Applying Retinal Vascular Structures Characteristics Coupling with Cortical Visual System in Alzheimer’s Disease Spectrum Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Assessments
2.3. Optical Coherence Tomography Imagine
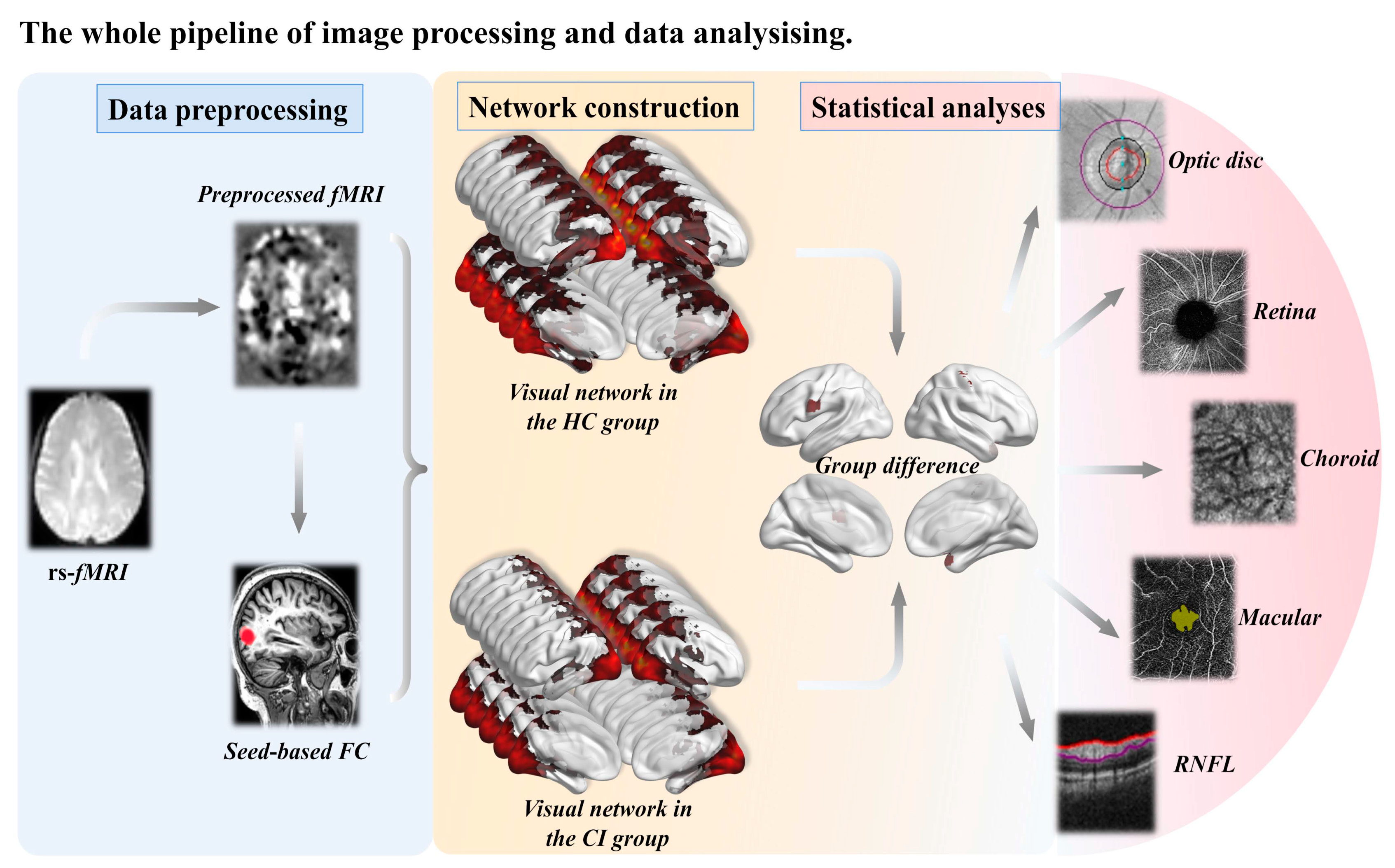
2.4. Imaging Analysis
2.4.1. Neuroimaging Data Acquisition
2.4.2. Image Preprocessing
2.4.3. Definition of Visual Network
2.5. Statistics Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Retinal Vascular Structures Data
3.3. Cortical Visual System Reconstruction
3.4. Associations of Retinal Vascular Structures Characteristics with Cortical Visual System
4. Discussion
4.1. Underlying Mechanisms on Cognitive Impairment of Aberrant Cortical Visual System
4.2. Retinal Markers Might Be Used as Potential Biomarkers for Diagnosing and Monitoring the Progression of AD
4.3. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carazo-Barrios, L.; Archidona-Arranz, A.; Claros-Ruiz, A.; García-Basterra, I.; Garzón-Maldonado, F.J.; Serrano-Castro, V.; Gutiérrez-Bedmar, M.; Barbancho, M.; De la Cruz Cosme, C.; García-Campos, J.M.; et al. Correlation between retinal nerve fibre layer thickness and white matter lesions in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2021, 36, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Jorge, L.; Canário, N.; Santiago, B.; Santana, I.; Castelhano, J.; Ambrósio, A.F.; Bernardes, R.; Castelo-Branco, M. Interplay Between Macular Retinal Changes and White Matter Integrity in Early Alzheimer’s Disease. J. Alzheimers Dis. 2019, 70, 723–732. [Google Scholar] [CrossRef]
- Fonte, C.; Smania, N.; Pedrinolla, A.; Munari, D.; Gandolfi, M.; Picelli, A.; Varalta, V.; Benetti, M.V.; Brugnera, A.; Federico, A.; et al. Comparison between physical and cognitive treatment in patients with MCI and Alzheimer’s disease. Aging 2019, 11, 3138–3155. [Google Scholar] [CrossRef] [PubMed]
- Risacher, S.L.; Saykin, A.J. Neuroimaging and other biomarkers for Alzheimer’s disease: The changing landscape of early detection. Annu. Rev. Clin. Psychol. 2013, 9, 621–648. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Pillai, J.A.; Bermel, R.; Jones, S.E.; Fernandez, H.; Leverenz, J.B.; Srivastava, S.K.; Ehlers, J.P. Correlation between brain volume and retinal photoreceptor outer segment volume in normal aging and neurodegenerative diseases. PLoS ONE 2020, 15, e0237078. [Google Scholar] [CrossRef] [PubMed]
- Tzekov, R.; Mullan, M. Vision function abnormalities in Alzheimer disease. Surv. Ophthalmol. 2014, 59, 414–433. [Google Scholar] [CrossRef]
- Yu, M.; Sporns, O.; Saykin, A.J. The human connectome in Alzheimer disease—Relationship to biomarkers and genetics. Nat. Rev. Neurol. 2021, 17, 545–563. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Van Stavern, G.P. Current opinion neurology: Visual pathway biomarkers in Alzheimer’s disease. Curr. Opin. Neurol. 2020, 33, 79–86. [Google Scholar] [CrossRef]
- Freud, E.; Plaut, D.C.; Behrmann, M. ‘What’ Is Happening in the Dorsal Visual Pathway. Trends Cogn. Sci. 2016, 20, 773–784. [Google Scholar] [CrossRef]
- den Haan, J.; Janssen, S.F.; van de Kreeke, J.A.; Scheltens, P.; Verbraak, F.D.; Bouwman, F.H. Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer’s disease and controls. Alzheimer’s Dement. 2018, 10, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.T.; Hilal, S.; Cheung, C.Y.; Venketasubramanian, N.; Niessen, W.J.; Vrooman, H.; Anuar, A.R.; Chew, M.; Chen, C.; Wong, T.Y.; et al. Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neurosci. Lett. 2015, 584, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2, e93621. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Cao, X.; Hu, J.; Jiang, L.; Mei, X.; Zheng, H.; Chen, Y.; Wang, M.; Cao, J.; Li, W.; et al. Retinal nerve fiber layer thickness is associated with hippocampus and lingual gyrus volumes in nondemented older adults. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109824. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Gómez, J.L.; Pelletier, A.; Rougier, M.B.; Korobelnik, J.F.; Schweitzer, C.; Delyfer, M.N.; Catheline, G.; Monfermé, S.; Dartigues, J.F.; Delcourt, C.; et al. Association of Retinal Nerve Fiber Layer Thickness With Brain Alterations in the Visual and Limbic Networks in Elderly Adults Without Dementia. JAMA Netw. Open 2018, 1, e184406. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.P.; Lopes, L.C.; Costa-Cunha, L.V.; Costa, C.F.; Pires, L.A.; Almeida, A.L.; Monteiro, M.L. Macular Thickness Measurements with Frequency Domain-OCT for Quantification of Retinal Neural Loss and its Correlation with Cognitive Impairment in Alzheimer’s Disease. PLoS ONE 2016, 11, e0153830. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.P.; Thompson, A.C.; Polascik, B.W.; Calixte, C.; Burke, J.R.; Petrella, J.R.; Grewal, D.S.; Fekrat, S. Correlation of OCTA and Volumetric MRI in Mild Cognitive Impairment and Alzheimer’s Disease. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 709–718. [Google Scholar] [CrossRef]
- Byun, M.S.; Park, S.W.; Lee, J.H.; Yi, D.; Jeon, S.Y.; Choi, H.J.; Joung, H.; Ghim, U.H.; Park, U.C.; Kim, Y.K.; et al. Association of Retinal Changes With Alzheimer Disease Neuroimaging Biomarkers in Cognitively Normal Individuals. JAMA Ophthalmol. 2021, 139, 548–556. [Google Scholar] [CrossRef]
- Lahme, L.; Esser, E.L.; Mihailovic, N.; Schubert, F.; Lauermann, J.; Johnen, A.; Eter, N.; Duning, T.; Alnawaiseh, M. Evaluation of Ocular Perfusion in Alzheimer’s Disease Using Optical Coherence Tomography Angiography. J. Alzheimers Dis. 2018, 66, 1745–1752. [Google Scholar] [CrossRef]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef]
- Romano, G.L.; Platania, C.B.M.; Forte, S.; Salomone, S.; Drago, F.; Bucolo, C. MicroRNA target prediction in glaucoma. Prog. Brain Res. 2015, 220, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, R.; Lyu, J.H.; Chen, J.H.; Wang, W.; Gao, M.L.; Li, W.J.; De, J.; Mu, H.Y.; Pan, W.G.; et al. Relationship Between Alzheimer’s Disease and Retinal Choroidal Thickness: A Cross-Sectional Study. J. Alzheimers Dis. 2021, 80, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Ghosh, P.M.; Madison, C.; Laforce, R., Jr.; Corbetta-Rastelli, C.; Weiner, M.W.; Greicius, M.D.; Seeley, W.W.; Gorno-Tempini, M.L.; Rosen, H.J.; et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain J. Neurol. 2013, 136, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Cerquera-Jaramillo, M.A.; Nava-Mesa, M.O.; González-Reyes, R.E.; Tellez-Conti, C.; de-la-Torre, A. Visual Features in Alzheimer’s Disease: From Basic Mechanisms to Clinical Overview. Neural Plast. 2018, 2018, 2941783. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Dinkin, M. Higher Cortical Visual Disorders. Continuum 2019, 25, 1329–1361. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Xu, J.; Chen, L.; Hirschler, L.; Barbier, E.L.; Li, T.; Wong, P.C.; Lu, H. Brain metabolism in tau and amyloid mouse models of Alzheimer’s disease: An MRI study. NMR Biomed. 2021, 34, e4568. [Google Scholar] [CrossRef]
- Chibhabha, F.; Yaqi, Y.; Li, F. Retinal involvement in Alzheimer’s disease (AD): Evidence and current progress on the non-invasive diagnosis and monitoring of AD-related pathology using the eye. Rev. Neurosci. 2020, 31, 883–904. [Google Scholar] [CrossRef]
- Parker, T.D.; Slattery, C.F.; Yong, K.X.X.; Nicholas, J.M.; Paterson, R.W.; Foulkes, A.J.M.; Malone, I.B.; Thomas, D.L.; Cash, D.M.; Crutch, S.J.; et al. Differences in hippocampal subfield volume are seen in phenotypic variants of early onset Alzheimer’s disease. NeuroImage. Clin. 2019, 21, 101632. [Google Scholar] [CrossRef]
- Johnson, E.B.; Rees, E.M.; Labuschagne, I.; Durr, A.; Leavitt, B.R.; Roos, R.A.; Reilmann, R.; Johnson, H.; Hobbs, N.Z.; Langbehn, D.R.; et al. The impact of occipital lobe cortical thickness on cognitive task performance: An investigation in Huntington’s Disease. Neuropsychologia 2015, 79, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Irish, M.; Foxe, D.; Hodges, J.R.; Piguet, O.; Burrell, J.R. Can visuospatial measures improve the diagnosis of Alzheimer’s disease? Alzheimer’s Dement. 2018, 10, 66–74. [Google Scholar] [CrossRef]
- Sapkota, R.P.; van der Linde, I.; Pardhan, S. How does aging influence object-location and name-location binding during a visual short-term memory task? Aging Ment. Health 2020, 24, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Cheong, E.N.; Jo, S.; Lee, S.; Shim, W.H.; Kang, D.W.; Kwon, M.; Kim, J.S.; Lee, J.H. Early Impairment in the Ventral Visual Pathway Can Predict Conversion to Dementia in Patients With Amyloid-negative Amnestic Mild Cognitive Impairment. Alzheimer Dis. Assoc. Disord. 2021, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Pourzinal, D.; Yang, J.H.J.; Byrne, G.J.; O’Sullivan, J.D.; Mitchell, L.; McMahon, K.L.; Copland, D.A.; Dissanayaka, N.N. Identifying subtypes of mild cognitive impairment in Parkinson’s disease using cluster analysis. J. Neurol. 2020, 267, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Scheff, M.A.; Mufson, E.J. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2011, 24, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Stoeckel, C.; Gough, P.M.; Watkins, K.E.; Devlin, J.T. Supramarginal gyrus involvement in visual word recognition. Cortex J. Devoted Study Nerv. Syst. Behav. 2009, 45, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Philipose, L.E.; Gottesman, R.F.; Newhart, M.; Kleinman, J.T.; Herskovits, E.H.; Pawlak, M.A.; Marsh, E.B.; Davis, C.; Heidler-Gary, J.; Hillis, A.E. Neural regions essential for reading and spelling of words and pseudowords. Ann. Neurol. 2007, 62, 481–492. [Google Scholar] [CrossRef]
- Damasio, A.R.; Damasio, H. The anatomic basis of pure alexia. Neurology 1983, 33, 1573–1583. [Google Scholar] [CrossRef]
- Iwamura, Y. Hierarchical somatosensory processing. Curr. Opin. Neurobiol. 1998, 8, 522–528. [Google Scholar] [CrossRef]
- Hart, N.J.; Koronyo, Y.; Black, K.L.; Koronyo-Hamaoui, M. Ocular indicators of Alzheimer’s: Exploring disease in the retina. Acta Neuropathol. 2016, 132, 767–787. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, C.; Cerri, E.; Fabiani, C.; Capsoni, S.; Cattaneo, A.; Domenici, L. The retina as a window to early dysfunctions of Alzheimer’s disease following studies with a 5xFAD mouse model. Neurobiol. Aging 2018, 67, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.B.; Chitranshi, N.; den Haan, J.; Mirzaei, M.; You, Y.; Lim, J.K.; Basavarajappa, D.; Godinez, A.; Di Angelantonio, S.; Sachdev, P.; et al. Retinal changes in Alzheimer’s disease- integrated prospects of imaging, functional and molecular advances. Prog. Retin. Eye Res. 2021, 82, 100899. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Pillai, J.A.; Bermel, R.; Bonner-Jackson, A.; Rae-Grant, A.; Fernandez, H.; Bena, J.; Jones, S.E.; Leverenz, J.B.; Srivastava, S.K.; et al. Outer Retinal Assessment Using Spectral-Domain Optical Coherence Tomography in Patients With Alzheimer’s and Parkinson’s Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2768–2777. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Kurtuluş, F.; Gözkaya, O.; Erol, M.K.; Cengiz, A.; Akıdan, M.; Yaman, A. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br. J. Ophthalmol. 2018, 102, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, Y.; Shi, Y.; Wright, C.B.; Sun, X.; Gregori, G.; Zheng, F.; Vanner, E.A.; Lam, B.L.; Rundek, T.; et al. Altered Macular Microvasculature in Mild Cognitive Impairment and Alzheimer Disease. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2018, 38, 292–298. [Google Scholar] [CrossRef]
- O’Bryhim, B.E.; Apte, R.S.; Kung, N.; Coble, D.; Van Stavern, G.P. Association of Preclinical Alzheimer Disease with Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018, 136, 1242–1248. [Google Scholar] [CrossRef]
- Hays, C.C.; Zlatar, Z.Z.; Wierenga, C.E. The Utility of Cerebral Blood Flow as a Biomarker of Preclinical Alzheimer’s Disease. Cell. Mol. Neurobiol. 2016, 36, 167–179. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Akinyemi, R.; Ihara, M. Does vascular pathology contribute to Alzheimer changes? J. Neurol. Sci. 2012, 322, 141–147. [Google Scholar] [CrossRef]
- Bambo, M.P.; Garcia-Martin, E.; Otin, S.; Pinilla, J.; Larrosa, J.M.; Polo, V.; Pablo, L.E. Visual function and retinal nerve fibre layer degeneration in patients with Alzheimer disease: Correlations with severity of dementia. Acta Ophthalmol. 2015, 93, e507–e508. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | HC | CI | p Value |
|---|---|---|---|
| (n = 20) | (n = 22) | ||
| Demogrraphics | |||
| Gender(female/male) | 11/9 | 7/15 | 0.129 |
| Age, years | 62.1 ± 7.56 | 66.91 ± 8.29 | 0.057 |
| Education, years | 13.65 ± 2.13 | 10.36 ± 2.57 | <0.001 * |
| General cognitive | |||
| MMSE | 28.85 ± 1.18 | 25.41 ± 3.96 | 0.001 * |
| MoCA-BJ | 26.90 ± 1.52 | 19.50 ± 4.35 | <0.001 * |
| Composition scores of each cognitive domain | |||
| Episodic memory | 1.00 ± 1.63 | −0.93 ± 1.42 | <0.001 * |
| AVLT-DR | 5.75 ± 2.81 | 3.16 ± 2.44 | 0.003 * |
| VR-DR | 8.10 ± 4.22 | 3.39 ± 3.75 | <0.001 * |
| Visuospatial processing function | 4.15 ± 0.50 | 2.59 ± 2.04 | 0.002 * |
| CDT | 3.85 ± 0.49 | 2.86 ± 1.32 | 0.005 * |
| VR-C | 13.85 ± 0.49 | 12.05 ± 4.15 | 0.055 |
| Information processing speed | 1.50 ± 1.84 | −1.37 ± 2.44 | <0.001 * |
| TMT-A (inverse) | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.002 * |
| Stroop A (inverse) | 0.07 ± 0.02 | 0.05 ± 0.02 | 0.001 * |
| Stroop B (inverse) | 0.06 ± 0.02 | 0.04 ± 0.02 | 0.001 * |
| Executive function | 0.98 ± 1.37 | −0.89 ± 1.47 | <0.001 * |
| TMTB (inverse) | 0.012 ± 0.005 | 0.007 ± 0.004 | 0.001 * |
| Stroop C(inverse) | 0.036 ± 0.010 | 0.026 ± 0.01 | 0.003 * |
| Characteristic | HC (n = 20) | CI (n = 22) | * p Value |
|---|---|---|---|
| Optic disc area | 2.058 ± 0.542 | 1.956 ± 0.375 | 0.484 |
| Mean C/D | 0.568 ± 0.132 | 0.550 ± 0.112 | 0.647 |
| Vertical C/D | 0.512 ± 0.150 | 0.505 ± 0.108 | 0.86 |
| Optic cup size | 0.242 ± 0.218 | 0.194 ± 0.165 | 0.428 |
| RNFL | 94.475 ± 8.076 | 94.250 ± 10.689 | 0.599 |
| Angiography 3 mm × 3 mm | |||
| Intra-layer VD | 18.300 ± 2.713 | 17.757 ± 2.671 | 0.518 |
| Completed VD | 17.090 ± 2.661 | 16.502 ± 2.510 | 0.467 |
| Central PD | 0.132 ± 0.047 | 0.122 ± 0.045 | 0.470 |
| Intra-layer PD | 0.332 ± 0.046 | 0.322 ± 0.0453 | 0.468 |
| Completed PD | 0.310 ± 0.046 | 0.308 ± 0.053 | 0.398 |
| FAZ area | 0.267 ± 0.083 | 0.307 ± 0.246 | 0.555 |
| FAZ perimeter | 2.296 ± 0.319 | 2.332 ± 0.583 | 0.804 |
| FAZ circularity | 0.625 ± 0.108 | 0.617 ± 0.224 | 0.883 |
| Angiography 6 mm × 6 mm | |||
| FAZ area | 0.216 ± 0.082 | 0.246 ± 0.116 | 0.338 |
| FAZ perimeter | 1.984 ± 0.470 | 2.276 ± 0.733 | 0.136 |
| FAZ circularity | 0.672 ± 0.086 | 0.614 ± 0.123 | 0.087 |
| Intra-layer VD | 13.563 ± 2.923 | 12.911 ± 3.604 | 0.522 |
| Outer VD | 15.100 ± 2.261 | 14.323 ± 3.032 | 0.349 |
| Completed VD | 14.448 ± 2.305 | 13.646 ± 3.068 | 0.342 |
| Central PD | 0.102 ± 0.055 | 0.095 ± 0.062 | 0.723 |
| Intra-layer PD | 0.320 ± 0.075 | 0.307 ± 0.081 | 0.594 |
| Outer PD | 0.369 ± 0.060 | 0.357 ± 0.069 | 0.545 |
| Completed PD | 0.350 ± 0.060 | 0.338 ± 0.069 | 0.573 |
| Regions | Cluster Size (mm3) | BA | Peak F Value | Peak MNI Coordinate |
|---|---|---|---|---|
| x, y, z (mm) | ||||
| Right ITG | 324 | 37 | 3.6261 | 51 −51 −18 |
| Left SMG | 540 | 40 | 3.867 | −57 −42 27 |
| Right PCG | 1161 | 3 | 3.8698 | 45 −27 66 |
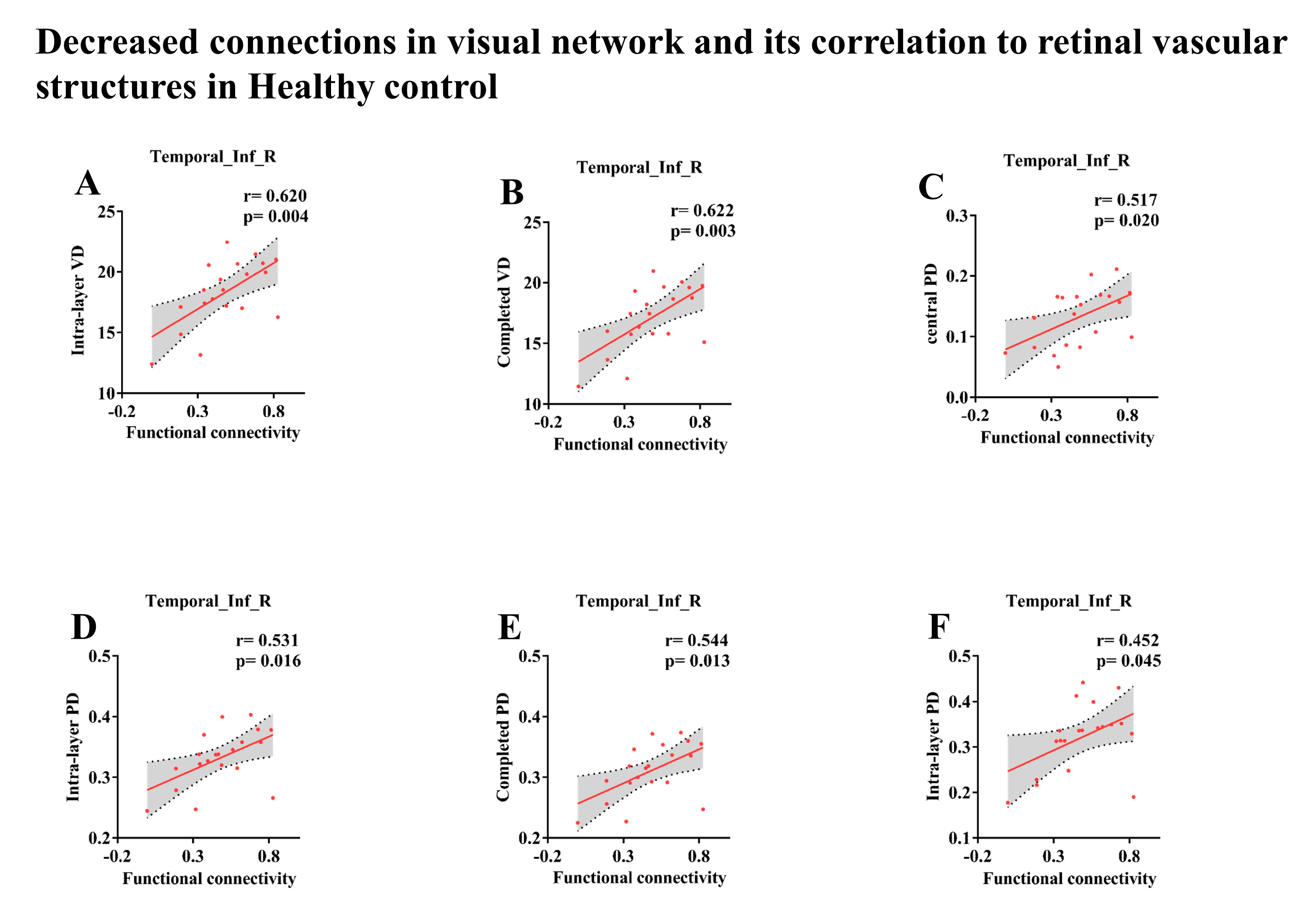
| Brain Regions | Visual Data | r-Value | p-Value | Effects of FC Changes on Vision |
|---|---|---|---|---|
| Temporal_Inf_R | ||||
| Intra-layer VD (3 mm × 3 mm) | 0.62 | 0.004 | Harmful | |
| Completed VD (3 mm × 3 mm) | 0.622 | 0.003 | Harmful | |
| Central PD (3 mm × 3 mm) | 0.517 | 0.02 | Harmful | |
| Intra-layer PD (3 mm × 3 m) | 0.531 | 0.016 | Harmful | |
| Completed PD (3 mm × 3 mm) | 0.544 | 0.013 | Harmful | |
| Intra-layer PD (6 mm × 6 mm) | 0.452 | 0.045 | Harmful | |
| SupraMarginal-L | ||||
| Intra-layer VD (6 mm × 6 mm) | 0.48 | 0.024 | Harmful | |
| Outer VD (6 mm × 6 mm) | 0.454 | 0.034 | Harmful | |
| Completed VD (6 mm × 6 mm) | 0.444 | 0.038 | Harmful | |
| Postcentral_R | ||||
| Intra-layer PD (3 mm × 3 mm) | 0.489 | 0.021 | Harmful | |
| Completed PD (3 mm × 3 mm) | 0.478 | 0.024 | Harmful |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Hu, Z.; Chen, H.; Sheng, X.; Qin, R.; Shao, P.; Yang, Z.; Yao, W.; Zhao, H.; Xu, Y.; et al. Applying Retinal Vascular Structures Characteristics Coupling with Cortical Visual System in Alzheimer’s Disease Spectrum Patients. Brain Sci. 2023, 13, 339. https://doi.org/10.3390/brainsci13020339
Wang L, Hu Z, Chen H, Sheng X, Qin R, Shao P, Yang Z, Yao W, Zhao H, Xu Y, et al. Applying Retinal Vascular Structures Characteristics Coupling with Cortical Visual System in Alzheimer’s Disease Spectrum Patients. Brain Sciences. 2023; 13(2):339. https://doi.org/10.3390/brainsci13020339
Chicago/Turabian StyleWang, Lianlian, Zheqi Hu, Haifeng Chen, Xiaoning Sheng, Ruomeng Qin, Pengfei Shao, Zhiyuan Yang, Weina Yao, Hui Zhao, Yun Xu, and et al. 2023. "Applying Retinal Vascular Structures Characteristics Coupling with Cortical Visual System in Alzheimer’s Disease Spectrum Patients" Brain Sciences 13, no. 2: 339. https://doi.org/10.3390/brainsci13020339
APA StyleWang, L., Hu, Z., Chen, H., Sheng, X., Qin, R., Shao, P., Yang, Z., Yao, W., Zhao, H., Xu, Y., & Bai, F. (2023). Applying Retinal Vascular Structures Characteristics Coupling with Cortical Visual System in Alzheimer’s Disease Spectrum Patients. Brain Sciences, 13(2), 339. https://doi.org/10.3390/brainsci13020339





