Resting-State Brain Activity Dysfunctions in Schizophrenia and Their Associations with Negative Symptom Domains: An fMRI Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Psychopathological Assessment
2.3. MRI Data Acquisition and Pre-Processing
2.4. ROI Selection
2.5. Statistical Analyses
3. Results
3.1. Sample Characteristics
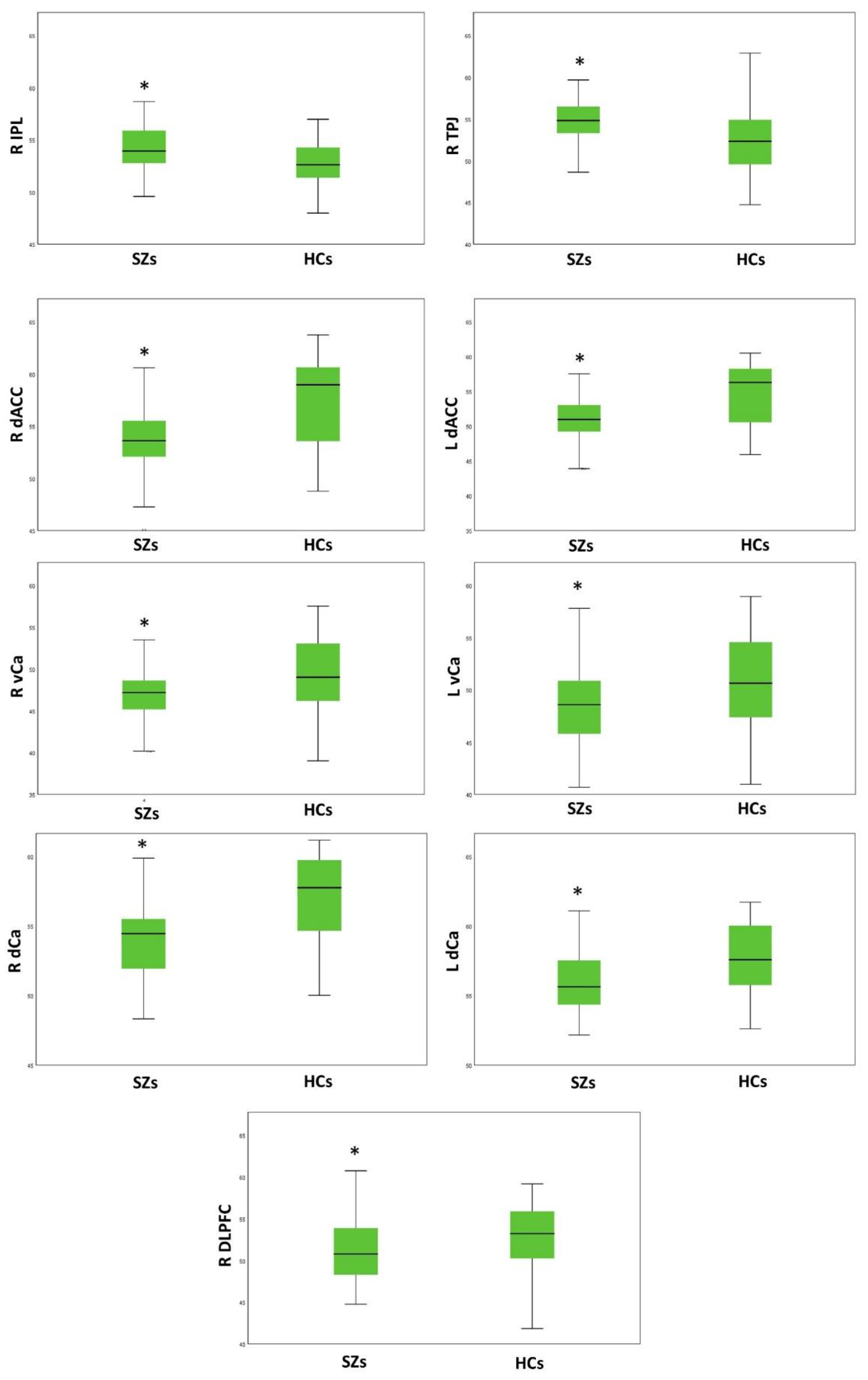
3.2. Group Comparison on Resting-State Activity
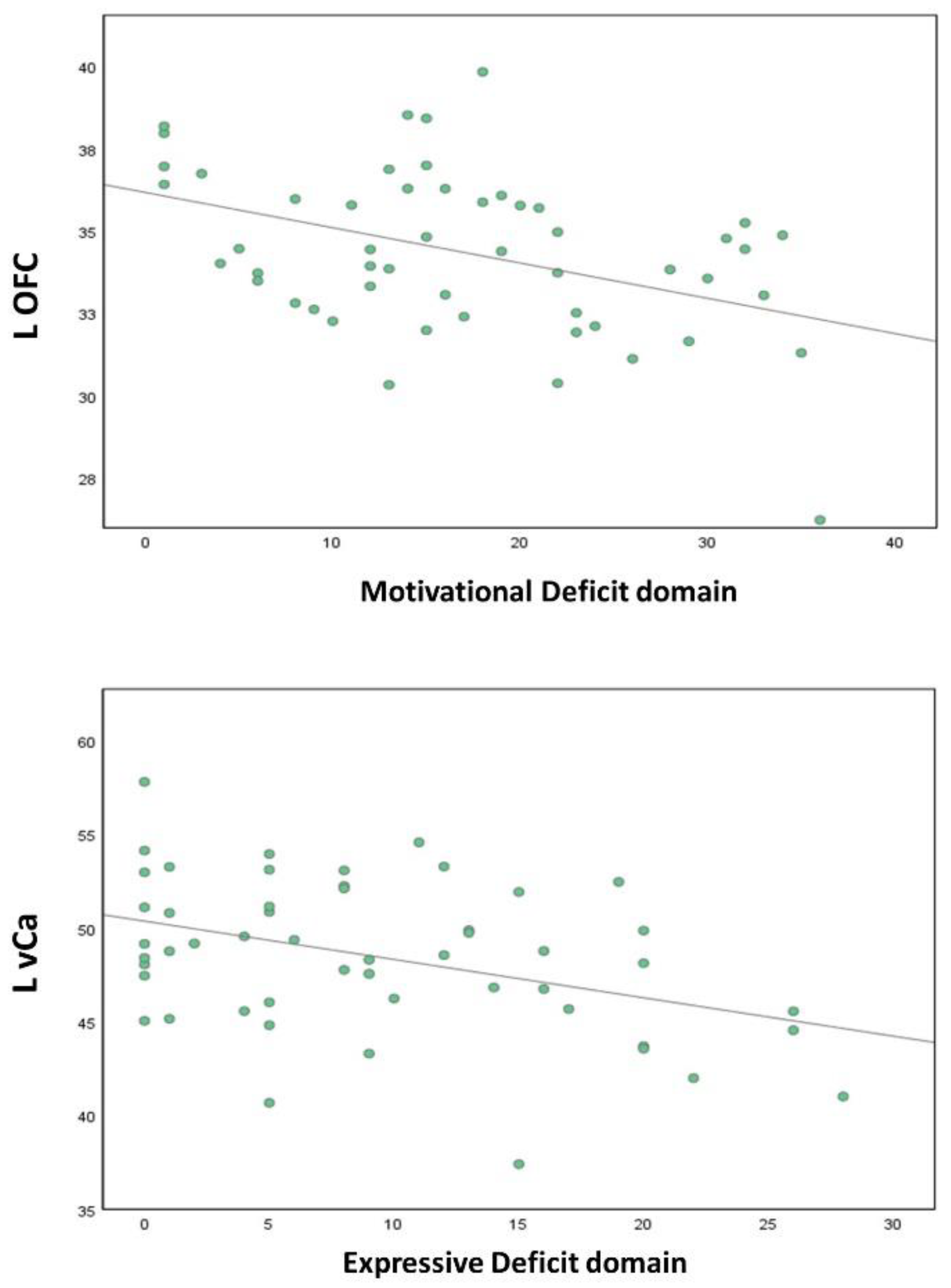
3.3. Correlation Analyses
3.4. Control Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galderisi, S.; Mucci, A.; Dollfus, S.; Nordentoft, M.; Falkai, P.; Kaiser, S.; Giordano, G.M.; Vandevelde, A.; Nielsen, M.; Glenthøj, L.B.; et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur. Psychiatry 2021, 64, e23. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.M.; Caporusso, E.; Pezzella, P.; Galderisi, S. Updated perspectives on the clinical significance of negative symptoms in patients with schizophrenia. Expert Rev. Neurother. 2022, 22, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; van Os, J.; De Hert, M.; Gaebel, W.; Galderisi, S.; Green, M.F.; Guloksuz, S.; Harvey, P.D.; Jones, P.B.; Malaspina, D.; et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry 2021, 20, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Novick, D.; Haro, J.M.; Suarez, D.; Vieta, E.; Naber, D. Recovery in the outpatient setting: 36-month results from the Schizophrenia Outpatients Health Outcomes (SOHO) study. Schizophr. Res. 2009, 108, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D.; Heaton, R.K.; Carpenter, W.T., Jr.; Green, M.F.; Gold, J.M.; Schoenbaum, M. Functional impairment in people with schizophrenia: Focus on employability and eligibility for disability compensation. Schizophr Res. 2012, 140, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galderisi, S.; Rossi, A.; Rocca, P.; Bertolino, A.; Mucci, A.; Bucci, P.; Rucci, P.; Gibertoni, D.; Aguglia, E.; Amore, M.; et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 2014, 13, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Galderisi, S.; Rucci, P.; Kirkpatrick, B.; Mucci, A.; Gibertoni, D.; Rocca, P.; Rossi, A.; Bertolino, A.; Strauss, G.P.; Aguglia, E.; et al. Interplay Among Psychopathologic Variables, Personal Resources, Context-Related Factors, and Real-life Functioning in Individuals with Schizophrenia: A Network Analysis. JAMA Psychiatry 2018, 75, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Galderisi, S.; Rucci, P.; Mucci, A.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Bozzatello, P.; et al. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: Stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry 2020, 19, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, L.; Giordano, G.M.; Bucci, P.; Pezzella, P.; Brando, F.; Galderisi, S. Improving Knowledge on Pathways to Functional Outcome in Schizophrenia: Main Results from the Italian Network for Research on Psychoses. Front. Psychiatry 2021, 12, 791117. [Google Scholar] [CrossRef]
- Mucci, A.; Galderisi, S.; Gibertoni, D.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Biondi, M.; et al. Factors Associated with Real-Life Functioning in Persons with Schizophrenia in a 4-Year Follow-up Study of the Italian Network for Research on Psychoses. JAMA Psychiatry 2021, 78, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Bitter, I.; Mohr, P.; Raspopova, N.; Szulc, A.; Samochowiec, J.; Micluia, I.V.; Skugarevsky, O.; Herold, R.; Mihaljevic-Peles, A.; Okribelashvili, N.; et al. Assessment and Treatment of Negative Symptoms in Schizophrenia-A Regional Perspective. Front. Psychiatry 2021, 12, 820801. [Google Scholar] [CrossRef] [PubMed]
- Căpățînă, O.O.; Micluția, I.V.; Fadgyas-Stănculete, M. Current perspectives in treating negative symptoms of schizophrenia: A narrative review (Review). Exp. Ther. Med. 2021, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Ostuzzi, G.; Bertolini, F.; Tedeschi, F.; Vita, G.; Brambilla, P.; del Fabro, L.; Gastaldon, C.; Papola, D.; Purgato, M.; Nosari, G.; et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: A network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry 2022, 21, 295–307. [Google Scholar] [CrossRef]
- Leichsenring, F.; Steinert, C.; Rabung, S.; Ioannidis, J.P.A. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: An umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry 2022, 21, 133–145. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Estradé, A.; Stanghellini, G.; Venables, J.; Onwumere, J.; Messas, G.; Gilardi, L.; Nelson, B.; Patel, V.; Bonoldi, I.; et al. The lived experience of psychosis: A bottom-up review co-written by experts by experience and academics. World Psychiatry 2022, 21, 168–188. [Google Scholar] [CrossRef]
- Sass, L. Subjectivity, psychosis and the science of psychiatry. World Psychiatry 2022, 21, 165–166. [Google Scholar] [CrossRef]
- Galderisi, S.; Kaiser, S.; Bitter, I.; Nordentoft, M.; Mucci, A.; Sabe, M.; Giordano, G.M.; Nielsen, M.; Glenthøj, L.B.; Pezzella, P.; et al. EPA guidance on treatment of negative symptoms in schizophrenia. Eur. Psychiatry 2021, 64, e21. [Google Scholar] [CrossRef]
- Giordano, G.M.; Brando, F.; Pezzella, P.; De Angelis, M.; Mucci, A.; Galderisi, S. Factors influencing the outcome of integrated therapy approach in schizophrenia: A narrative review of the literature. Front. Psychiatry 2022, 13, 970210. [Google Scholar] [CrossRef]
- Galderisi, S.; Riva, M.A.; Girardi, P.; Amore, M.; Carpiniello, B.; Aguglia, E.; Fagiolini, A.; Mucci, A.; Vita, A. Schizophrenia and “unmet needs”: From diagnosis to care in Italy. Eur. Psychiatry 2020, 63, e26. [Google Scholar] [CrossRef]
- Killaspy, H.; Harvey, C.; Brasier, C.; Brophy, L.; Ennals, P.; Fletcher, J.; Hamilton, B. Community-based social interventions for people with severe mental illness: A systematic review and narrative synthesis of recent evidence. World Psychiatry 2022, 21, 96–123. [Google Scholar] [CrossRef] [PubMed]
- Siskind, D.; Yung, A. After the acute crisis–engaging people with psychosis in rehabilitation-oriented care. World Psychiatry 2022, 21, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Fenton, W.S.; Carpenter, W.T., Jr.; Marder, S.R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 2006, 32, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Marder, S.R.; Galderisi, S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 2017, 16, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, W.T. Primary psychosis: More to know, much more to do. World Psychiatry. 2021, 20, 1–2. [Google Scholar] [CrossRef]
- Peralta, V.; Gil-Berrozpe, G.J.; Sánchez-Torres, A.; Cuesta, M.J. Clinical relevance of general and specific dimensions in bifactor models of psychotic disorders. World Psychiatry 2021, 20, 306–307. [Google Scholar] [CrossRef]
- Bègue, I.; Kaiser, S.; Kirschner, M. Pathophysiology of negative symptom dimensions of schizophrenia—Current developments and implications for treatment. Neurosci. Biobehav. Rev. 2020, 116, 74–88. [Google Scholar] [CrossRef]
- Kirschner, M.; Hager, O.M.; Bischof, M.; Hartmann, M.N.; Kluge, A.; Seifritz, E.; Tobler, P.N.; Kaiser, S. Ventral striatal hypoactivation is associated with apathy but not diminished expression in patients with schizophrenia. J. Psychiatry Neurosci. 2016, 41, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Mucci, A.; Dima, D.; Soricelli, A.; Volpe, U.; Bucci, P.; Frangou, S.; Prinster, A.; Salvatore, M.; Galderisi, S.; Maj, M. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol. Med. 2015, 45, 1765–1778. [Google Scholar] [CrossRef]
- Mucci, A.; Galderisi, S.; Amodio, A.; Dierks, T. Neuroimaging and Psychopathological Domains: Achievements and Perspectives. In Neuroimaging of Schizophrenia and Other Primary Psychotic Disorders; Springer: Berlin/Heidelberg, Germany, 2019; pp. 57–155. [Google Scholar] [CrossRef]
- Krueger, R.F.; Hobbs, K.A.; Conway, C.C.; Dick, D.M.; Dretsch, M.N.; Eaton, N.R.; Forbes, M.K.; Forbush, K.T.; Keyes, K.M.; Latzman, R.D.; et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): II. Externalizing superspectrum. World Psychiatry 2021, 20, 171–193. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Gaebel, W.; Maj, M.; Stein, D.J.; Kogan, C.S.; Saunders, J.B.; Poznyak, V.B.; Gureje, O.; Lewis-Fernández, R.; Maercker, A.; et al. An organization- and category-level comparison of diagnostic requirements for mental disorders in ICD-11 and DSM-5. World Psychiatry 2021, 20, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Lahey, B.B.; Moore, T.M.; Kaczkurkin, A.N.; Zald, D.H. Hierarchical models of psychopathology: Empirical support, implications, and remaining issues. World Psychiatry 2021, 20, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Amodio, A.; Quarantelli, M.; Mucci, A.; Prinster, A.; Soricelli, A.; Vignapiano, A.; Giordano, G.M.; Merlotti, E.; Nicita, A.; Galderisi, S. Avolition-Apathy and White Matter Connectivity in Schizophrenia: Reduced Fractional Anisotropy Between Amygdala and Insular Cortex. Clin. EEG Neurosci. 2018, 49, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.M.; Dowd, E.C. Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophr. Bull. 2010, 36, 919–934. [Google Scholar] [CrossRef] [Green Version]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [Green Version]
- Galderisi, S.; Merlotti, E.; Mucci, A. Neurobiological background of negative symptoms. Arch. Psychiatry Clin. Neurosci. 2015, 265, 543–558. [Google Scholar] [CrossRef]
- Giordano, G.M.; Pezzella, P.; Quarantelli, M.; Bucci, P.; Prinster, A.; Soricelli, A.; Perrottelli, A.; Giuliani, L.; Fabrazzo, M.; Galderisi, S. Investigating the Relationship between White Matter Connectivity and Motivational Circuits in Subjects with Deficit Schizophrenia: A Diffusion Tensor Imaging (DTI) Study. J. Clin. Med. 2021, 11, 61. [Google Scholar] [CrossRef]
- Giordano, G.M.; Stanziano, M.; Papa, M.; Mucci, A.; Prinster, A.; Soricelli, A.; Galderisi, S. Functional connectivity of the ventral tegmental area and avolition in subjects with schizophrenia: A resting state functional MRI study. Eur. Neuropsychopharmacol. 2018, 28, 589–602. [Google Scholar] [CrossRef]
- Morris, R.W.; Quail, S.; Griffiths, K.R.; Green, M.J.; Balleine, B.W. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol. Psychiatry 2015, 77, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Salamone, J.D.; Yohn, S.E.; Lopez-Cruz, L.; San Miguel, N.; Correa, M. Activational and effort-related aspects of motivation: Neural mechanisms and implications for psychopathology. Brain 2016, 139, 1325–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, D.J.; Shoptaw, S.J.; Vigo, D.V.; Lund, C.; Cuijpers, P.; Bantjes, J.; Sartorius, N.; Maj, M. Psychiatric diagnosis and treatment in the 21st century: Paradigm shifts versus incremental integration. World Psychiatry 2022, 21, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.M.; Levin-Aspenson, H.F.; Waszczuk, M.A.; Conway, C.C.; Dalgleish, T.; Dretschm, M.N.; Eaton, N.R.; Forbes, M.K.; Forbush, K.T.; Hobbs, K.A.; et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry 2022, 21, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Biller, A.; Walther, S.; Roesch-Ely, D.; Stippich, C.; Weisbrod, M.; Kaiser, S. Neural correlates of reward processing in schizophrenia--relationship to apathy and depression. Schizophr. Res. 2010, 118, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, E.K.; Culbreth, A.J.; Kandala, S.; Barch, D.M. From neuroimaging to daily functioning: A multimethod analysis of reward anticipation in people with schizophrenia. J. Abnorm. Psychol. 2019, 128, 723–734. [Google Scholar] [CrossRef]
- Stepien, M.; Manoliu, A.; Kubli, R.; Schneider, K.; Tobler, P.N.; Seifritz, E.; Herdener, M.; Kaiser, S.; Kirschner, M. Investigating the association of ventral and dorsal striatal dysfunction during reward anticipation with negative symptoms in patients with schizophrenia and healthy individuals. PLoS ONE 2018, 13, e0198215. [Google Scholar] [CrossRef] [Green Version]
- Wolf, D.H.; Satterthwaite, T.D.; Kantrowitz, J.J.; Katchmar, N.; Vandekar, L.; Elliott, M.A.; Ruparel, K. Amotivation in schizophrenia: Integrated assessment with behavioral, clinical, and imaging measures. Schizophr. Bull. 2014, 40, 1328–1337. [Google Scholar] [CrossRef]
- Schneider, K.; Michels, L.; Hartmann-Riemer, M.N.; Burrer, A.; Tobler, P.N.; Stampfli, P.; Kirschner, M.; Seifritz, E.; Kaiser, S. Cerebral blood flow in striatal regions is associated with apathy in patients with schizophrenia. J. Psychiatry Neurosci. 2019, 44, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Waltz, J.A.; Xu, Z.; Brown, E.C.; Ruiz, R.R.; Frank, M.J.; Gold, J.M. Motivational Deficits in Schizophrenia Are Associated with Reduced Differentiation Between Gain and Loss-Avoidance Feedback in the Striatum. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 239–247. [Google Scholar] [CrossRef]
- Waltz, J.A.; Schweitzer, J.B.; Ross, T.J.; Kurup, P.K.; Salmeron, B.J.; Rose, E.J.; Gold, J.M.; Stein, E.A. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology 2010, 35, 2427–2439. [Google Scholar] [CrossRef]
- Dowd, E.C.; Barch, D.M. Anhedonia and emotional experience in schizophrenia: Neural and behavioral indicators. Biol. Psychiatry 2010, 67, 902–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrondo, G.; Segarra, N.; Metastasio, A.; Ziauddeen, H.; Spencer, J.; Reinders, N.R.; Dudas, R.B.; Robbins, T.W.; Fletcher, P.C.; Murray, G.K. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: A replicated cross-diagnostic finding. Front. Psychol. 2015, 6, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, A.; Amad, A.; D’Hondt, F.; Pins, D.; Jaafari, N.; Thomas, P.; Jardri, R. Reward anticipation in schizophrenia: A coordinate-based meta-analysis. Schizophr. Res. 2020, 218, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Waltz, J.A.; Schweitzer, J.B.; Gold, J.M.; Kurup, P.K.; Ross, T.J.; Salmeron, B.J.; Rose, E.; McClure, S.M.; Stein, E.A. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology 2009, 34, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.O.; Rostrup, E.; Wulff, S.; Bak, N.; Broberg, B.V.; Lublin, H.; Kapur, S.; Glenthoj, B. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch. Gen. Psychiatry 2012, 69, 1195–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juckel, G. Inhibition of the reward system by antipsychotic treatment. Dialogues Clin. Neurosci. 2016, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kluge, A.; Kirschner, M.; Hager, O.M.; Bischof, M.; Habermeyer, B.; Seifritz, E.; Walther, S.; Kaiser, S. Combining actigraphy, ecological momentary assessment and neuroimaging to study apathy in patients with schizophrenia. Schizophr. Res. 2018, 195, 176–182. [Google Scholar] [CrossRef]
- Dowd, E.C.; Barch, D.M. Pavlovian reward prediction and receipt in schizophrenia: Relationship to anhedonia. PLoS ONE 2012, 7, e35622. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.S.; Barch, D.M. Frontal-striatum dysfunction during reward processing: Relationships to amotivation in schizophrenia. J. Abnorm. Psychol. 2016, 125, 453–469. [Google Scholar] [CrossRef]
- Koch, K.; Rus, O.G.; Reeß, T.J.; Schachtzabel, C.; Wagner, G.; Schultz, C.C.; Sorg, C.; Schlösser, R.G.M. Functional connectivity and grey matter volume of the striatum in schizophrenia. Br. J. Psychiatry 2014, 205, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Gradin, V.B.; Waiter, G.; O’Connor, A.; Romaniuk, L.; Stickle, C.; Matthews, K.; Hall, J.; Steele, J.D. Salience network-midbrain dysconnectivity and blunted reward signals in schizophrenia. Psychiatry Res. 2013, 211, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, A.; Palaniyappan, L.; Smieskova, R.; Simon, A.; Riecher-Rössler, A.; Lang, U.E.; Fusar-Poli, P.; McGuire, P.; Borgwardt, S.J. Dysfunctional insular connectivity during reward prediction in patients with first-episode psychosis. J. Psychiatry Neurosci. 2016, 41, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, D.K.; Chiappelli, J.J.; Sampath, H.; Kochunov, P.; Hare, S.M.; Wisner, K.; Rowland, L.M.; Hong, L.E. Aberrant Frontostriatal Connectivity in Negative Symptoms of Schizophrenia. Schizophr. Bull. 2019, 45, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.C.; Hsieh, J.C.; Li, C.T.; Bai, Y.M.; Su, T.P. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: A resting fMRI study. Neuroimage 2012, 59, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Harrison, B.J.; Goodby, E.; Dean, A.; Ooi, C.; Nathan, P.J.; Lennox, B.R.; Jones, P.B.; Suckling, J.; Bullmore, E.T. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 2013, 70, 1143–1151. [Google Scholar] [CrossRef] [Green Version]
- Forlim, C.G.; Klock, L.; Bächle, J.; Stoll, L.; Giemsa, P.; Fuchs, M.; Schoofs, N.; Montag, C.; Gallinat, J.; Kühn, S. Reduced Resting-State Connectivity in the Precuneus is correlated with Apathy in Patients with Schizophrenia. Sci. Rep. 2020, 10, 2616. [Google Scholar] [CrossRef] [Green Version]
- Abram, S.V.; Wisner, K.M.; Fox, J.M.; Barch, D.M.; Wang, L.; Csernansky, J.G.; MacDonald, A.W.; Smith, M.J. Fronto-temporal connectivity predicts cognitive empathy deficits and experiential negative symptoms in schizophrenia. Hum. Brain Mapp. 2017, 38, 1111–1124. [Google Scholar] [CrossRef]
- Mucci, A.; Merlotti, E.; Üçok, A.; Aleman, A.; Galderisi, S. Primary and persistent negative symptoms: Concepts, assessments and neurobiological bases. Schizophr. Res. 2017, 186, 19–28. [Google Scholar] [CrossRef]
- Giordano, G.M.; Brando, F.; Perrottelli, A.; Di Lorenzo, G.; Siracusano, A.; Giuliani, L.; Pezzella, P.; Altamura, M.; Bellomo, A.; Cascino, G.; et al. Tracing Links Between Early Auditory Information Processing and Negative Symptoms in Schizophrenia: An ERP Study. Front. Psychiatry 2021, 12, 790745. [Google Scholar] [CrossRef]
- Kaiser, S.; Lyne, J.; Agartz, I.; Clarke, M.; Mørch-Johnsen, L.; Faerden, A. Individual negative symptoms and domains—Relevance for assessment, pathomechanisms and treatment. Schizophr. Res. 2017, 186, 39–45. [Google Scholar] [CrossRef]
- Moritz, S.; Silverstein, S.M.; Dietrichkeit, M.; Gallinat, J. Neurocognitive deficits in schizophrenia are likely to be less severe and less related to the disorder than previously thought. World Psychiatry 2020, 19, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Kotov, R.; Jonas, K.G.; Carpenter, W.T.; Dretsch, M.N.; Eaton, N.R.; Forbes, M.K.; Forbush, K.T.; Hobbs, K.; Reininghaus, U.; Slade, T.; et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry 2020, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Lysaker, P.H.; Hasson-Ohayon, I. Metacognition in psychosis: A renewed path to understanding of core disturbances and recovery-oriented treatment. World Psychiatry 2021, 20, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J. Computer-based virtual reality assessment of functional capacity in primary psychosis. World Psychiatry 2022, 21, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, T.; Judd, N.; Sauce, B. Assessing the impact of environmental factors on the adolescent brain: The importance of regional analyses and genetic controls. World Psychiatry 2022, 21, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Peralta, V.; Moreno-Izco, L.; Sanchez-Torres, A.; García de Jalón, E.; Campos, M.S.; Cuesta, M.J. Characterization of the deficit syndrome in drug-naive schizophrenia patients: The role of spontaneous movement disorders and neurological soft signs. Schizophr. Bull. 2014, 40, 214–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hager, O.M.; Kirschner, M.; Bischof, M.; Hartmann-Riemer, M.N.; Kluge, A.; Seifritz, E.; Tobler, P.N.; Kaiser, S. Reward-dependent modulation of working memory is associated with negative symptoms in schizophrenia. Schizophr. Res. 2015, 168, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Rahm, C.; Liberg, B.; Reckless, G.; Ousdal, O.; Melle, I.; Andreassen, O.A.; Agartz, I. Negative symptoms in schizophrenia show association with amygdala volumes and neural activation during affective processing. Acta Neuropsychiatr. 2015, 27, 213–220. [Google Scholar] [CrossRef]
- Gur, R.E.; Loughead, J.; Kohler, C.G.; Elliott, M.A.; Lesko, K.; Ruparel, K.; Wolf, D.H.; Bilker, W.B.; Gur, R.C. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch. Gen. Psychiatry 2007, 64, 1356–1366. [Google Scholar] [CrossRef]
- Lepage, M.; Sergerie, K.; Benoit, A.; Czechowska, Y.; Dickie, E.; Armony, J.L. Emotional face processing and flat affect in schizophrenia: Functional and structural neural correlates. Psychol. Med. 2011, 41, 1833–1844. [Google Scholar] [CrossRef]
- Lindner, C.; Dannlowski, U.; Bauer, J.; Ohrmann, P.; Lencer, R.; Zwitserlood, P.; Kugel, H.; Suslow, T. Affective Flattening in Patients with Schizophrenia: Differential Association with Amygdala Response to Threat-Related Facial Expression under Automatic and Controlled Processing Conditions. Psychiatry Investig. 2016, 13, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stip, E.; Fahim, C.; Liddle, P.; Mancini-Marïe, A.; Mensour, B.; Bentaleb, L.A.; Beauregard, M. Neural correlates of sad feelings in schizophrenia with and without blunted affect. Can. J. Psychiatry 2005, 50, 909–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingoia, G.; Wagner, G.; Langbein, K.; Maitra, R.; Smesny, S.; Dietzek, M.; Burmeister, H.P.; Reichenbach, J.R.; Schlösser, R.G.; Gaser, C.; et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr. Res. 2012, 138, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hare, S.M.; Ford, J.M.; Mathalon, D.H.; Damaraju, E.; Bustillo, J.; Belger, A.; Lee, H.J.; Mueller, B.M.; Lim, K.; Brown, G.G.; et al. Salience-Default Mode Functional Network Connectivity Linked to Positive and Negative Symptoms of Schizophrenia. Schizophr. Bull. 2019, 45, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J.J.; Peterson, M.J.; McMahon, M.A.; Bizzell, J.; Calhoun, V.; van Erp, T.G.; Ford, J.M.; Lauriello, J.; Lim, K.O.; Manoach, D.S.; et al. Neural Correlates of Schizophrenia Negative Symptoms: Distinct Subtypes Impact Dissociable Brain Circuits. Mol. Neuropsychiatry 2015, 1, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Andreasen, N.C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br. J. Psychiatry Suppl. 1989, 155, 49–58. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Strauss, G.P.; Nguyen, L.; Fischer, B.A.; Daniel, D.G.; Cienfuegos, A.; Marder, S.R. The brief negative symptom scale: Psychometric properties. Schizophr. Bull. 2011, 37, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Wallwork, R.S.; Fortgang, R.; Hashimoto, R.; Weinberger, D.R.; Dickinson, D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr. Res. 2012, 137, 246–250. [Google Scholar] [CrossRef]
- Mucci, A.; Galderisi, S.; Merlotti, E.; Rossi, A.; Rocca, P.; Bucci, P.; Piegari, G.; Chieffi, M.; Vignapiano, A.; Maj, M. The Brief Negative Symptom Scale (BNSS): Independent validation in a large sample of Italian patients with schizophrenia. Eur. Psychiatry 2015, 30, 641–647. [Google Scholar] [CrossRef]
- Addington, J.; Shah, H.; Liu, L.; Addington, D. Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophr. Res. 2014, 153, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlach, J.; Korsgaard, S.; Clemmesen, P.; Lauersen, A.M.; Magelund, G.; Noring, U.; Povlsen, U.J.; Bech, P.; Casey, D. The St. Hans Rating Scale for extrapyramidal syndromes: Reliability and validity. Acta Psychiatr. Scand. 1993, 87, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Venkatasubramanian, G.; Jayakumar, P.N.; Keshavan, M.S.; Gangadhar, B.N. Schneiderian first rank symptoms and inferior parietal lobule cortical thickness in antipsychotic-naïve schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 40–46. [Google Scholar] [CrossRef]
- Jardri, R.; Pouchet, A.; Pins, D.; Thomas, P. Cortical activations during auditory verbal hallucinations in schizophrenia: A coordinate-based meta-analysis. Am. J. Psychiatry 2011, 168, 73–81. [Google Scholar] [CrossRef]
- Taylor, S.F.; Kang, J.; Brege, I.S.; Tso, I.F.; Hosanagar, A.; Johnson, T.D. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol. Psychiatry 2012, 71, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Tikka, S.K.; Nizamie, S.H.; Venkatesh Babu, G.M.; Aggarwal, N.; Das, A.K.; Goyal, N. Safety and Efficacy of Adjunctive Θ Burst Repetitive Transcranial Magnetic Stimulation to Right Inferior Parietal Lobule in Schizophrenia Patients with First-Rank Symptoms: A Pilot, Exploratory Study. J. ECT 2017, 33, 43–51. [Google Scholar] [CrossRef]
- Farrer, C.; Franck, N.; Georgieff, N.; Frith, C.D.; Decety, J.; Jeannerod, M. Modulating the experience of agency: A positron emission tomography study. Neuroimage 2003, 18, 324–333. [Google Scholar] [CrossRef]
- Ruby, P.; Decety, J. Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nat. Neurosci. 2001, 4, 546–550. [Google Scholar] [CrossRef]
- Plaze, M.; Mangin, J.F.; Paillère-Martinot, M.L.; Artiges, E.; Olié, J.P.; Krebs, M.O.; Gaillard, R.; Martinot, J.-L.; Cachia, A. “Who is talking to me?”—Self-other attribution of auditory hallucinations and sulcation of the right temporoparietal junction. Schizophr. Res. 2015, 169, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.J.; Frith, C. Self-awareness and action. Curr. Opin. Neurobiol. 2003, 13, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Chaminade, T.; Decety, J. Leader or follower? Involvement of the inferior parietal lobule in agency. NeuroReport 2002, 13, 1975–1978. [Google Scholar] [CrossRef] [PubMed]
- Decety, J.; Chaminade, T.; Grèzes, J.; Meltzoff, A.N. A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage 2002, 15, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.S.; Yan, P.; Bergquist, K.L.; Sinha, R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage 2007, 38, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, V.; Adleman, N.E.; White, C.D.; Glover, G.H.; Reiss, A.L. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain Mapp. 2001, 12, 131–143. [Google Scholar] [CrossRef]
- Salgado-Pineda, P.; Fuentes-Claramonte, P.; Spanlang, B.; Pomes, A.; Landin-Romero, R.; Portillo, F.; Bosque, C.; Franquelo, J.C.; Teixido, C.; Sarró, S.; et al. Neural correlates of disturbance in the sense of agency in schizophrenia: An fMRI study using the ‘enfacement’ paradigm. Schizophr. Res. 2022, 243, 395–401. [Google Scholar] [CrossRef]
- Walter, H.; Ciaramidaro, A.; Adenzato, M.; Vasic, N.; Ardito, R.B.; Erk, S.; Bara, B.G. Dysfunction of the social brain in schizophrenia is modulated by intention type: An fMRI study. Soc. Cogn. Affect. Neurosci. 2009, 4, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Penner, J.; Osuch, E.A.; Schaefer, B.; Théberge, J.; Neufeld, R.W.J.; Menon, R.S.; Rajakumar, N.; Williamson, P.C. Temporoparietal Junction Functional Connectivity in Early Schizophrenia and Major Depressive Disorder. Chronic Stress 2018, 2, 2470547018815232. [Google Scholar] [CrossRef]
- Lee, J.; Horan, W.P.; Wynn, J.K.; Green, M.F. Neural Correlates of Belief and Emotion Attribution in Schizophrenia. PLoS ONE 2016, 11, e0165546. [Google Scholar] [CrossRef] [Green Version]
- Bedford, N.J.; Surguladze, S.; Giampietro, V.; Brammer, M.J.; David, A.S. Self-evaluation in schizophrenia: An fMRI study with implications for the understanding of insight. BMC Psychiatry 2012, 12, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Minzenberg, M.J.; Ursu, S.; Ryan Walter, B.S.; Wendelken, C.; Ragland, J.D.; Carter, C.S.; Walter, B.S.R. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: Relationship with impaired cognition, behavioral disorganization, and global function. Am. J. Psychiatry 2008, 165, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minzenberg, M.J.; Laird, A.R.; Thelen, S.; Carter, C.S.; Glahn, D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry 2009, 66, 811–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesh, T.A.; Westphal, A.J.; Niendam, T.A.; Yoon, J.H.; Minzenberg, M.J.; Ragland, J.D.; Solomon, M.; Carter, C.S. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage Clin. 2013, 2, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Chechko, N.; Cieslik, E.C.; Müller, V.I.; Nickl-Jockschat, T.; Derntl, B.; Kogler, L.; Aleman, A.; Jardri, R.; Sommer, I.E.; Gruber, O.; et al. Differential Resting-State Connectivity Patterns of the Right Anterior and Posterior Dorsolateral Prefrontal Cortices (DLPFC) in Schizophrenia. Front. Psychiatry 2018, 9, 211. [Google Scholar] [CrossRef]
- Smucny, J.; Dienel, S.J.; Lewis, D.A.; Carter, C.S. Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology 2022, 47, 292–308. [Google Scholar] [CrossRef]
- Kristiansen, L.V.; Beneyto, M.; Haroutunian, V.; Meador-Woodruff, J.H. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol. Psychiatry 2006, 11, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.; David, A.S. Patterns of anterior cingulate activation in schizophrenia: A selective review. Neuropsychiatr. Dis. Treat. 2007, 3, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.B.; Liu, J.; Wang, L.X.; Li, C.; Xi, Y.B.; Guo, F.; Wang, H.-N.; Zhang, L.-C.; Liu, W.-M.; He, H.; et al. Anterior cingulate cortex-related connectivity in first-episode schizophrenia: A spectral dynamic causal modeling study with functional magnetic resonance imaging. Front. Hum. Neurosci. 2015, 9, 589. [Google Scholar] [CrossRef]
- Huang, M.L.; Khoh, T.T.; Lu, S.J.; Pan, F.; Chen, J.K.; Hu, J.B.; Hu, S.-H.; Xu, W.-J.; Zhou, W.-H.; Wei, N.; et al. Relationships between dorsolateral prefrontal cortex metabolic change and cognitive impairment in first-episode neuroleptic-naive schizophrenia patients. Medicine 2017, 96, e7228. [Google Scholar] [CrossRef]
- Giordano, G.M.; Koenig, T.; Mucci, A.; Vignapiano, A.; Amodio, A.; Di Lorenzo, G.; Siracusano, A.; Bellomo, A.; Altamura, M.; Monteleone, P.; et al. Neurophysiological correlates of Avolition-apathy in schizophrenia: A resting-EEG microstates study. Neuroimage Clin. 2018, 20, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Ballard, I.C.; Murty, V.P.; Carter, R.M.; MacInnes, J.J.; Huettel, S.A.; Adcock, R.A. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J. Neurosci. 2011, 31, 10340–10346. [Google Scholar] [CrossRef] [PubMed]
- Cadena, E.J.; White, D.M.; Kraguljac, N.V.; Reid, M.A.; Lahti, A.C. Evaluation of fronto-striatal networks during cognitive control in unmedicated patients with schizophrenia and the effect of antipsychotic medication. NPJ Schizophr. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornito, A.; Yücel, M.; Dean, B.; Wood, S.J.; Pantelis, C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: Bridging the gap between neuroimaging and neuropathology. Schizophr. Bull. 2009, 35, 973–993. [Google Scholar] [CrossRef] [PubMed]
- Palaniyappan, L.; Liddle, P.F. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012, 37, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Nguyen, P.T.; Schwab, N.A.; Tanner, J.J.; Price, C.C.; Ding, M. Mapping Dorsal and Ventral Caudate in Older Adults: Method and Validation. Front. Aging Neurosci. 2017, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Robbins, T.W.; Giardini, V.; Jones, G.H.; Reading, P.; Sahakian, B.J. Effects of dopamine depletion from the caudate-putamen and nucleus accumbens septi on the acquisition and performance of a conditional discrimination task. Behav. Brain Res. 1990, 38, 243–261. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Merritt, K.; Howes, O.D. Dopamine and glutamate in individuals at high risk for psychosis: A meta-analysis of in vivo imaging findings and their variability compared to controls. World Psychiatry 2021, 20, 405–416. [Google Scholar] [CrossRef]
- Haijma, S.V.; Van Haren, N.; Cahn, W.; Koolschijn, P.C.; Hulshoff Pol, H.E.; Kahn, R.S. Brain volumes in schizophrenia: A meta-analysis in over 18,000 subjects. Schizophr. Bull. 2013, 39, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, E.; Bellani, M.; Crespo-Facorro, B.; Brambilla, P. Basal ganglia anatomy and schizophrenia: The role of antipsychotic treatment. Epidemiol. Psychiatr. Sci. 2014, 23, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Duan, M.; Chen, X.; He, H.; Jiang, Y.; Jiang, S.; Xie, Q.; Lai, Y.; Luo, C.; Yao, D. Altered Basal Ganglia Network Integration in Schizophrenia. Front. Hum. Neurosci. 2015, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Costas, E.; Melendez-Ferro, M.; Roberts, R.C. Basal ganglia pathology in schizophrenia: Dopamine connections and anomalies. J Neurochem. 2010, 113, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Provost, J.S.; Hanganu, A.; Monchi, O. Neuroimaging studies of the striatum in cognition Part I: Healthy individuals. Front. Syst. Neurosci. 2015, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Howes, O.D.; Montgomery, A.J.; Asselin, M.-C.; Murray, R.M.; Valli, I.; Tabraham, P.; Bramon-Bosch, E.; Valmaggia, L.; Johns, L.; Broome, M.; et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry 2009, 66, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorg, C.; Manoliu, A.; Neufang, S.; Myers, N.; Peters, H.; Schwerthöffer, D.; Scherr, M.; Mühlau, M.; Zimmer, C.; Drzezga, A.; et al. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr. Bull. 2013, 39, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kirino, E.; Tanaka, S.; Fukuta, M.; Inami, R.; Inoue, R.; Aoki, S. Functional Connectivity of the Caudate in Schizophrenia Evaluated with Simultaneous Resting-State Functional MRI and Electroencephalography Recordings. Neuropsychobiology 2019, 77, 165–175. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Feng, J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef]
- Rolls, E.T. The Orbitofrontal Cortex; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for action, emotion, and memory. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 166, pp. 23–37. [Google Scholar] [CrossRef]
- Rushworth, M.F.; Kolling, N.; Sallet, J.; Mars, R.B. Valuation and decision-making in frontal cortex: One or many serial or parallel systems? Curr. Opin. Neurobiol. 2012, 22, 946–955. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013, 37, 1946–1954. [Google Scholar] [CrossRef] [Green Version]
- Strauss, G.P.; Waltz, J.A.; Gold, J.M. A review of reward processing and motivational impairment in schizophrenia. Schizophr. Bull. 2014, 40 (Suppl. 2), S107–S116. [Google Scholar] [CrossRef] [Green Version]
- Mørch-Johnsen, L.; Nesvåg, R.; Faerden, A.; Haukvik, U.K.; Jørgensen, K.N.; Lange, E.H.; Andreassen, O.A.; Melle, I.; Agartz, I. Brain structure abnormalities in first-episode psychosis patients with persistent apathy. Schizophr. Res. 2015, 164, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtani, T.; Bouix, S.; Hosokawa, T.; Saito, Y.; Eckbo, R.; Ballinger, T.; Rausch, A.; Melonakos, E.; Kubicki, M. Abnormalities in white matter connections between orbitofrontal cortex and anterior cingulate cortex and their associations with negative symptoms in schizophrenia: A DTI study. Schizophr. Res. 2014, 157, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demjaha, A.; Galderisi, S.; Glenthøj, B.; Arango, C.; Mucci, A.; Lawrence, A.; O’Daly, O.; Kempton, M.; Ciufolini, S.; Baandrup, L.; et al. Negative symptoms in First-Episode Schizophrenia related to morphometric alterations in orbitofrontal and superior temporal cortex: The OPTiMiSE study. Psychol. Med. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.C.; Frost, L.; Linsenbardt, D.; McIlroy, J.R.; Müller, R.A. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav. Brain Funct. 2006, 2, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huettel, S.A.; Misiurek, J. Modulation of prefrontal cortex activity by information toward a decision rule. NeuroReport 2004, 15, 1883–1886. [Google Scholar] [CrossRef] [Green Version]
- Parkes, S.L.; Bradfield, L.A.; Balleine, B.W. Interaction of insular cortex and ventral striatum mediates the effect of incentive memory on choice between goal-directed actions. J. Neurosci. 2015, 35, 6464–6471. [Google Scholar] [CrossRef] [Green Version]
- Haber, S.N.; Fudge, J.L.; McFarland, N.R. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000, 20, 2369–2382. [Google Scholar] [CrossRef] [Green Version]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef] [Green Version]
- Tricomi, E.M.; Delgado, M.R.; Fiez, J.A. Modulation of caudate activity by action contingency. Neuron 2004, 41, 281–292. [Google Scholar] [CrossRef] [Green Version]
- O’Doherty, J.; Dayan, P.; Schultz, J.; Deichmann, R.; Friston, K.; Dolan, R.J. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 2004, 304, 452–454. [Google Scholar] [CrossRef] [Green Version]
- da Silva Alves, F.; Bakker, G.; Schmitz, N.; Abeling, N.; Hasler, G.; van der Meer, J.; Nederveen, A.; de Haan, L.; Linszen, D.; van Amelsvoort, T. Dopaminergic modulation of the reward system in schizophrenia: A placebo-controlled dopamine depletion fMRI study. Eur. Neuropsychopharmacol. 2013, 23, 1577–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekroud, A.M.; Bondar, J.; Delgadillo, J.; Doherty, G.; Wasil, A.; Fokkema, M.; Cohen, Z.; Belgrave, D.; DeRubeis, R.; Iniesta, R.; et al. The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry 2021, 20, 154–170. [Google Scholar] [CrossRef] [PubMed]
| SZs (N = 62) | HCs (N = 46) | p | |
|---|---|---|---|
| Age | 37.92 ± 10.58 | 30.07 ± 8.11 | 6.3 × 10−5 |
| Education | 12.56 ± 3.15 | 16.37 ± 3.62 | 6.0 × 10−8 |
| Gender (M/F) | 37/25 | 25/21 | 0.580 |
| PANSS Total score | 60.20 ± 19.54 | ||
| PANSS Positive | 7.59 ± 3.64 | ||
| PANSS Negative | 12.87 ± 6.57 | ||
| PANSS Disorganization (item P2) | 1.84 ± 0.97 | ||
| BNSS Total score | 28.00 ± 17.61 | ||
| BNSS Motivational Deficit | 16.98 ± 9.77 | ||
| BNSS Expressive Deficit | 9.21 ± 7.94 | ||
| CDSS total score | 3.95 ± 3.98 | ||
| SHRS global Parkinsonism | 0.40 ± 0.88 | ||
| Type of AP medication (%) | 77.4 % second-generation AP; 10.5% first-generation AP; and 12.1% both |
| Brain Regions | SZs | HCs | F | p | ||
|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | |||
| Right Hemisphere | ||||||
| DLPFC | 51.55 | 4.04 | 52.94 | 3.93 | 10.029 | 0.002 |
| VLPFC | 51.61 | 2.34 | 50.73 | 2.47 | 3.786 | 0.054 |
| OFC | 35.18 | 3.19 | 37.05 | 3.61 | 5.847 | 0.017 * |
| STG | 49.43 | 2.55 | 49.52 | 2.28 | 0.008 | 0.929 |
| IPL | 54.38 | 2.58 | 52.82 | 2.41 | 10.711 | 0.001 |
| TPJ | 55.18 | 2.88 | 52.49 | 3.76 | 16.723 | 8.5 × 10−5 |
| Pcun | 59.05 | 2.57 | 57.88 | 3.00 | 5.262 | 0.024 * |
| daIC | 59.04 | 2.10 | 59.28 | 2.51 | 0.018 | 0.894 |
| vaIC | 57.00 | 3.52 | 56.23 | 3.82 | 1.093 | 0.298 |
| pIC | 58.76 | 2.40 | 59.04 | 2.41 | 0.179 | 0.673 |
| daCC | 54.27 | 3.85 | 57.74 | 3.92 | 28.201 | 6.24 × 10−7 |
| LOC | 50.28 | 3.75 | 47.97 | 4.34 | 7.842 | 0.006 * |
| Amy | 50.71 | 5.39 | 48.62 | 5.50 | 3.653 | 0.059 |
| NA | 44.37 | 5.34 | 44.03 | 6.76 | 0.273 | 0.602 |
| vCa | 46.75 | 3.11 | 49.36 | 4.61 | 14.000 | 3 × 10−4 |
| dCa | 54.05 | 2.68 | 57.20 | 2.82 | 32.945 | 9.44 × 10−8 |
| Pu | 48.83 | 3.66 | 52.25 | 4.10 | 8.128 | 0.005 * |
| Left Hemisphere | ||||||
| DLPFC | 52.33 | 2.93 | 52.12 | 3.09 | 0.685 | 0.410 |
| VLPFC | 51.80 | 2.99 | 50.10 | 2.67 | 7.091 | 0.009 * |
| OFC | 34.29 | 2.43 | 35.36 | 2.82 | 2.687 | 0.104 |
| STG | 52.88 | 3.13 | 52.87 | 2.29 | 0.171 | 0.680 |
| IPL | 52.38 | 3.18 | 52.34 | 2.58 | 0.275 | 0.601 |
| TPJ | 54.97 | 3.77 | 54.76 | 2.90 | 0.075 | 0.784 |
| Pcun | 57.50 | 1.92 | 57.51 | 1.82 | 0.215 | 0.644 |
| daIC | 59.20 | 3.07 | 59.58 | 2.15 | 0.005 | 0.941 |
| vaIC | 57.57 | 4.29 | 56.30 | 2.95 | 3.233 | 0.075 |
| pIC | 60.55 | 3.72 | 60.69 | 2.36 | 0.725 | 0.396 |
| daCC | 51.51 | 4.06 | 54.95 | 4.22 | 23.107 | 5 × 10−6 |
| LOC | 48.34 | 4.93 | 47.91 | 4.59 | 0.091 | 0.764 |
| Amy | 51.28 | 5.59 | 48.78 | 5.38 | 5.802 | 0.018 * |
| NA | 45.55 | 3.52 | 46.60 | 3.96 | 1.011 | 0.317 |
| vCa | 48.39 | 3.81 | 50.62 | 4.64 | 9.465 | 0.003 |
| dCa | 55.88 | 2.46 | 57.70 | 2.57 | 9.950 | 0.002 |
| Pu | 50.10 | 3.87 | 53.24 | 3.42 | 5.617 | 0.020 * |
| Brain Regions | BNSS Motivational Deficit | BNSS Expressive Deficit | ||
|---|---|---|---|---|
| Pearson’s Coefficient | p | Pearson’s Coefficient | p | |
| R STG | 0.178 | 0.208 | 0.363 | 0.008 * |
| R Amy | −0.260 | 0.063 | −0.266 | 0.057 |
| L OFC | −0.424 | 0.002 ** | −0.344 | 0.013 * |
| L IPL | 0.323 | 0.020 * | 0.205 | 0.145 |
| L vaIC | −0.284 | 0.041 * | −0.295 | 0.033 * |
| L vCa | −0.367 | 0.007 * | −0.401 | 0.003 ** |
| R dCa | −0.343 | 0.013 * | −0.225 | 0.108 |
| L dCa | −0.346 | 0.012 * | −0.240 | 0.086 |
| Left OFC | Left vCa | |||
|---|---|---|---|---|
| Pearson’s Coefficient | p | Pearson’s Coefficient | p | |
| BNSS Total score | −0.420 | 0.002 * | −0.407 | 0.003 * |
| Motivational Deficit | −0.424 | 0.002 * | −0.367 | 0.007 # |
| Avolition | −0.442 | 0.001 * | - | - |
| Asociality | −0.432 | 0.001 * | - | - |
| Anhedonia | −0.333 | 0.016 * | - | - |
| Expressive Deficit | −0.344 | 0.013 * | −0.401 | 0.003 * |
| Blunted affect | - | - | −0.394 | 0.004 * |
| Alogia | - | - | −0.378 | 0.006 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, G.M.; Pezzella, P.; Giuliani, L.; Fazio, L.; Mucci, A.; Perrottelli, A.; Blasi, G.; Amore, M.; Rocca, P.; Rossi, A.; et al. Resting-State Brain Activity Dysfunctions in Schizophrenia and Their Associations with Negative Symptom Domains: An fMRI Study. Brain Sci. 2023, 13, 83. https://doi.org/10.3390/brainsci13010083
Giordano GM, Pezzella P, Giuliani L, Fazio L, Mucci A, Perrottelli A, Blasi G, Amore M, Rocca P, Rossi A, et al. Resting-State Brain Activity Dysfunctions in Schizophrenia and Their Associations with Negative Symptom Domains: An fMRI Study. Brain Sciences. 2023; 13(1):83. https://doi.org/10.3390/brainsci13010083
Chicago/Turabian StyleGiordano, Giulia Maria, Pasquale Pezzella, Luigi Giuliani, Leonardo Fazio, Armida Mucci, Andrea Perrottelli, Giuseppe Blasi, Mario Amore, Paola Rocca, Alessandro Rossi, and et al. 2023. "Resting-State Brain Activity Dysfunctions in Schizophrenia and Their Associations with Negative Symptom Domains: An fMRI Study" Brain Sciences 13, no. 1: 83. https://doi.org/10.3390/brainsci13010083








