On the Micromixing Behavior of a Spinning Disk Reactor for Metallic Cu Nanoparticles Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Copper Nanoparticles
2.3. Characterization
2.4. Methods
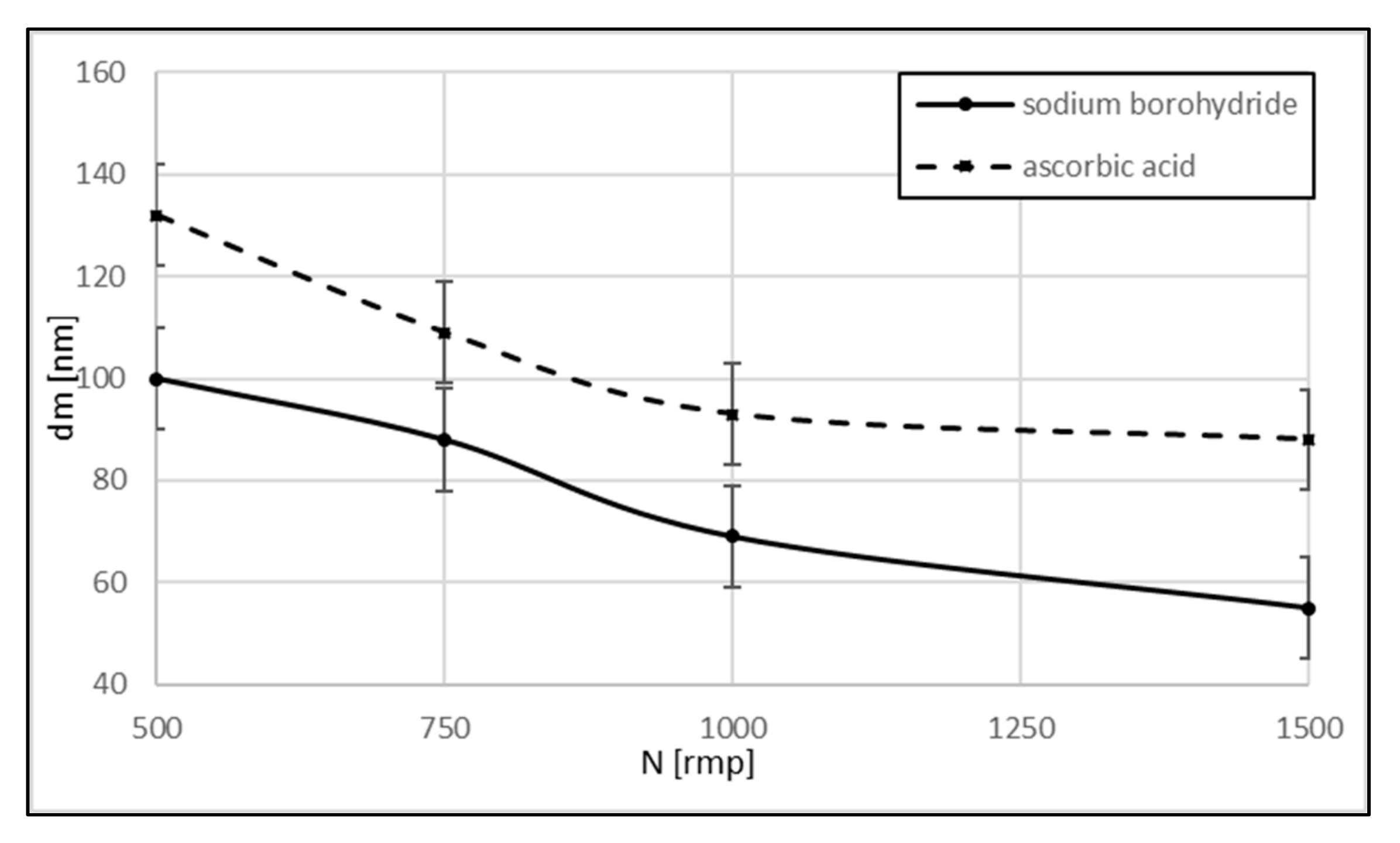
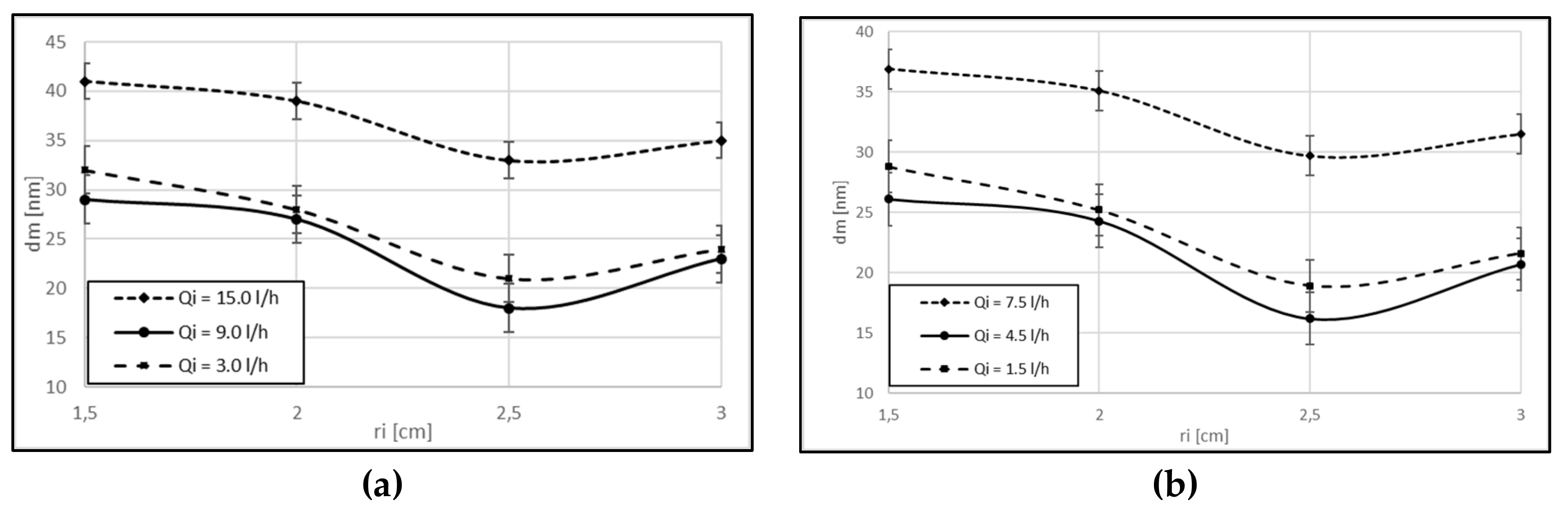
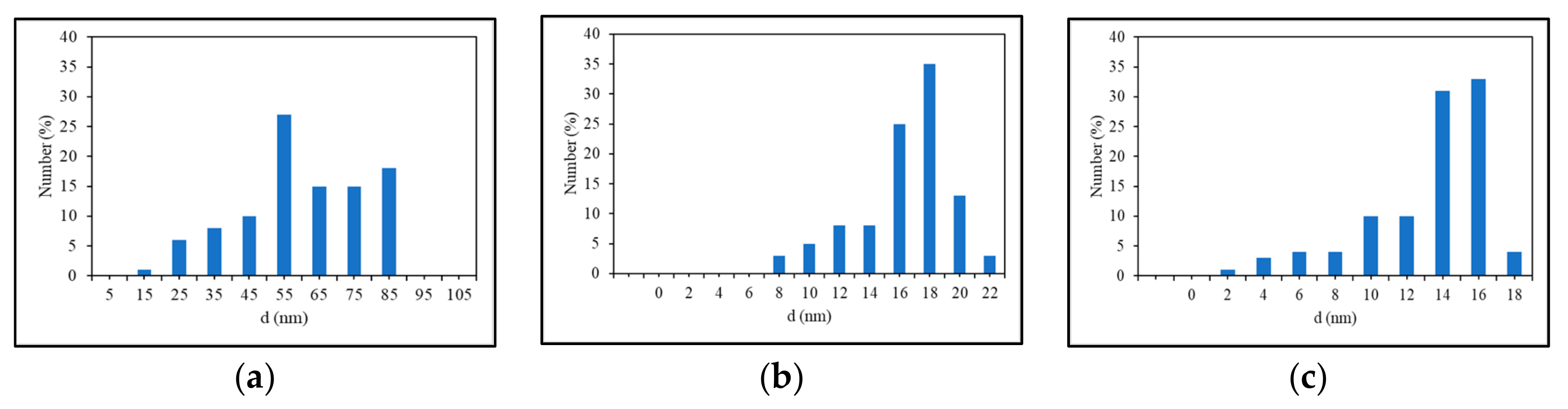
3. Results and Discussion
Experimental Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vilardi, G.; Di Palma, L.; Verdone, N. Competitive reaction modelling in aqueous systems: The case of contemporary reduction of dichromates and nitrates by nZVI. Chem. Eng. Trans. 2017, 60, 175–180. [Google Scholar]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Vilardi, G. Mathematical modelling of simultaneous nitrate and dissolved oxygen reduction by Cu-nZVI using a bi-component shrinking core model. Powder Technol. 2019, 343, 613–618. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.; Li, G.; Zhao, T.; Fu, X.; Sun, R.; Feng, Z.; Wong, C.P. Facile preparation of monodisperse, impurity-free, and antioxidation copper nanoparticles on a large scale for application in conductive ink. ACS Appl. Mater. Interfaces 2013, 6, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.M.D.; Le, T.T.T.; Fribourg-Blanc, E.; Dang, M.C. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 015009. [Google Scholar] [CrossRef]
- Badiei, E.; Sangpour, P.; Bagheri, M.; Pazouki, M. Graphene Oxide Antibacterial Sheets: Synthesis and Characterization. Int. J. Eng. Trans. C 2014, 27, 1803–1808. [Google Scholar]
- Stoller, M.; Mescia, M.; Peroni, V.C.; Chianese, A. Production of nanoparticles of titanium dioxide by using a spinning disc reactor. Chem. Eng. Trans. 2007, 11, 71–76. [Google Scholar]
- De Caprariis, B.; Stoller, M.; Chianesea, A.; Verdone, N. CFD model of a spinning disk reactor for nanoparticle production. Chem. Eng. 2015, 43, 757–762. [Google Scholar] [CrossRef]
- Stoller, M.; Di Palma, L.; Vuppala, S.; Verdone, N.; Vilardi, G. Process intensification techniques for the production of nano-and submicronic particles for food and medical applications. Curr. Pharm. Des. 2018, 24, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Ochando Pulido, J.M.; Stoller, M. Kinetics and boundary flux optimization of integrated photocatalysis and ultrafiltration process for two-phase vegetation and olive washing wastewaters treatment. Chem. Eng. J. 2015, 279, 387–395. [Google Scholar] [CrossRef]
- Vilardi, G. Bimetallic nZVI-induced chemical denitrification modelling using the shrinking core model. Chem. Eng. Trans. 2018, 70, 235–240. [Google Scholar]
- Stoller, M.; Miranda, L.; Chianese, A. Optimal feed location in a spinning disc reactor for the production of TiO2 nanoparticles. Chem. Eng. Trans. 2009, 17, 993–998. [Google Scholar]
- Vilardi, G.; Stoller, M.; Verdone, N.; Di Palma, L. Production of nano Zero Valent Iron particles by means of a spinning disk reactor. Chem. Eng. Trans. 2017, 57, 751–756. [Google Scholar]
- Ochando-Pulido, J.M.; Stoller, M.; Di Palma, L.; Martínez-Férez, A.; Vilardi, G. Spinning Disk Reactor Technology in Photocatalysis: Nanostructured Catalysts Intensified Production and Applications. Nanophotocatal. Environ. Appl. 2019, 29, 303–333. [Google Scholar] [CrossRef]
- Vilardi, G.; Stoller, M.; Di Palma, L.; Verdone, N. CFD Model of Agitated Vessel for the Removal of Cr(vi) by Nano-hematite Particles. Chem. Eng. Trans. 2019, 73, 157–162. [Google Scholar]


| N (rpm) | Dm (nm) |
|---|---|
| 500 | 45 |
| 1000 | 41 |
| 1500 | 39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, A.; Stoller, M. On the Micromixing Behavior of a Spinning Disk Reactor for Metallic Cu Nanoparticles Production. Appl. Sci. 2019, 9, 3311. https://doi.org/10.3390/app9163311
Marchetti A, Stoller M. On the Micromixing Behavior of a Spinning Disk Reactor for Metallic Cu Nanoparticles Production. Applied Sciences. 2019; 9(16):3311. https://doi.org/10.3390/app9163311
Chicago/Turabian StyleMarchetti, Angela, and Marco Stoller. 2019. "On the Micromixing Behavior of a Spinning Disk Reactor for Metallic Cu Nanoparticles Production" Applied Sciences 9, no. 16: 3311. https://doi.org/10.3390/app9163311






