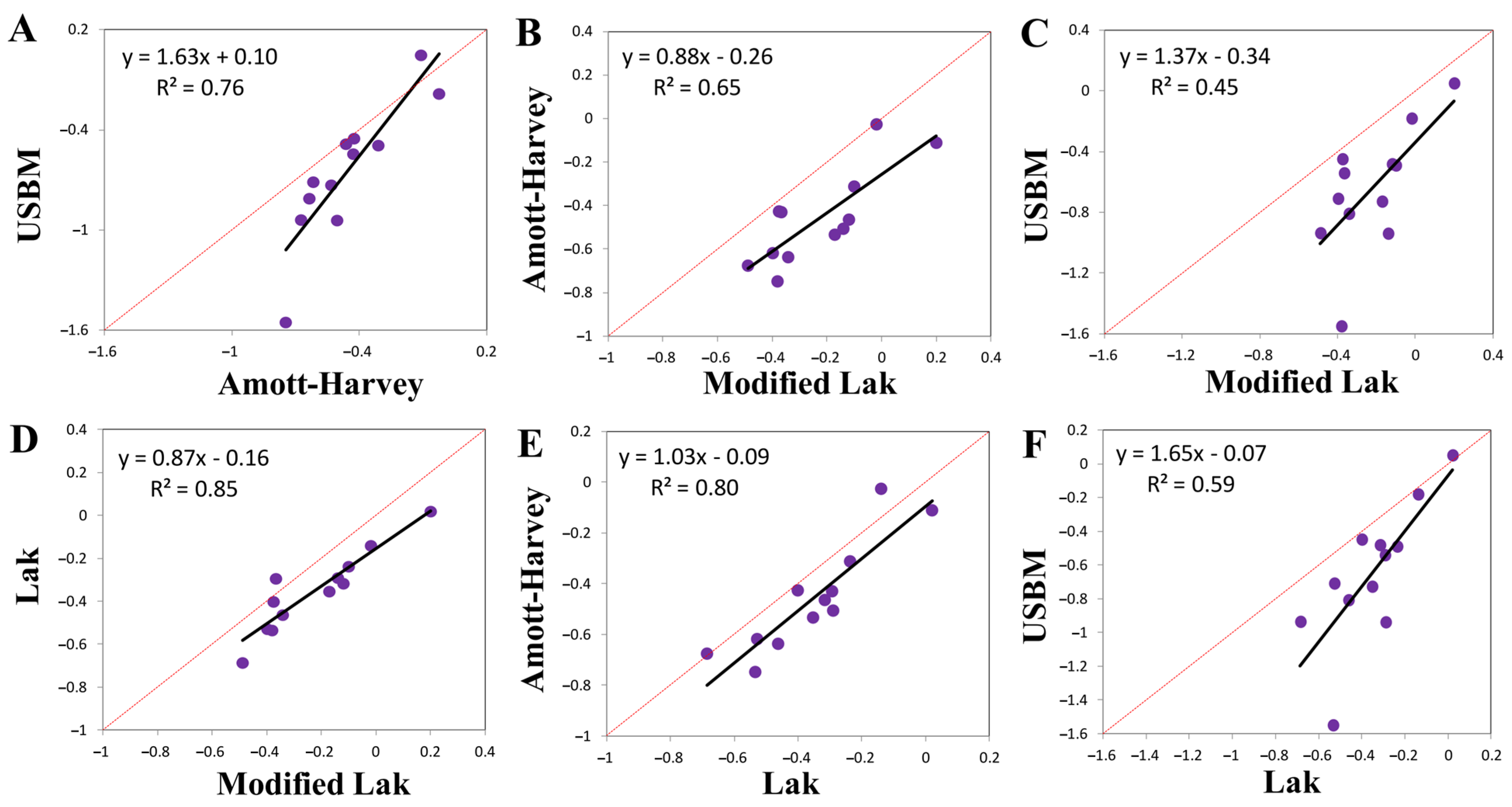
2.4.1. Lak Method
2.4.2. Modified Lak Method
3. Wettability Alteration
3.1. Injection of Low-Salinity Water
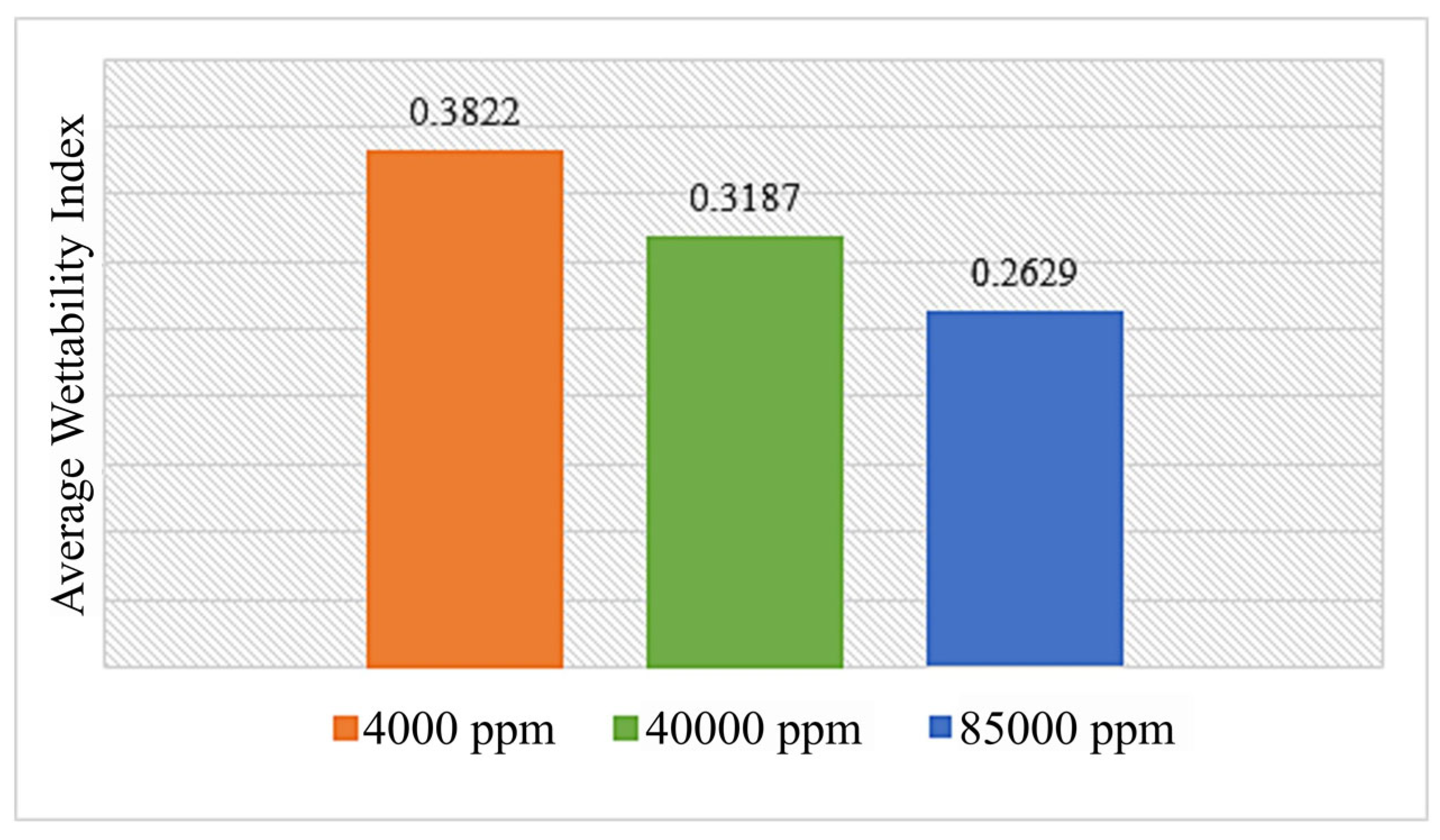
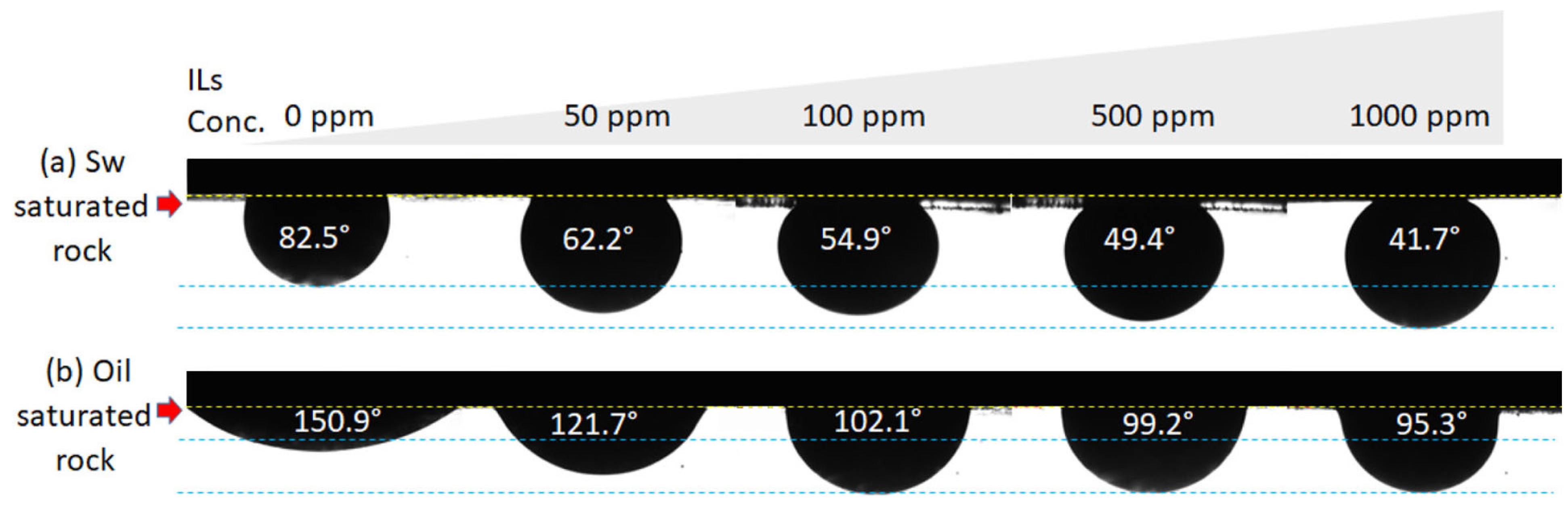
3.2. Injection of Surfactants and Ionized Fluids
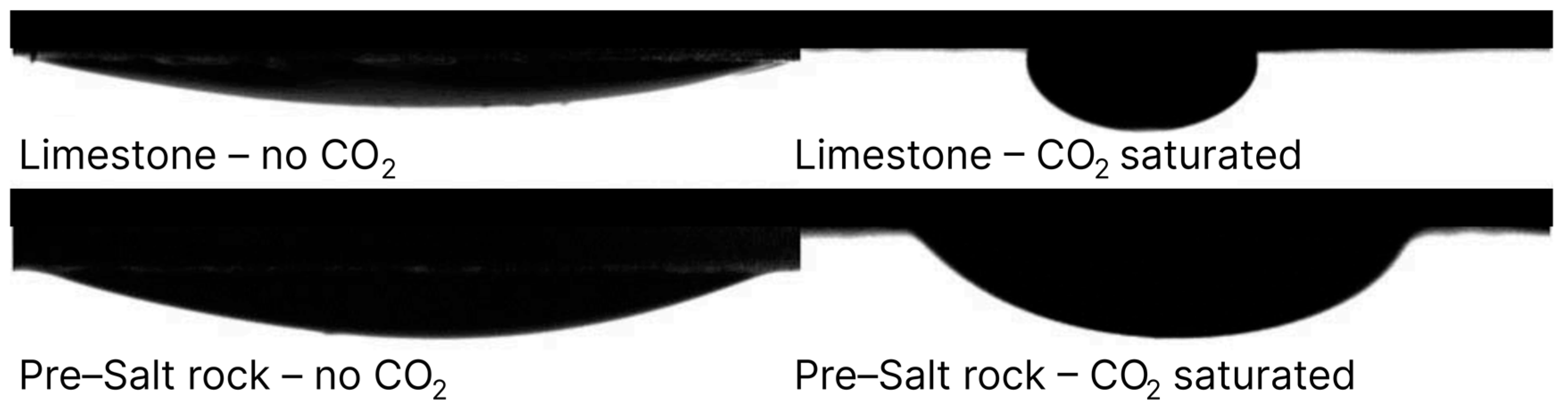
3.3. Injection of Carbonated Water
4. Wettability Alteration Due to Rock Dissolution and Precipitation
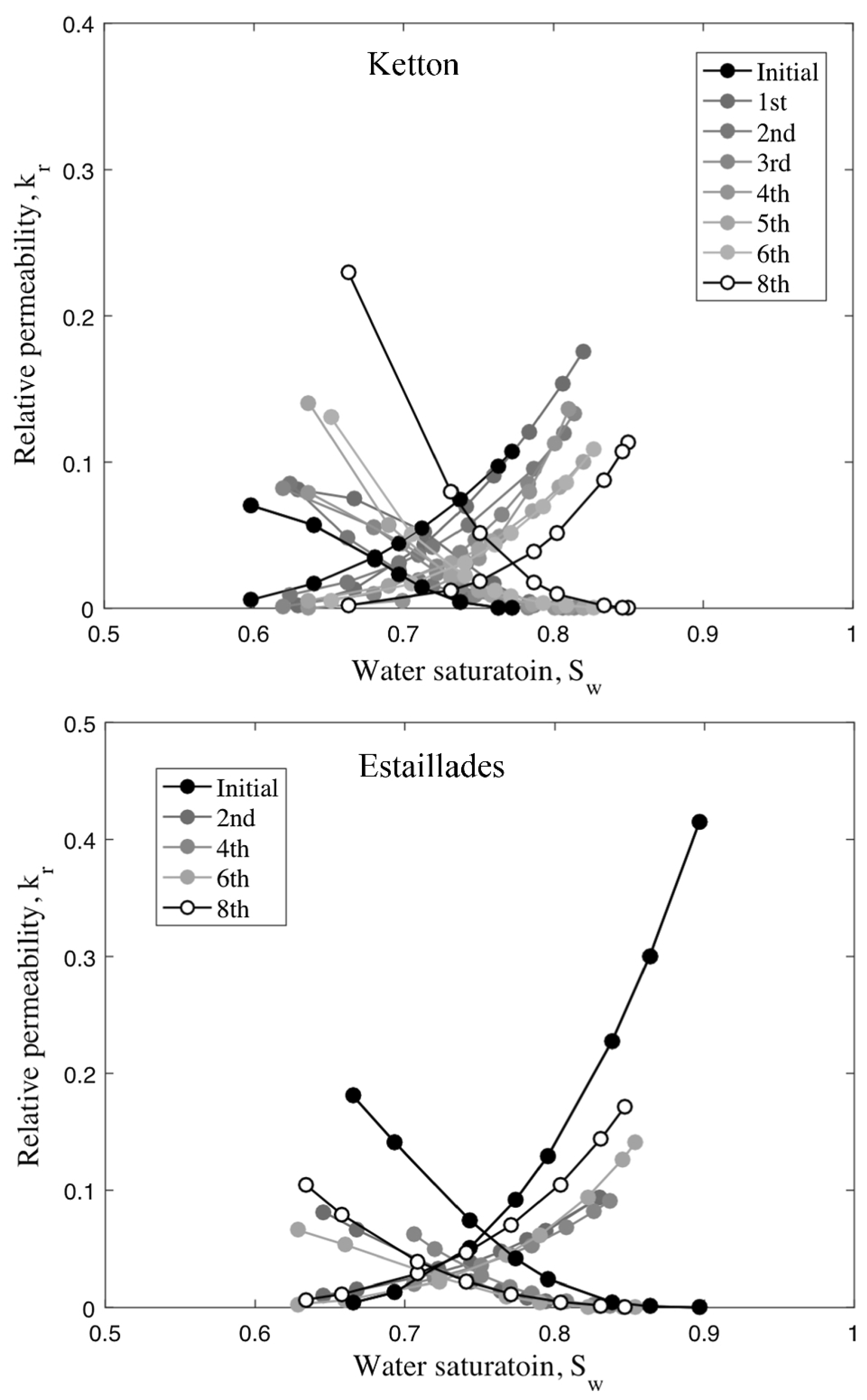
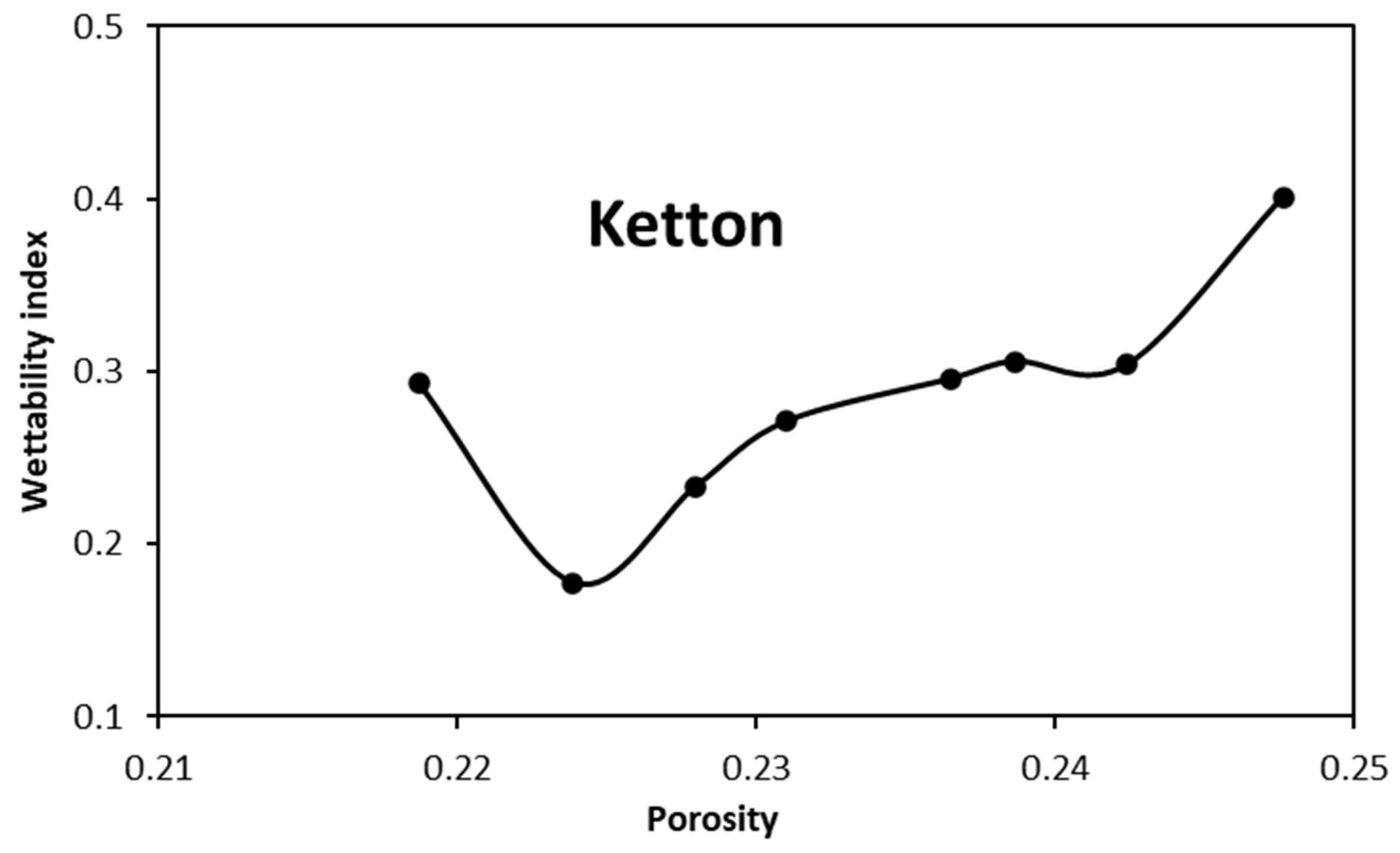
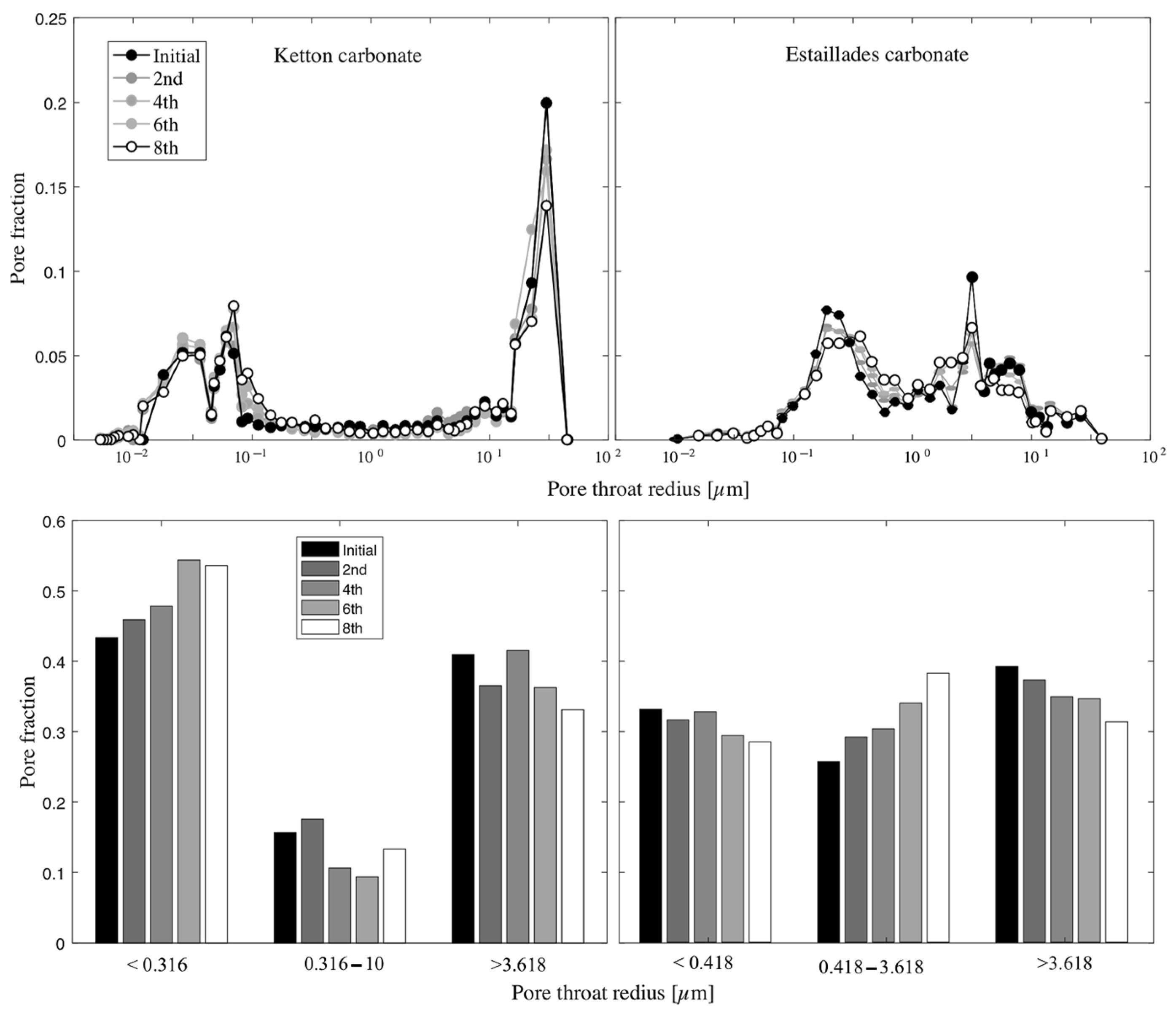
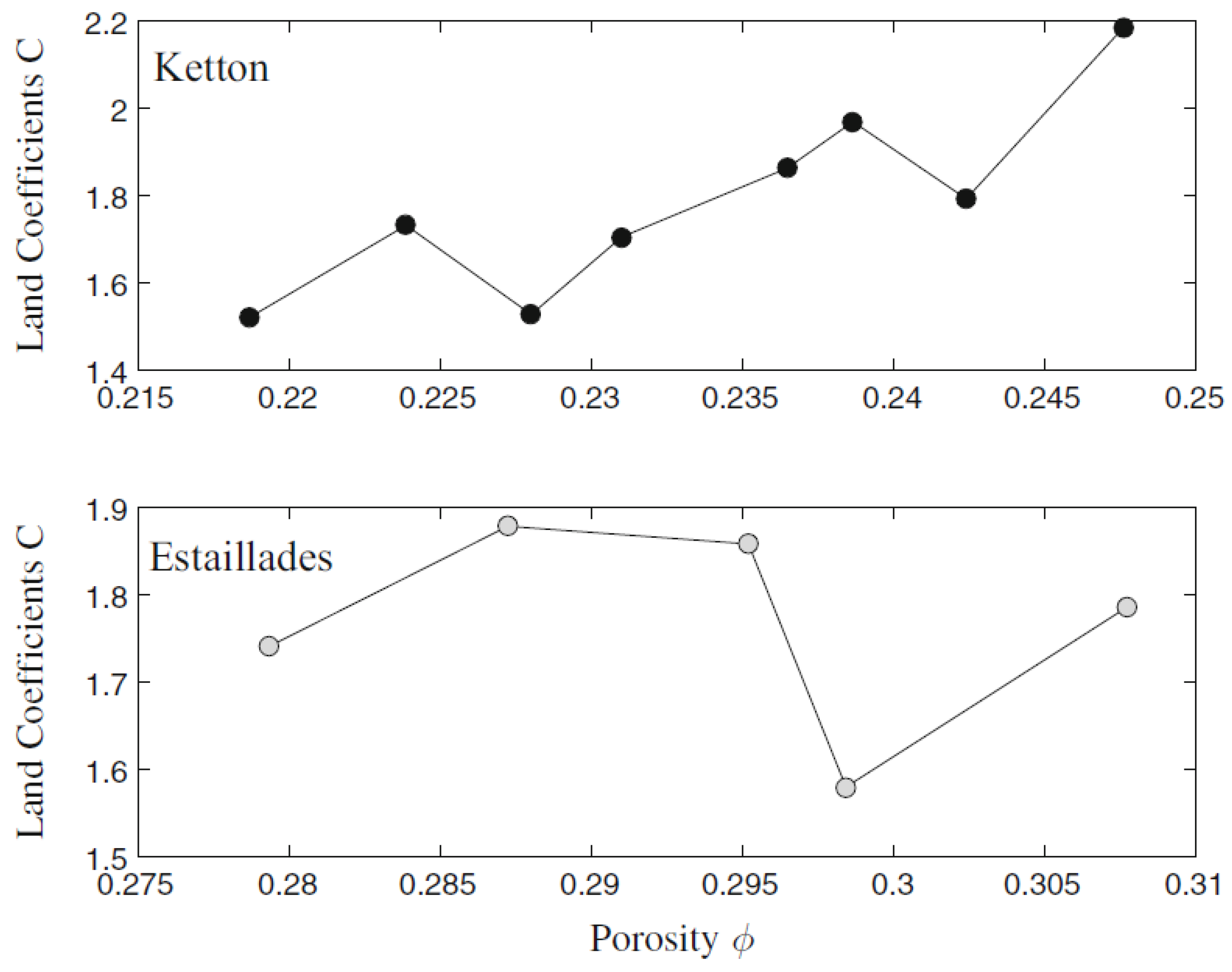
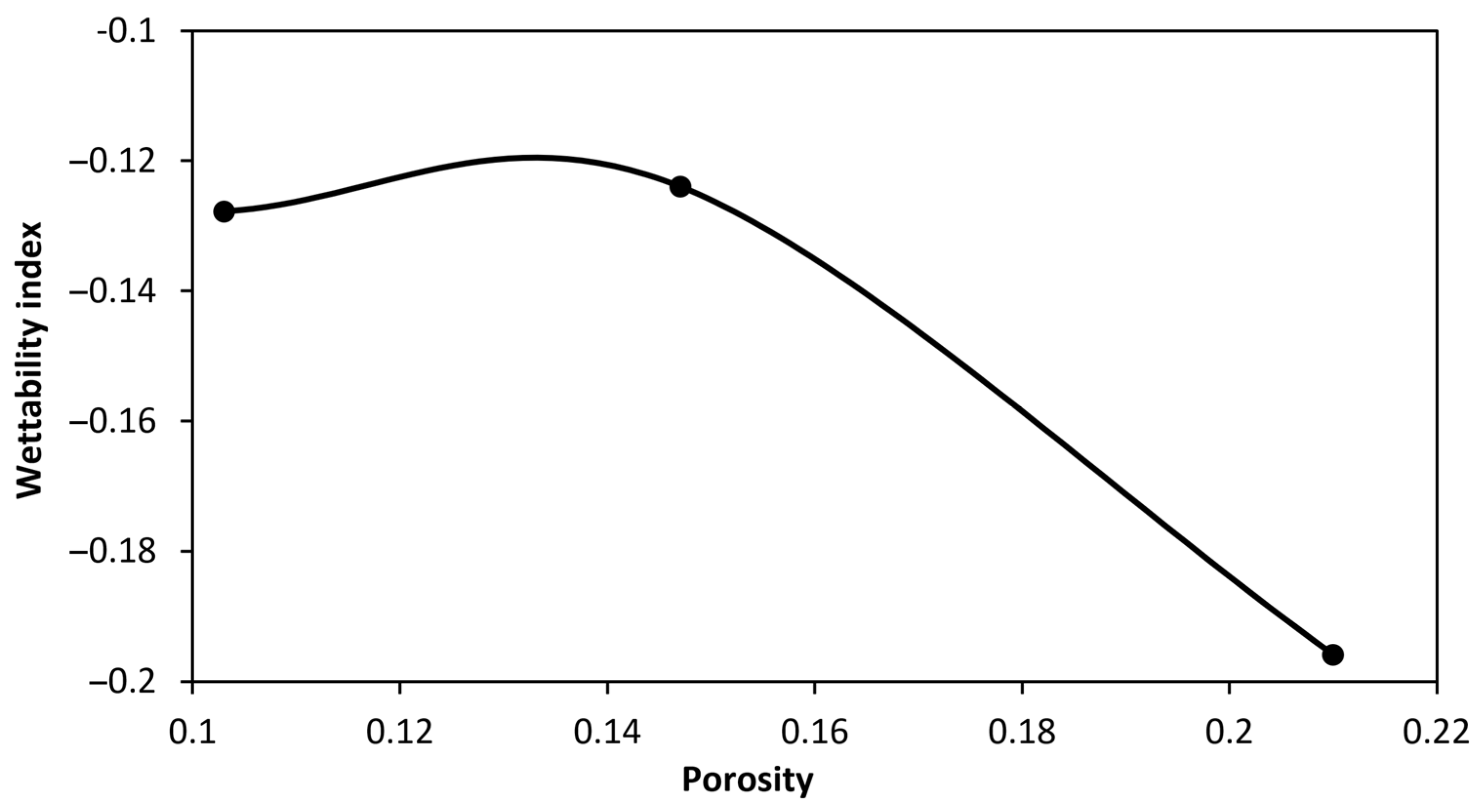
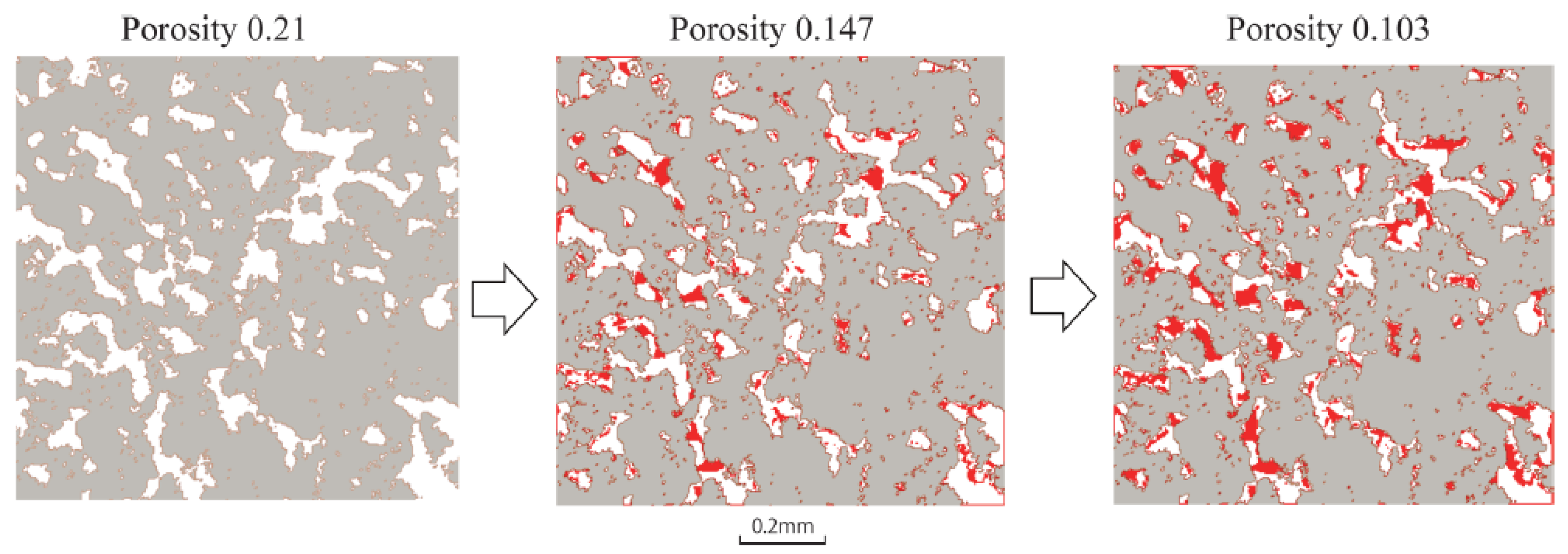
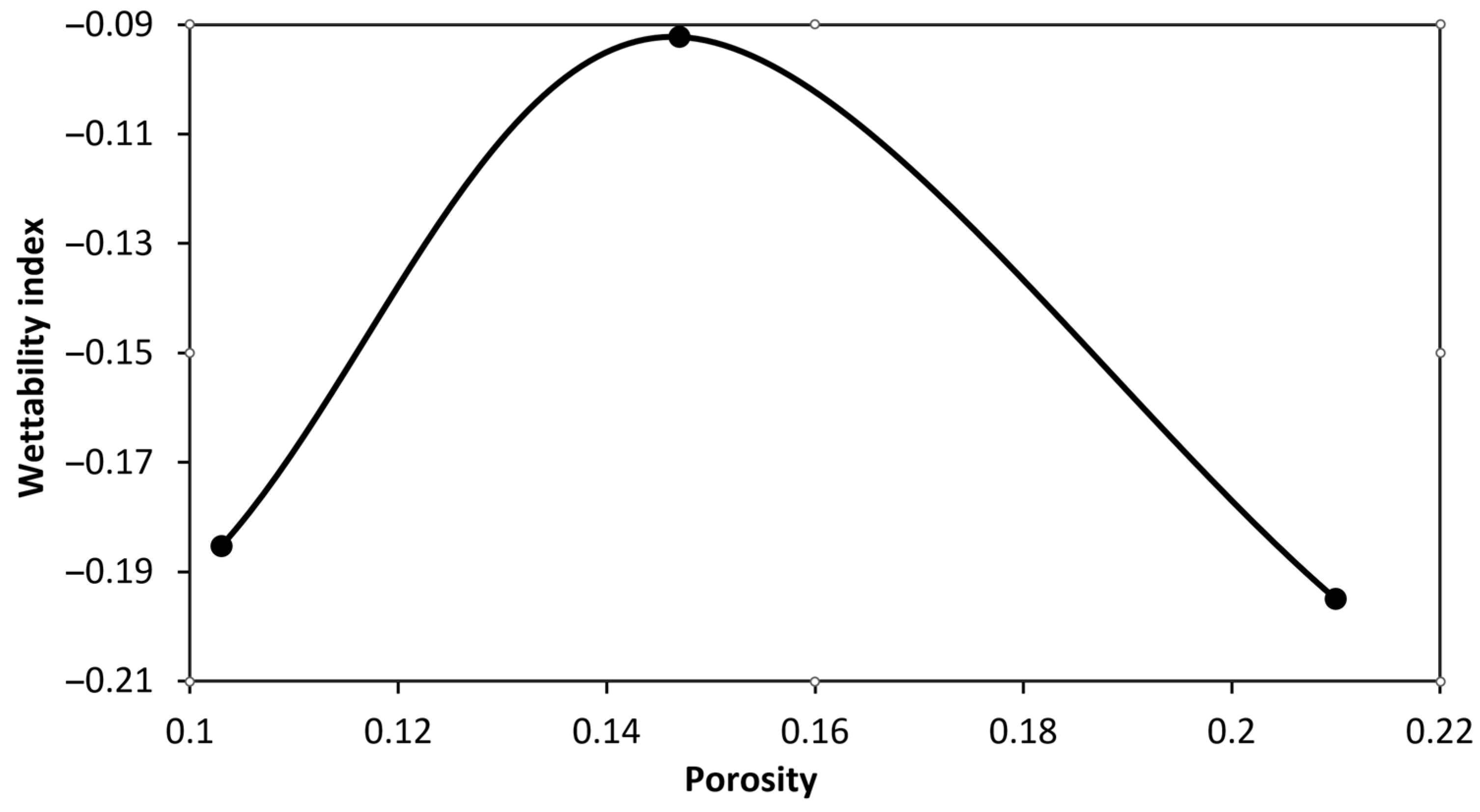
4.1. Wettability Alteration Due to Rock Dissolution
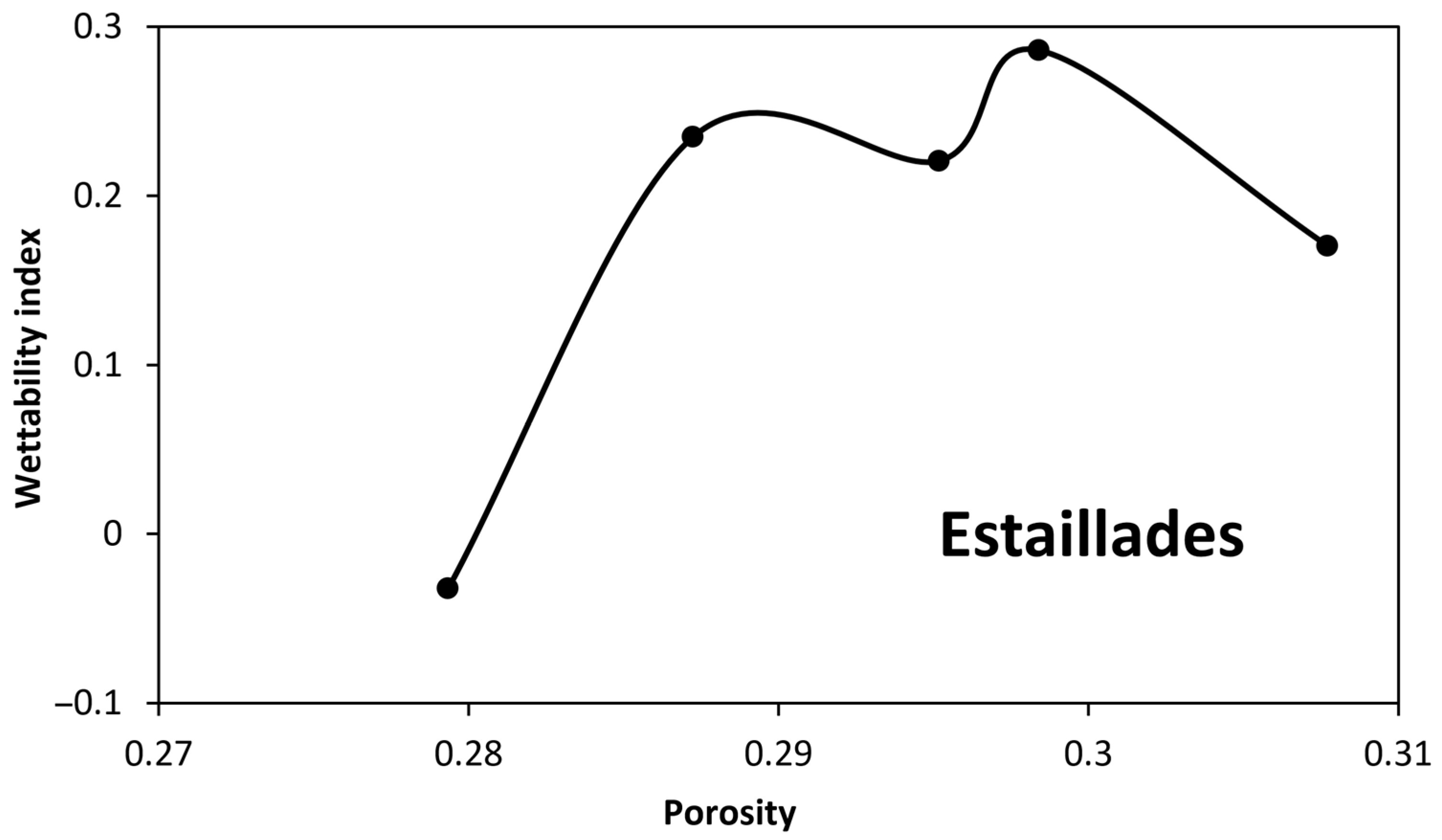
4.2. Wettability Alteration Due to Precipitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aslanidis, P. Experimental Reproduction of Wettability for Core Analysis. Ph.D. Thesis, Universitetet i Stavanger, Stavanger, Norway, 2022. ISBN 9788284391106. [Google Scholar]
- Wang, Z.; Chu, Y.; Zhao, G.; Yin, Z.; Kuang, T.; Yan, F.; Zhang, L.; Zhang, L. Study of Surface Wettability of Mineral Rock Particles by an Improved Washburn Method. ACS Omega 2023, 8, 15721–15729. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lu, S.; Li, J.; Hu, Y.; Zhang, P.; Chen, J.; Zhang, P. Experimental Investigations of the Mineral Wettability in Shale and Its Influence Factors. Acta Geol. Sin. Engl. Ed. 2015, 89, 170–171. [Google Scholar] [CrossRef]
- Kułynycz, V. The Influence of Wettability on the Petrophysical Parameters of Reservoir Rocks. AGH Drill. Oil Gas 2017, 34, 775. [Google Scholar] [CrossRef]
- Faramarzi-Palangar, M.; Mirzaei-Paiaman, A.; Ghoreishi, S.A.; Ghanbarian, B. Wettability of Carbonate Reservoir Rocks: A Comparative Analysis. Appl. Sci. 2021, 12, 131. [Google Scholar] [CrossRef]
- Amott, E. Observations Relating to the Wettability of Porous Rock. Trans. AIME 1959, 216, 156–162. [Google Scholar] [CrossRef]
- Glover, P.W.J. Formation Evaluation MSc Course Notes; Department of Geologyand Petroleum Geology University of Aberdeen UK CiNii Labs. Available online: https://homepages.see.leeds.ac.uk/~earpwjg/PG_EN/CD%20Contents/Formation%20Evaluation%20English/Contentsfinal.pdf (accessed on 3 December 2023).
- Morrow, N.R. Wettability and Its Effect on Oil Recovery. J. Pet. Technol. 1990, 42, 1476–1484. [Google Scholar] [CrossRef]
- Donaldson, E.C.; Thomas, R.D.; Lorenz, P.B. Wettability Determination and Its Effect on Recovery Efficiency. Soc. Pet. Eng. J. 1969, 9, 13–20. [Google Scholar] [CrossRef]
- Amirpour, M.; Shadizadeh, S.R.; Esfandyari, H.; Ahmadi, S. Experimental Investigation of Wettability Alteration on Residual Oil Saturation Using Nonionic Surfactants: Capillary Pressure Measurement. Petroleum 2015, 1, 289–299. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Galedarzadeh, M.; Shadizadeh, S.R. Wettability Alteration in Carbonate Rocks by Implementing New Derived Natural Surfactant: Enhanced Oil Recovery Applications. Transp. Porous Media 2015, 106, 645–667. [Google Scholar] [CrossRef]
- Sohal, M.A.; Thyne, G.; Søgaard, E.G. Novel Application of the Flotation Technique to Measure the Wettability Changes by Ionically Modified Water for Improved Oil Recovery in Carbonates. Energy Fuels 2016, 30, 6306–6320. [Google Scholar] [CrossRef]
- Ghosh, B.; Belhaj, H.; Alhashmi, H.; Idachaba, F.; Joshi, P.; Rahman, M.M.; Haroun, M. Standardization of Particle Size for Floating Particle Wettability Measurement for Carbonate Rocks. ACS Omega 2023, 8, 11837–11851. [Google Scholar] [CrossRef] [PubMed]
- Shedid, S.A.; Ghannam, M.T. Factors Affecting Contact-Angle Measurement of Reservoir Rocks. J. Pet Sci. Eng. 2004, 44, 193–203. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Mitiurev, N.A.; Shilobreeva, S.N.; Cheremisin, A.N. Experimental Methods for Studying the Wetting Properties of Oil Reservoirs: A Review. Izv. Phys. Solid Earth 2019, 55, 496–508. [Google Scholar] [CrossRef]
- Deng, X.; Kamal, M.S.; Patil, S.; Hussain, S.M.S.; Zhou, X. A Review on Wettability Alteration in Carbonate Rocks: Wettability Modifiers. Energy Fuels 2020, 34, 31–54. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Yu, W.; Bai, B.; Song, X.; Zhang, J. Surfactant Induced Reservoir Wettability Alteration: Recent Theoretical and Experimental Advances in Enhanced Oil Recovery. Pet Sci. 2011, 8, 463–476. [Google Scholar] [CrossRef]
- Zou, S.; Sun, C. X-ray Micro-Computed Imaging of Wettability Characterization for Multiphase Flow in Porous Media: A Review. Capillarity 2020, 3, 36–44. [Google Scholar] [CrossRef]
- Blunt, M.J.; Lin, Q.; Akai, T.; Bijeljic, B. A Thermodynamically Consistent Characterization of Wettability in Porous Media Using High-Resolution Imaging. J. Colloid Interface Sci. 2019, 552, 59–65. [Google Scholar] [CrossRef]
- Andrew, M.; Bijeljic, B.; Blunt, M.J. Pore-Scale Contact Angle Measurements at Reservoir Conditions Using X-Ray Microtomography. Adv. Water Resour 2014, 68, 24–31. [Google Scholar] [CrossRef]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral Characterization Using Scanning Electron Microscopy (SEM): A Review of the Fundamentals, Advancements, and Research Directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Deglint, H.J.; Clarkson, C.R.; DeBuhr, C.; Ghanizadeh, A. Live Imaging of Micro-Wettability Experiments Performed for Low-Permeability Oil Reservoirs. Sci. Rep. 2017, 7, 4347. [Google Scholar] [CrossRef]
- Ivanova, A.; Orekhov, A.; Markovic, S.; Iglauer, S.; Grishin, P.; Cheremisin, A. Live Imaging of Micro and Macro Wettability Variations of Carbonate Oil Reservoirs for Enhanced Oil Recovery and CO2 Trapping/Storage. Sci. Rep. 2022, 12, 1262. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ning, Z.F.; Li, N.; Zhang, B.; Ding, G.Y. Microscopic Surface Wettability Electrochemical Characterization of Tight Sandstone with Infrared Spectra Testing. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Qingdao, China, 26–29 June 2017; Institute of Physics Publishing: Bristol, UK, 2017; Volume 82. [Google Scholar]
- Al-Khafaji, A.; Wen, D. Quantification of Wettability Characteristics for Carbonates Using Different Salinities. J. Pet Sci. Eng. 2019, 173, 501–511. [Google Scholar] [CrossRef]
- Jarrahian, K.; Seiedi, O.; Sheykhan, M.; Sefti, M.V.; Ayatollahi, S. Wettability Alteration of Carbonate Rocks by Surfactants: A Mechanistic Study. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 1–10. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Zhou, G.; Wang, H.; Bai, Y.; Liu, Y. Influence Mechanism of Surfactants on Wettability of Coal with Different Metamorphic Degrees Based on Infrared Spectrum Experiments. ACS Omega 2021, 6, 22248–22258. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Wang, D.; He, X. Experimental Investigation on the Wettability of Respirable Coal Dust Based on Infrared Spectroscopy and Contact Angle Analysis. Adv. Powder Technol. 2017, 28, 3130–3139. [Google Scholar] [CrossRef]
- Kim, T.W.; Kovscek, A.R. Wettability Alteration of a Heavy Oil/Brine/Carbonate System with Temperature. Energy Fuels 2013, 27, 2984–2998. [Google Scholar] [CrossRef]
- Valori, A.; Nicot, B. A Review of 60 Years of NMR Wettability. Petrophysics SPWLA J. Form. Eval. Reserv. Descr. 2019, 60, 255–263. [Google Scholar] [CrossRef]
- Al-Garadi, K.; El-Husseiny, A.; Elsayed, M.; Connolly, P.; Mahmoud, M.; Johns, M.; Adebayo, A. A Rock Core Wettability Index Using NMR T2 Measurements. J. Pet Sci. Eng. 2022, 208, 109386. [Google Scholar] [CrossRef]
- Baban, A.; Al-Yaseri, A.; Keshavarz, A.; Amin, R.; Iglauer, S. CO2—Brine—Sandstone Wettability Evaluation at Reservoir Conditions via Nuclear Magnetic Resonance Measurements. Int. J. Greenh. Gas Control. 2021, 111, 103435. [Google Scholar] [CrossRef]
- Legens, C.; Palermo, T.; Toulhoat, H.; Fafet, A.; Koutsoukos, P.; Malmaison, R.; Francé, F.F. Carbonate Rock Wettability Changes Induced by Organic Compound Adsorption. J. Pet Sci. Eng. 1998, 20, 277–282. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hosseini, M.; Tangestani, E.; Mousavi, S.E.; Niazi, M. Wettability Alteration and Oil Recovery by Spontaneous Imbibition of Smart Water and Surfactants into Carbonates. Pet. Sci. 2020, 17, 712–721. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Ayirala, S.; Yousef, A. Smart Water Effects on Wettability, Adhesion, and Oil Liberation in Carbonates. SPE J. 2020, 25, 1771–1783. [Google Scholar] [CrossRef]
- Mirzaei-Paiaman, A. New Methods for Qualitative and Quantitative Determination of Wettability from Relative Permeability Curves: Revisiting Craig’s Rules of Thumb and Introducing Lak Wettability Index. Fuel 2021, 288, 119623. [Google Scholar] [CrossRef]
- Mirzaei-Paiaman, A.; Faramarzi-Palangar, M.; Djezzar, S.; Kord, S. A New Approach to Measure Wettability by Relative Permeability Measurements. J. Pet. Sci. Eng. 2022, 208, 109191. [Google Scholar] [CrossRef]
- Craig, F.F. The Reservoir Engineering Aspects of Waterflooding; Monograph Series; Society of Petroleum Engineers (SPE)CiNii Labs: Calgary, AB, Canada, 1971. [Google Scholar]
- Mahani, H.; Keya, A.L.; Berg, S.; Bartels, W.B.; Nasralla, R.; Rossen, W.R. Insights into the Mechanism of Wettability Alteration by Low-Salinity Flooding (LSF) in Carbonates. Energy Fuels 2015, 29, 1352–1367. [Google Scholar] [CrossRef]
- Alomair, O.; Al-Dousari, M.; Azubuike, N.; Garrouch, A.A. Evaluation of the Impact of Low-Salinity Water on Wettability Alteration and Oil Recovery in Berea Sandstones. Fuel 2023, 337, 127151. [Google Scholar] [CrossRef]
- Tang, G.Q.; Morrow, N.R. Salinity, Temperature, Oil Composition, and Oil Recovery by Waterflooding. SPE Reserv. Eng. 1997, 12, 269–276. [Google Scholar] [CrossRef]
- Nasralla, R.A.; Bataweel, M.A.; Nasr-El-Din, H.A. Investigation of Wettability Alteration and Oil-Recovery Improvement by Low-Salinity Water in Sandstone Rock. J. Can. Pet. Technol. 2013, 52, 144–154. [Google Scholar] [CrossRef]
- Morrow, N.; Buckley, J.; Morrow, N.R. Improved Oil Recovery by Low-Salinity Waterflooding. J. Pet. Technol. 2011, 63, 106–112. [Google Scholar] [CrossRef]
- Rezaeidoust, A.; Puntervold, T.; Strand, S.; Austad, T. Smart Water as Wettability Modifier in Carbonate and Sandstone: A Discussion of Similarities/Differences in the Chemical Mechanisms. Energy Fuels 2009, 23, 4479–4485. [Google Scholar] [CrossRef]
- Kallel, W.; van Dijke, M.I.J.; Sorbie, K.S.; Wood, R.; Jiang, Z.; Harland, S. Modelling the Effect of Wettability Distributions on Oil Recovery from Microporous Carbonate Reservoirs. Adv. Water Resour. 2016, 95, 317–328. [Google Scholar] [CrossRef]
- Boampong, L.O.; Rafati, R.; Haddad, A.S. Modelling of Carbonate Rock Wettability Based on Surface Charge and Calcite Dissolution. Fuel 2023, 331, 125856. [Google Scholar] [CrossRef]
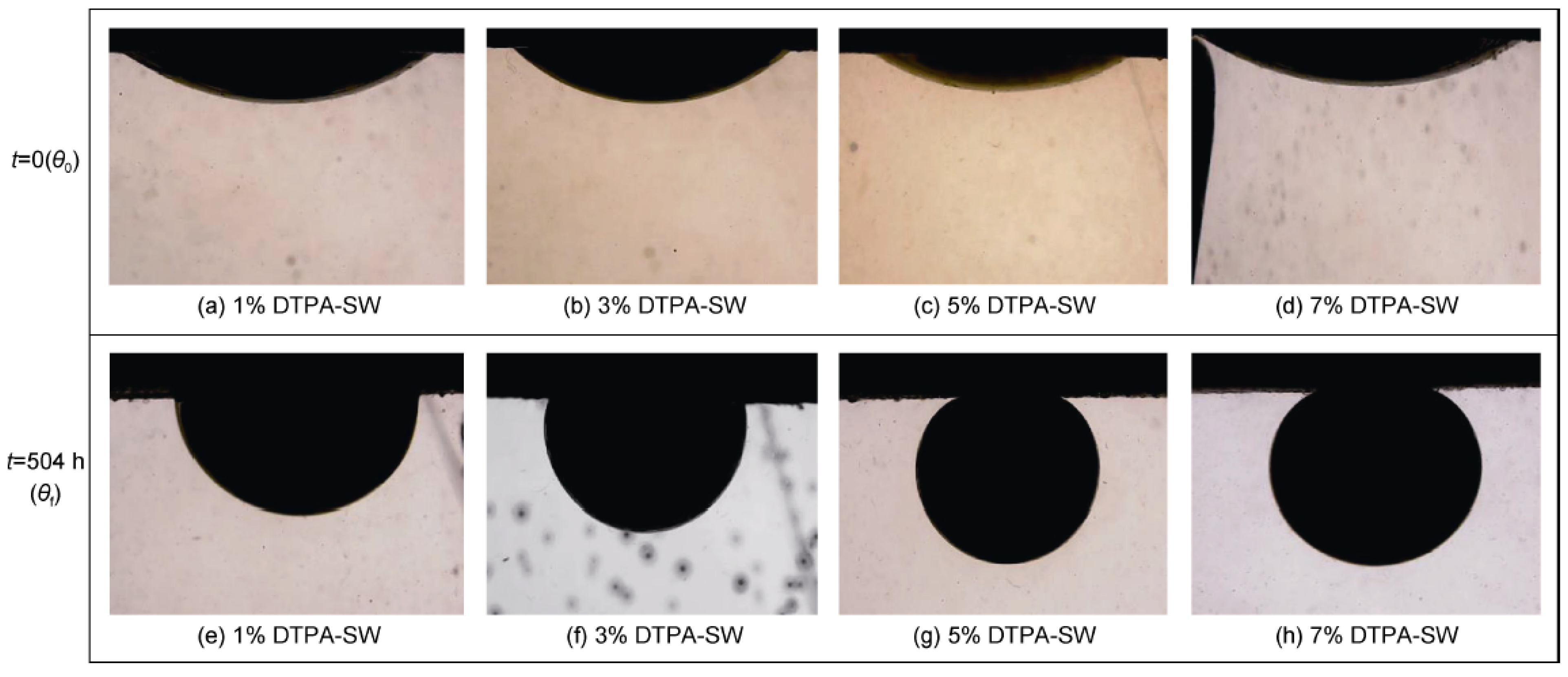
- Keradeh, M.P.; Tabatabaei-Nezhad, S.A. Investigation of the Effect of Diethylene Triamine Pentaacetic Acid Chelating Agent as an Enhanced Oil Recovery Fluid on Wettability Alteration of Sandstone Rocks. Pet. Explor. Dev. 2023, 50, 675–687. [Google Scholar] [CrossRef]
- Standnes, D.C.; Nogaret, L.A.D.; Chen, H.L.; Austad, T. An Evaluation of Spontaneous Imbibition of Water into Oil-Wet Carbonate Reservoir Cores Using a Nonionic and a Cationic Surfactant. Energy Fuels 2002, 16, 1557–1564. [Google Scholar] [CrossRef]
- Chen, P. Enhanced Oil Recovery in Fractured Vuggy Carbonates. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2014. [Google Scholar]
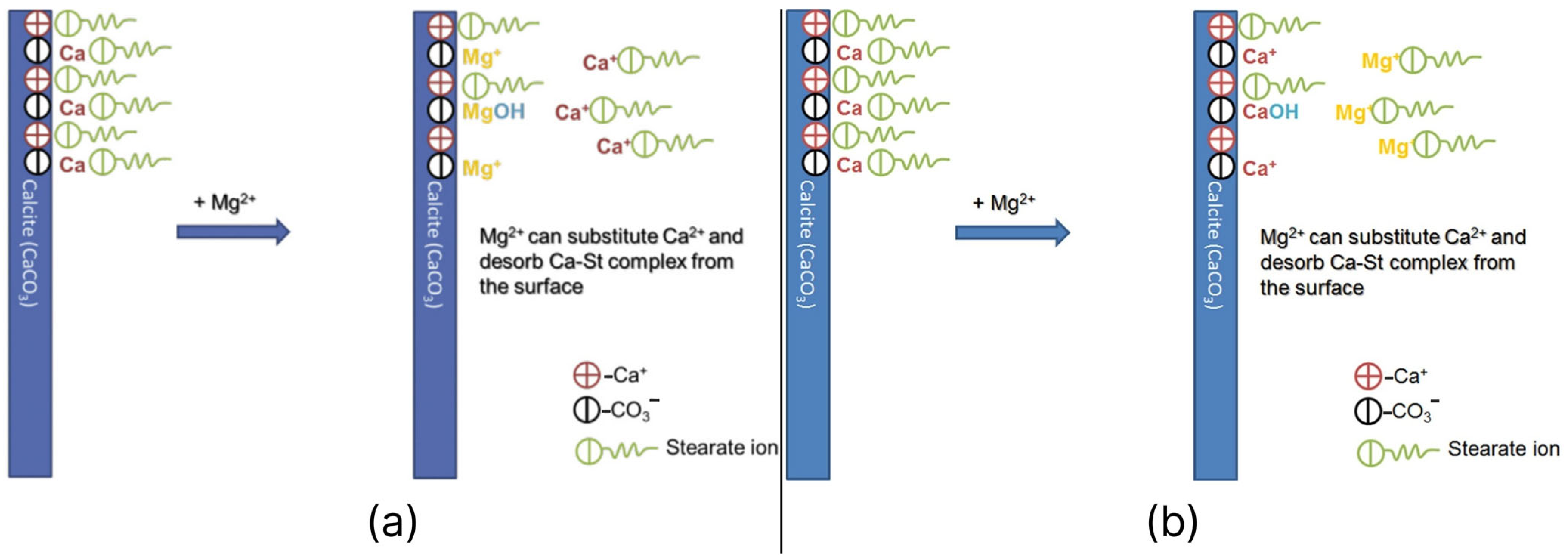
- Karimi, M.; Al-Maamari, R.S.; Ayatollahi, S.; Mehranbod, N. Mechanistic Study of Wettability Alteration of Oil-Wet Calcite: The Effect of Magnesium Ions in the Presence and Absence of Cationic Surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 403–415. [Google Scholar] [CrossRef]
- Ahmadali, T.; Gonzalez, M.V.; Harwell, J.H.; Scamehorn, J.F. Reducing Surfactant Adsorption in Carbonate Reservoirs. SPE Reserv. Eng. 1993, 8, 117–122. [Google Scholar] [CrossRef]
- Seethepalli, A.; Adibhatla, B.; Mohanty, K.K. Physicochemical Interactions During Surfactant Flooding of Fractured Carbonate Reservoirs. SPE J. 2004, 9, 411–418. [Google Scholar] [CrossRef]
- Golabi, E.; Seyedeyn-Azad, F.; Ayatollahi, S.; Hosseni, S.; Dastanian, M. Experimental Study of Anionic and Cationic Surfactants Effects on Reduce of IFT and Wettability Alteration in Carbonate Rock. Int. J. Sci. Eng. Res. 2008, 3, 1–8. [Google Scholar]
- Wu, Y.; Shuler, P.J.; Blanco, M.; Tang, Y.; Goddard, W.A. An Experimental Study of Wetting Behavior and Surfactant EOR in Carbonates with Model Compounds. SPE J. 2008, 13, 26–34. [Google Scholar] [CrossRef]
- Kubelka, J.; Bai, S.; Piri, M. Effects of Surfactant Charge and Molecular Structure on Wettability Alteration of Calcite: Insights from Molecular Dynamics Simulations. J. Phys. Chem. B 2021, 125, 1293–1305. [Google Scholar] [CrossRef]
- Bordeaux Rego, F.; Mehrabi, M.; Sanaei, A.; Sepehrnoori, K. Improvements on Modelling Wettability Alteration by Engineered Water Injection: Geochemical Pore-Scale Approach. In Proceedings of the SPE Improved Oil Recovery, Virtual, 30 August 2020. [Google Scholar]
- Gupta, R.; Mohanty, K.K. Wettability Alteration of Fractured Carbonate Reservoirs. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008; pp. 19–23. [Google Scholar]
- Zahid, A.; Stenby, E.H.; Shapiro, A.A. Improved Oil Recovery in Chalk: Wettability Alteration or Something Else? In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Barcelona, Spain, 14–17 June 2010; pp. 14–17. [Google Scholar]
- Gupta, R.; Mohanty, K.K. Wettability Alteration Mechanism for Oil Recovery from Fractured Carbonate Rocks. Transp. Porous Media 2011, 87, 635–652. [Google Scholar] [CrossRef]
- Abdallah, W.; Gmira, A. Wettability Assessment and Surface Compositional Analysis of Aged Calcite Treated with Dynamic Water. Energy Fuels 2014, 28, 1652–1663. [Google Scholar] [CrossRef]
- Høgnesen, E.J.; Strand, S.; Austad, T. Waterflooding of Preferential Oil-Wet Carbonates: Oil Recovery Related to Reservoir Temperature and Brine Composition. In Proceedings of the SPE Europec/EAGE Annual Conference, Madrid, Spain, 13–16 June 2005. [Google Scholar]
- Sakthivel, S. Wettability Alteration of Carbonate Reservoirs Using Imidazolium-Based Ionic Liquids. ACS Omega 2021, 6, 30315–30326. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Wang, F.; Wu, J.; Usman Tahir, M.; Li, Q.; Yuan, L.; Liu, Z. Effect of Thickener and Reservoir Parameters on the Filtration Property of CO2 Fracturing Fluid. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 1705–1715. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.; Li, Q.; Zhang, C.; Ansari, U.; Song, B. Establishment and Evaluation of Strength Criterion for Clayey Silt Hydrate-Bearing Sediments. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 742–750. [Google Scholar] [CrossRef]
- Drexler, S.; Hoerlle, F.; Godoy, W.; Boyd, A.; Couto, P. Wettability Alteration by Carbonated Brine Injection and Its Impact on Pore-Scale Multiphase Flow for Carbon Capture and Storage and Enhanced Oil Recovery in a Carbonate Reservoir. Appl. Sci. 2020, 10, 6496. [Google Scholar] [CrossRef]
- Drexler, S.; Silveira, T.M.G.; De Belli, G.; Couto, P. Experimental Study of the Effect of Carbonated Brine on Wettability and Oil Displacement for EOR Application in the Brazilian Pre-Salt Reservoirs. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 3282–3296. [Google Scholar] [CrossRef]
- Ruidiaz, E.M.; Winter, A.; Trevisan, O. V Oil Recovery and Wettability Alteration in Carbonates Due to Carbonate Water Injection. J. Pet. Explor. Prod. Technol. 2018, 8, 249–258. [Google Scholar] [CrossRef]
- Mehdiyev, F.; Erzuah, S.; Omekeh, A.; Fjelde, I. Surface Complexation Modelling of Wettability Alteration during Carbonated Water Flooding. Energies 2022, 15, 20. [Google Scholar] [CrossRef]
- Arjomand, E.; Easton, C.D.; Myers, M.; Tian, W.; Saeedi, A.; Wood, C.D. Changing Sandstone Rock Wettability with Supercritical CO2-Based Silylation. Energy Fuels 2020, 34, 2015–2027. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Manshad, A.K.; Mohammadi, A.H. Effects of Ions and Dissolved Carbon Dioxide in Brine on Wettability Alteration, Contact Angle and Oil Production in Smart Water and Carbonated Smart Water Injection Processes in Carbonate Oil Reservoirs. Fuel 2019, 235, 1039–1051. [Google Scholar] [CrossRef]
- Niu, B.; Krevor, S. The Impact of Mineral Dissolution on Drainage Relative Permeability and Residual Trapping in Two Carbonate Rocks. Transp. Porous Media 2020, 131, 363–380. [Google Scholar] [CrossRef]
- Jiang, F.; Tsuji, T. Changes in Pore Geometry and Relative Permeability Caused by Carbonate Precipitation in Porous Media. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2014, 90, 053306. [Google Scholar] [CrossRef] [PubMed]
- McPhee, C.; Reed, J.; Zubizarreta, I. Wettability and Wettability Tests; Elsevier: Amsterdam, The Netherlands, 2015; pp. 313–345. [Google Scholar]
- Sagbana, P.I.; Sarkodie, K.; Nkrumah, W.A. A Critical Review of Carbonate Reservoir Wettability Modification during Low Salinity Waterflooding. Petroleum 2023, 9, 317–330. [Google Scholar] [CrossRef]
- Anderson, W. Wettability Literature Survey—Part 2: Wettability Measurement. J. Pet. Technol. 1986, 38, 1246–1262. [Google Scholar] [CrossRef]
- Abdallah, W.; Buckley, J.S.; Carnegie, A.; Edwards, J.; Herold, B.; Fordham, E.; Graue, A.; Habashy, T.; Seleznev, N.; Signer, C.; et al. Fundamentals of Wettability. Technology 1986, 38, 1125–1144. [Google Scholar]
- León Pabón, J.A.; Mejía Pilonieta, T.J.; Carrillo Moreno, L.F.; Buendía Lombana, H.; Zapata, J.F.; Díaz Prada, C.A. Experimental Comparison for the Calculation of Rock Wettability Using the Amott-Harvey Method and a New Visual Method. CTF Cienc. Tecnol. Y Futuro 2014, 5, 5–22. [Google Scholar] [CrossRef]
- Thyne, G.D. A Review of the Measurement of Wettability. Available online: https://www.researchgate.net/publication/283055682_A_review_of_the_measurement_of_wettability (accessed on 3 December 2023).
- Rücker, M.; Bartels, W.-B.; Garfi, G.; Shams, M.; Bultreys, T.; Boone, M.; Pieterse, S.; Maitland, G.C.; Krevor, S.; Cnudde, V.; et al. Relationship between Wetting and Capillary Pressure in a Crude Oil/Brine/Rock System: From Nano-Scale to Core-Scale. J. Colloid Interface Sci. 2020, 562, 159–169. [Google Scholar] [CrossRef]
- Isah, A.; Arif, M.; Mahmoud, M.; Kamal, M.S. Influence of Rock Permeability and Surface Conditioning on Carbonate Wettability: A Link between Contact Angle and Amott-Index. Geoenergy Sci. Eng. 2023, 227, 211892. [Google Scholar] [CrossRef]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A Review of Factors That Affect Contact Angle and Implications for Flotation Practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef]
- Meng, W.; Zhang, Y.; Pei, H.; Yu, J.; Hu, Y.; Gu, Z.; Su, J. Pore-Scale Investigation on the Effect of Capillary Barrier on Two-Phase Displacement in Dual-Structure Porous Media. Phys. Fluids 2024, 36, 012003. [Google Scholar] [CrossRef]
- Bolysbek, D.A.; Kuljabekov, A.B.; Uzbekaliyev, K.S.; Assilbekov, B.K. Effect of Rock Dissolution on Two-Phase Relative Permeabilities: Pore-Scale Simulations Based on Experimental Data. Appl. Sci. 2023, 13, 11385. [Google Scholar] [CrossRef]
- Ning, T.; Xi, M.; Hu, B.; Wang, L.; Huang, C.; Su, J. Effect of Viscosity Action and Capillarity on Pore-Scale Oil–Water Flowing Behaviors in a Low-Permeability Sandstone Waterflood. Energies 2021, 14, 8200. [Google Scholar] [CrossRef]






| Type | Amott–Harvey Wettability Index |
|---|---|
| water-wet | 0.3–1 |
| mixed-wet | −0.3–0.3 |
| oil-wet | −1~−0.3 |
| Type | USBM Wettability Index |
|---|---|
| water-wet | >0 |
| mixed-wet | ~0 |
| oil-wet | <0 |
| Type | Contact Angle (deg.) |
|---|---|
| water-wet | 0–80 |
| intermediate-wet | 80–100 |
| oil-wet | 100–160 |
| strongly oil-wet | 160–180 |
| Rock | Diameter (cm) | Length (cm) | Porosity | Permeability (mD) |
|---|---|---|---|---|
| Ketton | 38 | 16.4 | 0.218 | 2840 |
| Estaillades | 38 | 16.5 | 0.279 | 196 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolysbek, D.; Uzbekaliyev, K.; Assilbekov, B. Rock Wettability Alteration Induced by the Injection of Various Fluids: A Review. Appl. Sci. 2024, 14, 8663. https://doi.org/10.3390/app14198663
Bolysbek D, Uzbekaliyev K, Assilbekov B. Rock Wettability Alteration Induced by the Injection of Various Fluids: A Review. Applied Sciences. 2024; 14(19):8663. https://doi.org/10.3390/app14198663
Chicago/Turabian StyleBolysbek, Darezhat, Kenbai Uzbekaliyev, and Bakytzhan Assilbekov. 2024. "Rock Wettability Alteration Induced by the Injection of Various Fluids: A Review" Applied Sciences 14, no. 19: 8663. https://doi.org/10.3390/app14198663






