The Effect of Diagonal Exercise Training for Neurorehabilitation on Functional Activity in Stroke Patients: A Pilot Study
Abstract
:1. Introduction
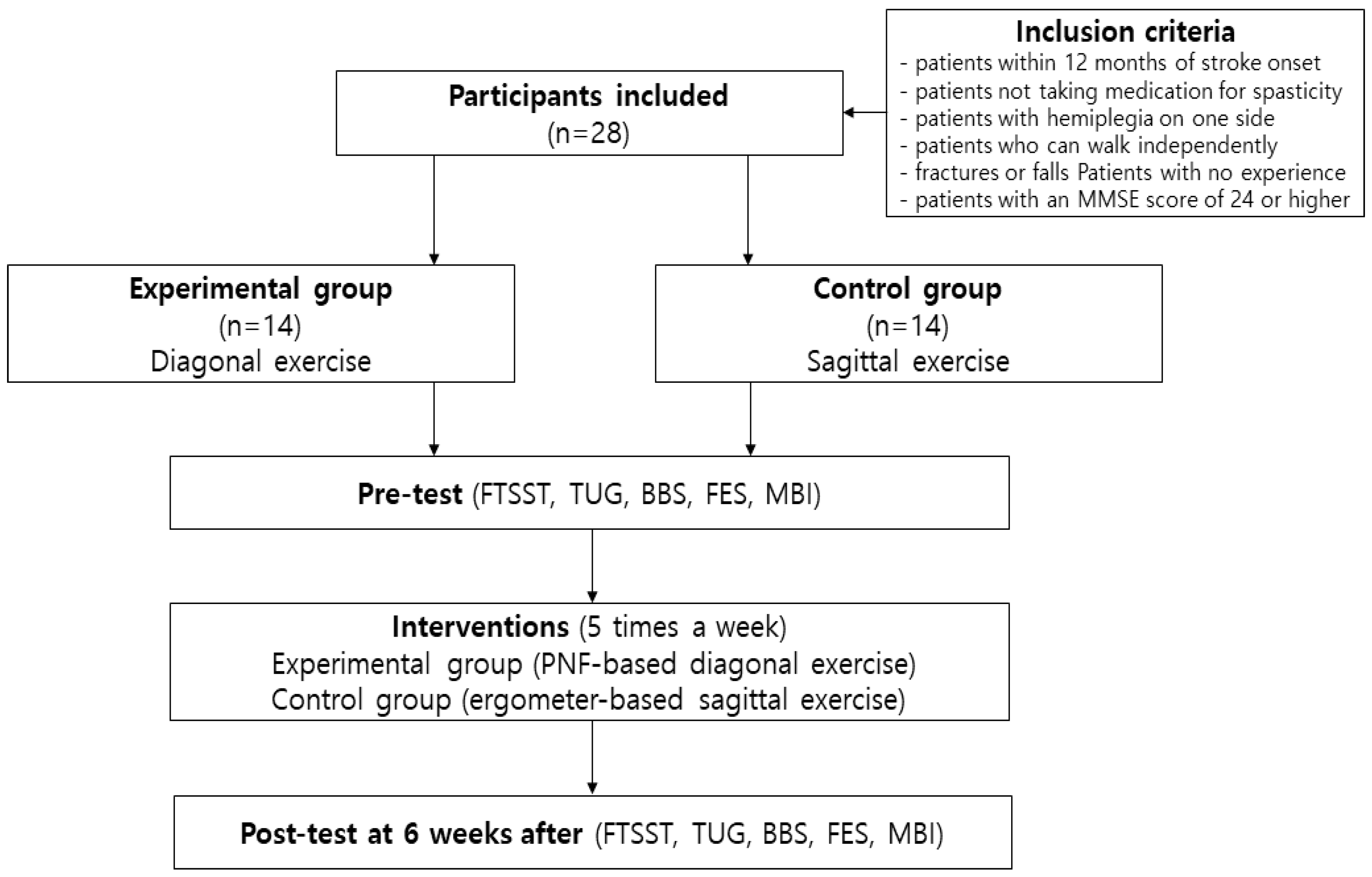
2. Materials and Methods
2.1. Subjects
2.2. Designs
2.3. Experimental Procedures
2.3.1. Diagonal Exercise Using Proprioceptive Neuromuscular Facilitation Therapy
2.3.2. Sagittal Exercise Using Upper and Lower Limb Ergometers
2.4. Assessment Methods
2.4.1. Balance
2.4.2. Confidence in Falling
2.4.3. Activities of Daily Living
2.5. Statistical Analysis Method
3. Results
3.1. General Characteristics of Subjects and Homogeneity Test of Pre-Test
3.2. Clinical Outcomes of the Experimental Group and Control Group
3.3. Comparative Analysis between Groups Using the Amount of Change between Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Battaglini, D.; Robba, C.; Lopes da Silva, A.; Dos Santos Samary, C.; Leme Silva, P.; Dal Pizzol, F.; Pelosi, P.; Rocco, P.R.M. Brain-Heart Interaction after Acute Ischemic Stroke. Crit. Care Lond. Engl. 2020, 24, 163. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic Stroke. Nat. Rev. Dis. Primer 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Sivolap, Y.P.; Damulin, I.V. [Stroke and depression]. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 2019, 119, 143–147. [Google Scholar] [CrossRef]
- Li, J.; Zhong, D.; Ye, J.; He, M.; Liu, X.; Zheng, H.; Jin, R.; Zhang, S.-L. Rehabilitation for Balance Impairment in Patients after Stroke: A Protocol of a Systematic Review and Network Meta-Analysis. BMJ Open 2019, 9, e026844. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M. Prediction of Motor Recovery after Stroke: Advances in Biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Lo Coco, D.; Lopez, G.; Corrao, S. Cognitive Impairment and Stroke in Elderly Patients. Vasc. Health Risk Manag. 2016, 12, 105–116. [Google Scholar]
- Longley, V.; Hazelton, C.; Heal, C.; Pollock, A.; Woodward-Nutt, K.; Mitchell, C.; Pobric, G.; Vail, A.; Bowen, A. Non-Pharmacological Interventions for Spatial Neglect or Inattention Following Stroke and Other Non-Progressive Brain Injury. Cochrane Database Syst. Rev. 2021, 7, CD003586. [Google Scholar]
- Denissen, S.; Staring, W.; Kunkel, D.; Pickering, R.M.; Lennon, S.; Geurts, A.C.; Weerdesteyn, V.; Verheyden, G.S. Interventions for Preventing Falls in People after Stroke. Cochrane Database Syst. Rev. 2019, 10, CD008728. [Google Scholar] [CrossRef]
- Mansfield, A.; Inness, E.L.; Mcilroy, W.E. Stroke. Handb. Clin. Neurol. 2018, 159, 205–228. [Google Scholar]
- Tasseel-Ponche, S.; Delafontaine, A.; Godefroy, O.; Yelnik, A.P.; Doutrellot, P.-L.; Duchossoy, C.; Hyra, M.; Sader, T.; Diouf, M. Walking Speed at the Acute and Subacute Stroke Stage: A Descriptive Meta-Analysis. Front. Neurol. 2022, 13, 989622. [Google Scholar] [CrossRef]
- Eraifej, J.; Clark, W.; France, B.; Desando, S.; Moore, D. Effectiveness of Upper Limb Functional Electrical Stimulation after Stroke for the Improvement of Activities of Daily Living and Motor Function: A Systematic Review and Meta-Analysis. Syst. Rev. 2017, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- García-Rudolph, A.; Sánchez-Pinsach, D.; Salleras, E.O.; Tormos, J.M. Subacute Stroke Physical Rehabilitation Evidence in Activities of Daily Living Outcomes: A Systematic Review of Meta-Analyses of Randomized Controlled Trials. Medicine 2019, 98, e14501. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A. Treatment of Acute Ischemic Stroke. Contin. Minneap. Minn 2017, 23, 62–81. [Google Scholar] [CrossRef]
- Srithumsuk, W.; Chaleoykitti, S.; Jaipong, S.; Pattayakorn, P.; Podimuang, K. Association between Depression and Medication Adherence in Stroke Survivor Older Adults. Jpn. J. Nurs. Sci. JJNS 2021, 18, e12434. [Google Scholar] [CrossRef] [PubMed]
- Klamroth-Marganska, V. Stroke Rehabilitation: Therapy Robots and Assistive Devices. Adv. Exp. Med. Biol. 2018, 1065, 579–587. [Google Scholar]
- Le Danseur, M. Stroke Rehabilitation. Crit. Care Nurs. Clin. N. Am. 2020, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Guiu-Tula, F.X.; Cabanas-Valdés, R.; Sitjà-Rabert, M.; Urrútia, G.; Gómara-Toldrà, N. The Efficacy of the Proprioceptive Neuromuscular Facilitation (PNF) Approach in Stroke Rehabilitation to Improve Basic Activities of Daily Living and Quality of Life: A Systematic Review and Meta-Analysis Protocol. BMJ Open 2017, 7, e016739. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Chou, L.-W.; Hsieh, Y.-L. Proprioceptive Neuromuscular Facilitation-Based Physical Therapy on the Improvement of Balance and Gait in Patients with Chronic Stroke: A Systematic Review and Meta-Analysis. Life Basel Switz. 2022, 12, 882. [Google Scholar] [CrossRef]
- Alahmari, K.A.; Silvian, P.; Ahmad, I.; Reddy, R.S.; Tedla, J.S.; Kakaraparthi, V.N.; Rengaramanujam, K. Effectiveness of Low-Frequency Stimulation in Proprioceptive Neuromuscular Facilitation Techniques for Post Ankle Sprain Balance and Proprioception in Adults: A Randomized Controlled Trial. BioMed. Res. Int. 2020, 2020, 9012930. [Google Scholar] [CrossRef]
- Hindle, K.B.; Whitcomb, T.J.; Briggs, W.O.; Hong, J. Proprioceptive Neuromuscular Facilitation (PNF): Its Mechanisms and Effects on Range of Motion and Muscular Function. J. Hum. Kinet. 2012, 31, 105–113. [Google Scholar] [CrossRef]
- Davis, D.S.; Ashby, P.E.; McCale, K.L.; McQuain, J.A.; Wine, J.M. The Effectiveness of 3 Stretching Techniques on Hamstring Flexibility Using Consistent Stretching Parameters. J. Strength Cond. Res. 2005, 19, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Lial, L.; Teles Monteiro, M.G.; Aragão, A.; Santos David, L.; Coertjens, M.; Silva-Júnior, F.L.; Dias, G.; Velasques, B.; Ribeiro, P.; et al. Diagonal Movement of the Upper Limb Produces Greater Adaptive Plasticity than Sagittal Plane Flexion in the Shoulder. Neurosci. Lett. 2017, 643, 8–15. [Google Scholar] [CrossRef]
- Smedes, F.; Giacometti da Silva, L. Motor Learning with the PNF-Concept, an Alternative to Constrained Induced Movement Therapy in a Patient after a Stroke; a Case Report. J. Bodyw. Mov. Ther. 2019, 23, 622–627. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.M.; Meyer, S.; Sandstad, S.; Wiskerke, E.; Thuwis, R.; Vandekerckhove, C.; Myny, C.; Ghosh, N.; Beyens, H.; Dejaeger, E.; et al. A Cross-Sectional Study Comparing Lateral and Diagonal Maximum Weight Shift in People with Stroke and Healthy Controls and the Correlation with Balance, Gait and Fear of Falling. PLoS ONE 2017, 12, e0183020. [Google Scholar] [CrossRef]
- Muñoz-Bermejo, L.; Adsuar, J.C.; Mendoza-Muñoz, M.; Barrios-Fernández, S.; Garcia-Gordillo, M.A.; Pérez-Gómez, J.; Carlos-Vivas, J. Test-Retest Reliability of Five Times Sit to Stand Test (FTSST) in Adults: A Systematic Review and Meta-Analysis. Biology 2021, 10, 510. [Google Scholar] [CrossRef]
- Chan, P.P.; Si Tou, J.I.; Tse, M.M.; Ng, S.S. Reliability and Validity of the Timed Up and Go Test With a Motor Task in People With Chronic Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 2213–2220. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, Y.-S. The Diagnostic Accuracy of the Berg Balance Scale in Predicting Falls. West. J. Nurs. Res. 2017, 39, 1502–1525. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and Initial Validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef]
- Ohura, T.; Hase, K.; Nakajima, Y.; Nakayama, T. Validity and Reliability of a Performance Evaluation Tool Based on the Modified Barthel Index for Stroke Patients. BMC Med. Res. Methodol. 2017, 17, 131. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Liang, J.N.; Chen, B.; Aruin, A.S. Characteristics of Medial-Lateral Postural Control While Exposed to the External Perturbation in Step Initiation. Sci. Rep. 2019, 9, 16817. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, A.; Lobo-Prat, J.; Font-Llagunes, J.M. Systematic Review on Wearable Lower-Limb Exoskeletons for Gait Training in Neuromuscular Impairments. J. Neuroeng. Rehabil. 2021, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Or, C.K.; Chen, T. Effectiveness of Using Virtual Reality-Supported Exercise Therapy for Upper Extremity Motor Rehabilitation in Patients with Stroke: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Med. Internet Res. 2022, 24, e24111. [Google Scholar] [CrossRef] [PubMed]
- Darekar, A.; Lamontagne, A.; Fung, J. Locomotor Circumvention Strategies Are Altered by Stroke: II. Postural Coordination. J. Neuroeng. Rehabil. 2017, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Fukata, K.; Amimoto, K.; Inoue, M.; Sekine, D.; Inoue, M.; Fujino, Y.; Makita, S.; Takahashi, H. Effects of Diagonally Aligned Sitting Training with a Tilted Surface on Sitting Balance for Low Sitting Performance in the Early Phase after Stroke: A Randomised Controlled Trial. Disabil. Rehabil. 2021, 43, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Trisan, R. Balance Problems and Fall Risks in the Elderly. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 727–737. [Google Scholar] [CrossRef]
- Rubega, M.; Formaggio, E.; Di Marco, R.; Bertuccelli, M.; Tortora, S.; Menegatti, E.; Cattelan, M.; Bonato, P.; Masiero, S.; Del Felice, A. Cortical Correlates in Upright Dynamic and Static Balance in the Elderly. Sci. Rep. 2021, 11, 14132. [Google Scholar] [CrossRef]
- Han, P.; Zhang, W.; Kang, L.; Ma, Y.; Fu, L.; Jia, L.; Yu, H.; Chen, X.; Hou, L.; Wang, L.; et al. Clinical Evidence of Exercise Benefits for Stroke. Adv. Exp. Med. Biol. 2017, 1000, 131–151. [Google Scholar]
- Chiaramonte, R.; Bonfiglio, M.; Leonforte, P.; Coltraro, G.L.; Guerrera, C.S.; Vecchio, M. Proprioceptive and Dual-Task Training: The Key of Stroke Rehabilitation, A Systematic Review. J. Funct. Morphol. Kinesiol. 2022, 7, 53. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Truijen, S.; Schröder, J.; Maebe, Z.; Blanckaert, K.; van der Waal, C.; Vink, M.; Saeys, W. The Effectiveness of Trunk Training on Trunk Control, Sitting and Standing Balance and Mobility Post-Stroke: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2019, 33, 992–1002. [Google Scholar] [CrossRef]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of Different Exercise Interventions on Risk of Falls, Gait Ability, and Balance in Physically Frail Older Adults: A Systematic Review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef]
- Yang, F.; Lees, J.; Simpkins, C.; Butler, A. Interventions for Preventing Falls in People Post-Stroke: A Meta-Analysis of Randomized Controlled Trials. Gait Posture 2021, 84, 377–388. [Google Scholar] [CrossRef]
- Vahlberg, B.; Cederholm, T.; Lindmark, B.; Zetterberg, L.; Hellström, K. Short-Term and Long-Term Effects of a Progressive Resistance and Balance Exercise Program in Individuals with Chronic Stroke: A Randomized Controlled Trial. Disabil. Rehabil. 2017, 39, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, A.F.; Paul, G.; Hausdorff, J.M. Risk Factors for Falls among Older Adults: A Review of the Literature. Maturitas 2013, 75, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gazibara, T.; Kurtagic, I.; Kisic-Tepavcevic, D.; Nurkovic, S.; Kovacevic, N.; Gazibara, T.; Pekmezovic, T. Falls, Risk Factors and Fear of Falling among Persons Older than 65 Years of Age. Psychogeriatr. Off. J. Jpn. Psychogeriatr. Soc. 2017, 17, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Gschwind, Y.J.; Kressig, R.W.; Lacroix, A.; Muehlbauer, T.; Pfenninger, B.; Granacher, U. A Best Practice Fall Prevention Exercise Program to Improve Balance, Strength/Power, and Psychosocial Health in Older Adults: Study Protocol for a Randomized Controlled Trial. BMC Geriatr. 2013, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.W.; Bamford, C.; Deary, V.; Finch, T.L.; Gray, J.; MacDonald, C.; McMeekin, P.; Sabin, N.J.; Steen, I.N.; Whitney, S.L.; et al. Cognitive-Behavioural Therapy-Based Intervention to Reduce Fear of Falling in Older People: Therapy Development and Randomised Controlled Trial—The Strategies for Increasing Independence, Confidence and Energy (STRIDE) Study. Health Technol. Assess. Winch. Engl. 2016, 20, 1–206. [Google Scholar] [CrossRef]
- Sertel, M.; Aydoğan Arslan, S.; Tütün Yümin, E.; Demirci, C.S.; Tarsuslu Şimşek, T. Investigation of the Relationship between Physical Activity, Kinesiophobia and Fear of Falling in Older Adults with Chronic Pain. Somatosens. Mot. Res. 2021, 38, 241–247. [Google Scholar] [CrossRef]
- Toyoda, H.; Hayashi, C.; Okano, T. Associations between Physical Function, Falls, and the Fear of Falling among Older Adults Participating in a Community-Based Physical Exercise Program: A Longitudinal Multilevel Modeling Study. Arch. Gerontol. Geriatr. 2022, 102, 104752. [Google Scholar] [CrossRef]
- Lin, S.; Wang, C.; Wang, Q.; Xie, S.; Tu, Q.; Zhang, H.; Peng, M.; Zhou, J.; Redfern, J. The Experience of Stroke Survivors and Caregivers during Hospital-to-Home Transitional Care: A Qualitative Longitudinal Study. Int. J. Nurs. Stud. 2022, 130, 104213. [Google Scholar] [CrossRef]
- Chow, J.W.; Stokic, D.S. The Contribution of Walking Speed versus Recent Stroke to Temporospatial Gait Variability. Gait Posture 2023, 100, 216–221. [Google Scholar] [CrossRef]
- Nevisipour, M.; Honeycutt, C.F. Investigating the Underlying Biomechanical Mechanisms Leading to Falls in Long-Term Ankle-Foot Orthosis and Functional Electrical Stimulator Users with Chronic Stroke. Gait Posture 2022, 92, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Westerlind, E.K.; Lernfelt, B.; Hansson, P.-O.; Persson, C.U. Drug Treatment, Postural Control, and Falls: An Observational Cohort Study of 504 Patients with Acute Stroke, the Fall Study of Gothenburg. Arch. Phys. Med. Rehabil. 2019, 100, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Alexandre de Assis, I.S.; Luvizutto, G.J.; Bruno, A.C.M.; Sande de Souza, L.A.P. The Proprioceptive Neuromuscular Facilitation Concept in Parkinson Disease: A Systematic Review and Meta-Analysis. J. Chiropr. Med. 2020, 19, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Arcanjo, F.L.; Martins, J.V.P.; Moté, P.; Leporace, G.; de Oliveira, D.A.; de Sousa, C.S.; Saquetto, M.B.; Gomes-Neto, M. Proprioceptive Neuromuscular Facilitation Training Reduces Pain and Disability in Individuals with Chronic Low Back Pain: A Systematic Review and Meta-Analysis. Complement. Ther. Clin. Pract. 2022, 46, 101505. [Google Scholar] [CrossRef] [PubMed]
| EP (n=14) | CO (n = 14) | p-Value | |
|---|---|---|---|
| Age (years) | 68.21 ± 3.01 | 67.07 ± 2.58 | 0.292 |
| Weight (kg) | 59.21 ± 5.47 | 61.28 ± 5.31 | 0.319 |
| Height (cm) | 156.92 ± 2.22 | 159.00 ± 4.20 | 0.155 |
| FTSST (second) | 17.78 ± 2.54 | 16.57 ± 2.56 | 0.220 |
| TUG (second) | 23.42 ± 3.05 | 21.78 ± 2.15 | 0.112 |
| BBS (score) | 28.00 ± 2.57 | 29.21 ± 5.17 | 0.439 |
| FES (score) | 19.28 ± 3.12 | 19.85 ± 3.99 | 0.677 |
| MBI (score) | 35.64 ± 5.03 | 33.78 ± 6.25 | 0.395 |
| Variable | Group | T1 | T2 | t | p |
|---|---|---|---|---|---|
| FTSST (Second) | EP (n = 14) | 17.78 ± 2.54 | 13.85 ± 2.74 | 10.213 | 0.000 ** |
| CO (n = 14) | 16.57 ± 2.56 | 14.42 ± 2.13 | 5.491 | 0.000 ** | |
| TUG (Second) | EP (n = 14) | 23.42 ± 3.05 | 21.50 ± 2.68 | 5.434 | 0.000 ** |
| CO (n = 14) | 21.78 ± 2.15 | 20.35 ± 3.43 | 3.333 | 0.005 * | |
| BBS (Score) | EP (n = 14) | 28.00 ± 2.57 | 33.64 ± 3.34 | −14.593 | 0.000 ** |
| CO (n = 14) | 29.21 ± 5.17 | 32.50 ± 3.81 | −6.216 | 0.000 ** | |
| FES (Score) | EP (n = 14) | 19.28 ± 3.12 | 22.64 ± 2.70 | −6.209 | 0.000 ** |
| CO (n = 14) | 19.85 ± 3.99 | 21.42 ± 2.97 | −2.705 | 0.018 | |
| MBI (Score) | EP (n = 14) | 35.64 ± 5.03 | 39.50 ± 2.92 | −5.756 | 0.000 ** |
| CO (n = 14) | 33.78 ± 6.25 | 36.85 ± 4.05 | −3.466 | 0.004 * |
| Variable | EP (n = 14) | CO (n = 14) | t | p | Effect Size |
|---|---|---|---|---|---|
| FTSST (Second) | 3.92 ± 1.43 | 2.14 ± 1.46 | 3.259 | 0.003 * | 1.229 |
| TUG (Second) | 1.92 ± 1.32 | 1.42 ± 1.60 | 0.899 | 0.377 | 0.341 |
| BBS (Score) | −5.64 ± 1.44 | −3.28 ± 1.97 | −3.599 | 0.001 * | 1.364 |
| FES (Score) | −3.35 ± 2.02 | −1.57 ± 2.17 | −2.250 | 0.033 | 0.850 |
| MBI (Score) | −3.85 ± 2.50 | −3.07 ± 3.31 | −0.707 | 0.486 | 0.266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, E.-J. The Effect of Diagonal Exercise Training for Neurorehabilitation on Functional Activity in Stroke Patients: A Pilot Study. Brain Sci. 2023, 13, 799. https://doi.org/10.3390/brainsci13050799
Lee J-H, Kim E-J. The Effect of Diagonal Exercise Training for Neurorehabilitation on Functional Activity in Stroke Patients: A Pilot Study. Brain Sciences. 2023; 13(5):799. https://doi.org/10.3390/brainsci13050799
Chicago/Turabian StyleLee, Jung-Ho, and Eun-Ja Kim. 2023. "The Effect of Diagonal Exercise Training for Neurorehabilitation on Functional Activity in Stroke Patients: A Pilot Study" Brain Sciences 13, no. 5: 799. https://doi.org/10.3390/brainsci13050799





