Gait Analysis with Wearables Is a Potential Progression Marker in Parkinson’s Disease
Abstract
:1. Introduction
2. Method
2.1. Patients
2.2. Clinical Evaluation
2.3. Instruments
2.4. Gait Data Collection
2.5. Statistical Analysis
3. Results
3.1. Clinical Baseline Data of Participants
3.2. Spatiotemporal Gait Parameters
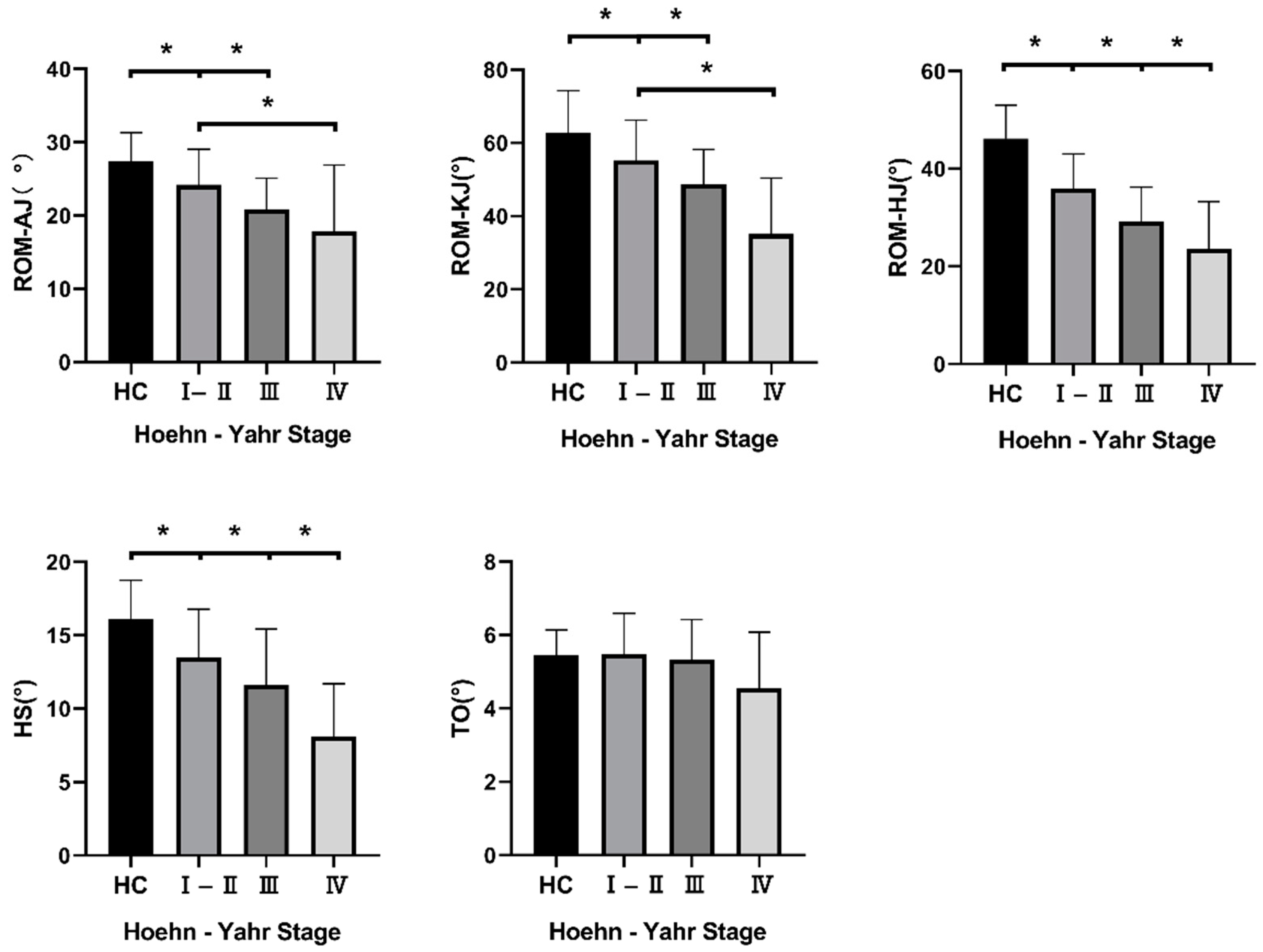
3.3. Kinematic Gait Parameters
3.4. Symmetry Analysis of Gait Parameters
3.5. Correlation Analysis between Gait Parameters and H–Y Stage, UPDRS Scores and PDQ39
4. Discussion
4.1. Spatiotemporal Gait Parameters
4.2. Kinematic Gait Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Pablo-Fernández, E.; Lees, A.; Holton, J.; Warner, T.T. Prognosis and Neuropathologic Correlation of Clinical Subtypes of Parkinson Disease. JAMA Neurol. 2019, 76, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Park, S.; Lee, H.; Park, Y.; Kim, J.; Kim, J.; Koh, S.B. Backward Gait is Associated with Motor Symptoms and Fear of Falling in Patients with De Novo Parkinson’s Disease. J. Clin. Neurol. 2019, 15, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Curtze, C.; Nutt, J.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Objective Gait and Balance Impairments Relate to Balance Confidence and Perceived Mobility in People with Parkinson Disease. Phys. Ther. 2016, 96, 1734–1743. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Yarnall, A.; Coleman, S.; Burn, D.; Rochester, L. Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naïve cohort. Mov. Disord. 2016, 31, 1829–1836. [Google Scholar] [CrossRef]
- Jellinger, K.; Logroscino, G.; Rizzo, G.; Copetti, M.; Arcuti, S.; Martino, D.; Fontana, A. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology 2016, 87, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Defebvre, L.; Bordet, R. Dopaminergic and non-dopaminergic pharmacological hypotheses for gait disorders in Parkinson’s disease. Fundam. Clin. Pharmacol. 2010, 24, 407–421. [Google Scholar] [CrossRef]
- Goetz, C.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.; Stern, M.; Tilley, B.; Dodel, R.; Dubois, B.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 2007, 22, 41–47. [Google Scholar] [CrossRef]
- Richards, M.; Marder, K.; Cote, L.; Mayeux, R. Interrater reliability of the Unified Parkinson’s Disease Rating Scale motor examination. Mov. Disord. 1994, 9, 89–91. [Google Scholar] [CrossRef]
- Heldman, D.; Giuffrida, J.; Chen, R.; Payne, M.; Mazzella, F.; Duker, A.; Sahay, A.; Kim, S.; Revilla, F.; Espay, A.J. The modified bradykinesia rating scale for Parkinson’s disease: Reliability and comparison with kinematic measures. Mov. Disord. 2011, 26, 1859–1863. [Google Scholar] [CrossRef]
- Bouca-Machado, R.; Jalles, C.; Guerreiro, D.; Pona-Ferreira, F.; Branco, D.; Guerreiro, T.; Matias, R.; Ferreira, J.J. Gait Kinematic Parameters in Parkinson’s Disease: A Systematic Review. J. Parkinsons Dis. 2020, 10, 843–853. [Google Scholar] [CrossRef]
- Dewey, D.; Miocinovic, S.; Bernstein, I.; Khemani, P.; Dewey, R.; Querry, R.; Chitnis, S.; Dewey, R.B., Jr. Automated gait and balance parameters diagnose and correlate with severity in Parkinson disease. J. Neurol. Sci. 2014, 345, 131–138. [Google Scholar] [CrossRef]
- Hobert, M.; Maetzler, W.; Aminian, K.; Chiari, L. Technical and clinical view on ambulatory assessment in Parkinson’s disease. Acta Neurol. Scand. 2014, 130, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Prieto, L.; Forjaz, M.J. Longitudinal metric properties of disability rating scales for Parkinson’s disease. Value Health 2006, 9, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hsu, W.; Wu, R.; Lu, T.; Lin, K. Motion analysis of axial rotation and gait stability during turning in people with Parkinson’s disease. Gait Posture 2016, 44, 83–88. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Tao, S.; Zhang, X.; Cai, H.; Lv, Z.; Hu, C.; Xie, H. Gait based biometric personal authentication by using MEMS inertial sensors. J. Ambient. Intell. Humaniz. Comput. 2018, 9, 1705–1712. [Google Scholar] [CrossRef]
- Mancini, M.; King, L.; Salarian, A.; Holmstrom, L.; McNames, J.; Horak, F.B. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J. Bioeng. Biomed. Sci. 2011, 007. [Google Scholar] [CrossRef]
- Galna, B.; Lord, S.; Rochester, L. Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol. Gait Posture 2013, 37, 580–585. [Google Scholar] [CrossRef]
- Bryant, M.; Rintala, D.; Hou, J.; Collins, R.; Protas, E.J. Gait variability in Parkinson’s disease: Levodopa and walking direction. Acta Neurol. Scand. 2016, 134, 83–86. [Google Scholar] [CrossRef]
- Patterson, K.; Gage, W.; Brooks, D.; Black, S.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Artan, N.; Gu, H.; Dong, Z.; Burina Ganatra, L.; Shermon, S.; Rabin, E. Gait Study of Parkinson’s Disease Subjects Using Haptic Cues with A Motorized Walker. Sensors 2018, 18, 3549. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, A.; de Bruin, E.; Bruins, N.; Zijlstra, W. The step length-frequency relationship in physically active community-dwelling older women. Eur. J. Appl. Physiol. 2008, 104, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, U. Spatiotemporal gait analysis of older persons in clinical practice and research: Which parameters are relevant? Z. Gerontol. Geriatr. 2020, 53, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Grajic, M.; Stankovic, I.; Radovanovic, S.; Kostic, V. Gait in drug naive patients with de novo Parkinson’s disease--altered but symmetric. Neurol. Res. 2015, 37, 712–716. [Google Scholar] [CrossRef]
- Burleigh-Jacobs, A.; Horak, F.; Nutt, J.; Obeso, J. Step initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Mov. Disord. 1997, 12, 206–215. [Google Scholar] [CrossRef]
- Smulders, K.; Dale, M.; Carlson-Kuhta, P.; Nutt, J.; Horak, F.B. Pharmacological treatment in Parkinson’s disease: Effects on gait. Parkinsonism Relat. Disord. 2016, 31, 3–13. [Google Scholar] [CrossRef]
- Valkanova, V.; Esser, P.; Demnitz, N.; Sexton, C.E.; Zsoldos, E.; Mahmood, A.; Griffanti, L.; Kivimaki, M.; Singh-Manoux, A.; Dawes, H.; et al. Association between gait and cognition in an elderly population based sample. Gait Posture 2018, 65, 240–245. [Google Scholar] [CrossRef]
- Welzel, J.; Wendtland, D.; Warmerdam, E.; Romijnders, R.; Elshehabi, M.; Geritz, J.; Berg, D.; Hansen, C.; Maetzler, W. Step Length Is a Promising Progression Marker in Parkinson’s Disease. Sensors 2021, 21, 2292. [Google Scholar] [CrossRef]
- Demnitz, N.; Zsoldos, E.; Mahmood, A.; Mackay, C.E.; Kivimaki, M.; Singh-Manoux, A.; Dawes, H.; Johansen-Berg, H.; Ebmeier, K.P.; Sexton, C.E. Associations between Mobility, Cognition, and Brain Structure in Healthy Older Adults. Front. Aging Neurosci. 2017, 9, 155. [Google Scholar] [CrossRef]
- Bohannon, R.; Glenney, S. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2014, 20, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Mody, S.; Woodman, R.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.; Cudkowicz, M.; Firtion, R.; Wei, J.; Goldberger, A.L. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov. Disord. 1998, 13, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Herman, T.; Giladi, N.; Hausdorff, J.M. Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLoS ONE 2014, 9, e96675. [Google Scholar] [CrossRef]
- Godi, M.; Giardini, M.; Schieppati, M. Walking Along Curved Trajectories. Changes With Age and Parkinson’s Disease. Hints to Rehabilitation. Front. Neurol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Morris, M.; Iansek, R.; Matyas, T.; Summers, J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov. Disord. 1998, 13, 61–69. [Google Scholar] [CrossRef]
- Zanardi, A.P.J.; da Silva, E.S.; Costa, R.R.; Passos-Monteiro, E.; Dos Santos, I.O.; Kruel, L.F.M.; Peyre-Tartaruga, L.A. Gait parameters of Parkinson’s disease compared with healthy controls: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 752. [Google Scholar] [CrossRef]
- Schlachetzki, J.C.M.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e0183989. [Google Scholar] [CrossRef]
- van Hoeve, S.; Poeze, M. Multisegment Foot Models and Clinical Application After Foot and Ankle Trauma: A Review. J. Foot Ankle Surg. 2019, 58, 748–754. [Google Scholar] [CrossRef]
- Dipaola, M.; Pavan, E.; Cattaneo, A.; Frazzitta, G.; Pezzoli, G.; Cavallari, P.; Frigo, C.; Isaias, I.U. Mechanical Energy Recovery during Walking in Patients with Parkinson Disease. PLoS ONE 2016, 11, e0156420. [Google Scholar] [CrossRef]
- Serrao, M.; Chini, G.; Caramanico, G.; Bartolo, M.; Castiglia, S.; Ranavolo, A.; Conte, C.; Venditto, T.; Coppola, G.; di Lorenzo, C.; et al. Prediction of Responsiveness of Gait Variables to Rehabilitation Training in Parkinson’s Disease. Front. Neurol. 2019, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Lee, J.; Kim, J.; Lee, P.; Sohn, Y.H. Is Dominant-Side Onset Associated with a Better Motor Compensation in Parkinson’s Disease? Mov. Disord. 2015, 30, 1921–1925. [Google Scholar] [CrossRef] [PubMed]
- Yogev, G.; Plotnik, M.; Peretz, C.; Giladi, N.; Hausdorff, J.M. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: When does the bilateral coordination of gait require attention? Exp. Brain Res. 2007, 177, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Elshehabi, M.; Galna, B.; Hobert, M.A.; Warmerdam, E.; Suenkel, U.; Brockmann, K.; Metzger, F.; Hansen, C.; Berg, D.; et al. Gait analysis with wearables predicts conversion to parkinson disease. Ann. Neurol. 2019, 86, 357–367. [Google Scholar] [CrossRef]
- Harrison, M.; Wylie, S.; Frysinger, R.; Patrie, J.; Huss, D.; Currie, L.; Wooten, G.F. UPDRS activity of daily living score as a marker of Parkinson’s disease progression. Mov. Disord. 2009, 24, 224–230. [Google Scholar] [CrossRef]
- Chae, D.; Chung, S.J.; Lee, P.H.; Park, K. Predicting the longitudinal changes of levodopa dose requirements in Parkinson’s disease using item response theory assessment of real-world Unified Parkinson’s Disease Rating Scale. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 611–621. [Google Scholar] [CrossRef]
- Liguori, C.; De Franco, V.; Cerroni, R.; Spanetta, M.; Mercuri, N.B.; Stefani, A.; Pierantozzi, M.; Di Pucchio, A. Sleep problems affect quality of life in Parkinson’s disease along disease progression. Sleep Med. 2021, 81, 307–311. [Google Scholar] [CrossRef]
- Van Uem, J.; Walgaard, S.; Ainsworth, E.; Hasmann, S.; Heger, T.; Nussbaum, S.; Hobert, M.; Micó-Amigo, E.; Van Lummel, R.; Berg, D.; et al. Quantitative Timed-Up-and-Go Parameters in Relation to Cognitive Parameters and Health-Related Quality of Life in Mild-to-Moderate Parkinson’s Disease. PLoS ONE 2016, 11, e0151997. [Google Scholar] [CrossRef]


| HC | H–Y Ⅰ–Ⅱ | H–Y Ⅲ | H–Y Ⅳ | p | |
|---|---|---|---|---|---|
| N | 56 | 64 | 53 | 21 | |
| Age (years) | 62.36 ± 6.60 | 65.61 ± 9.47 | 66.66 ± 10.44 | 65.43 ± 6.90 | 0.066 |
| Height (cm) | 163.36 ± 5.98 | 165.20 ± 6.61 | 163.70 ± 8.23 | 163.57 ± 7.89 | 0.536 |
| Weight (kg) | 63.02 ± 8.37 | 65.99 ± 10.65 | 65.47 ± 11.86 | 64.50 ± 9.41 | 0.386 |
| Male (%) | 28 (50%) | 41 (64.1%) | 26 (49.1%) | 8 (38.1%) | 0.136 |
| Education (%) | 0.360 | ||||
| Illiteracy | 2 (3.6%) | 6 (9.4%) | 2 (3.8%) | 2 (9.5%) | |
| Primary school | 9 (16.1%) | 10 (15.6%) | 9 (17.0%) | 3 (14.3%) | |
| Middle school | 41 (73.2%) | 35 (54.7%) | 35 (66.0%) | 15 (71.4%) | |
| College | 4 (7.1%) | 13 (20.3%) | 7 (13.2%) | 1 (4.8%) | |
| Shoes size | 39.18 ± 1.89 | 39.97 ± 2.09 | 39.66 ± 2.06 | 39.71 ± 2.08 | 0.213 |
| PD duration (years) | 3.51 ± 3.73 | 8.13 ± 7.69 | 10.14 ± 3.93 | ||
| UPDRS III total scores | 22.77 ± 9.24 | 35.72 ± 11.81 | 55.14 ± 15.93 |
| HC | H–Y Ⅰ–Ⅱ | H–Y Ⅲ | H–Y Ⅳ | p | Post-Hoc | ||
|---|---|---|---|---|---|---|---|
| AI–SL | 2.29 ± 0.95 | 2.57 ± 1.41 | 3.05 ± 1.92 | 5.39 ± 4.87 | <0.001 | <0.001 1 | 0.002 2 |
| AI–ST | 7.38 ± 8.62 | 9.34 ± 9.35 | 11.64 ± 15.53 | 13.82 ± 12.31 | 0.087 | ||
| AI–StPT (%) | 5.51 ± 6.29 | 5.37 ± 5.17 | 7.80 ± 12.82 | 7.53 ± 9.58 | 0.794 | ||
| AI–SwPT (%) | 8.94 ± 8.89 | 9.21 ± 8.10 | 11.62 ± 13.97 | 13.66 ± 11.85 | 0.351 | ||
| AI–HS | 14.27 ± 9.66 | 16.89 ± 12.26 | 16.96 ± 11.98 | 21.84 ± 13.29 | 0.125 | ||
| AI–TO | 8.84 ± 7.04 | 10.42 ± 8.76 | 11.33 ± 12.09 | 11.88 ± 8.08 | 0.445 | ||
| AI–ROM–AJ | 9.59 ± 7.35 | 10.95 ± 8.48 | 14.17 ± 11.58 | 10.87 ± 10.84 | 0.131 | ||
| AI–ROM–KJ | 13.49 ± 10.43 | 16.22 ± 13.49 | 18.44 ± 14.62 | 16.54 ± 17.25 | 0.338 | ||
| AI–ROM–HJ | 8.84 ± 7.12 | 12.00 ± 9.81 | 13.82 ± 15.94 | 18.68 ± 14.81 | 0.045 | 0.030 1 | |
| H–Y Stage | UPDRS I | UPDRS II | UPDRS III | PDQ39 | |
|---|---|---|---|---|---|
| SL (m) | −0.566 (<0.001) | −0.155 (0.070) | −0.453 (<0.001) | −0.454 (<0.001) | −0.433 (<0.001) |
| GV (m/s) | −0.458 (<0.001) | −0.040 (0.644) | −0.334 (<0.001) | −0.376 (<0.001) | −0.269 (0.001) |
| CA (steps/min) | −0.052 (0.545) | 0.212 (0.012) | 0.001 (0.987) | −0.069 (0.421) | 0.110 (0.201) |
| ST (s) | 0.055 (0.518) | −0.210 (0.013) | 0.003 (0.970) | 0.075 (0.383) | −0.109 (0.204) |
| StPT (%) | 0.247 (0.003) | −0.073 (0.396) | 0.199 (0.019) | 0.229 (0.007) | 0.092 (0.284) |
| SwPT (%) | −0.268 (0.001) | 0.057 (0.504) | −0.216 (0.011) | −0.253 (0.003) | −0.062 (0.469) |
| CV−SL | 0.552 (<0.001) | 0.219 (0.010) | 0.500 (<0.001) | 0.463 (<0.001) | 0.358 (<0.001) |
| CV−ST | 0.401 (<0.001) | 0.194 (0.023) | 0.369 (<0.001) | 0.371 (<0.001) | 0.224 (0.008) |
| CV−StPT | 0.380 (<0.001) | 0.153 (0.074) | 0.315 (<0.001) | 0.311 (<0.001) | 0.229 (0.007) |
| CV−SwPT | 0.455 (<0.001) | 0.107 (0.211) | 0.373 (<0.001) | 0.346 (<0.001) | 0.252 (0.003) |
| walk radio | −0.431 (<0.001) | −0.263 (0.002) | −0.380 (<0.001) | −0.343 (<0.001) | −0.441 (<0.001) |
| ROM−AJ (°) | −0.382 (<0.001) | −0.250 (0.003) | −0.287 (0.001) | −0.281 (0.001) | −0.375 (<0.001) |
| ROM−KJ (°) | −0.429 (<0.001) | −0.107 (0.210) | −0.327 (<0.001) | −0.340 (<0.001) | −0.434 (<0.001) |
| ROM−HJ (°) | −0.517 (<0.001) | −0.118 (0.168) | −0.408 (<0.001) | −0.422 (<0.001) | −0.332 (<0.001) |
| HS (°) | −0.454 (<0.001) | −0.133(0.121) | −0.398 (<0.001) | −0.379 (<0.001) | −0.246 (0.004) |
| TO (°) | −0.171 (0.044) | −0.089 (0.302) | −0.105 (0.220) | −0.144 (<0.001) | −0.067 (0.434) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Wu, Z.; Wang, Y.; Jiang, Y.; Gu, R.; Zhong, M.; Jiang, X.; Shen, B.; Zhu, J.; Yan, J.; et al. Gait Analysis with Wearables Is a Potential Progression Marker in Parkinson’s Disease. Brain Sci. 2022, 12, 1213. https://doi.org/10.3390/brainsci12091213
Zhu S, Wu Z, Wang Y, Jiang Y, Gu R, Zhong M, Jiang X, Shen B, Zhu J, Yan J, et al. Gait Analysis with Wearables Is a Potential Progression Marker in Parkinson’s Disease. Brain Sciences. 2022; 12(9):1213. https://doi.org/10.3390/brainsci12091213
Chicago/Turabian StyleZhu, Sha, Zhuang Wu, Yaxi Wang, Yinyin Jiang, Ruxin Gu, Min Zhong, Xu Jiang, Bo Shen, Jun Zhu, Jun Yan, and et al. 2022. "Gait Analysis with Wearables Is a Potential Progression Marker in Parkinson’s Disease" Brain Sciences 12, no. 9: 1213. https://doi.org/10.3390/brainsci12091213







