Spontaneously Hypertensive Rats Present Exacerbated Focal Stroke Behavioral Outcomes
Abstract
:1. Introduction
Stroke
2. Materials and Methods
2.1. Animals
2.2. Ethical Aspects
2.3. Focal Permanent Stroke by Thermocoagulation of Pial Blood Vessels
2.4. Sensorimotor Functions Evaluation
2.4.1. Cylinder Test
2.4.2. Adhesive Removal Test
2.5. Memory Evaluation—Open Field Task
2.6. Infarct Size Evaluation
2.7. Statistical Analysis
3. Results
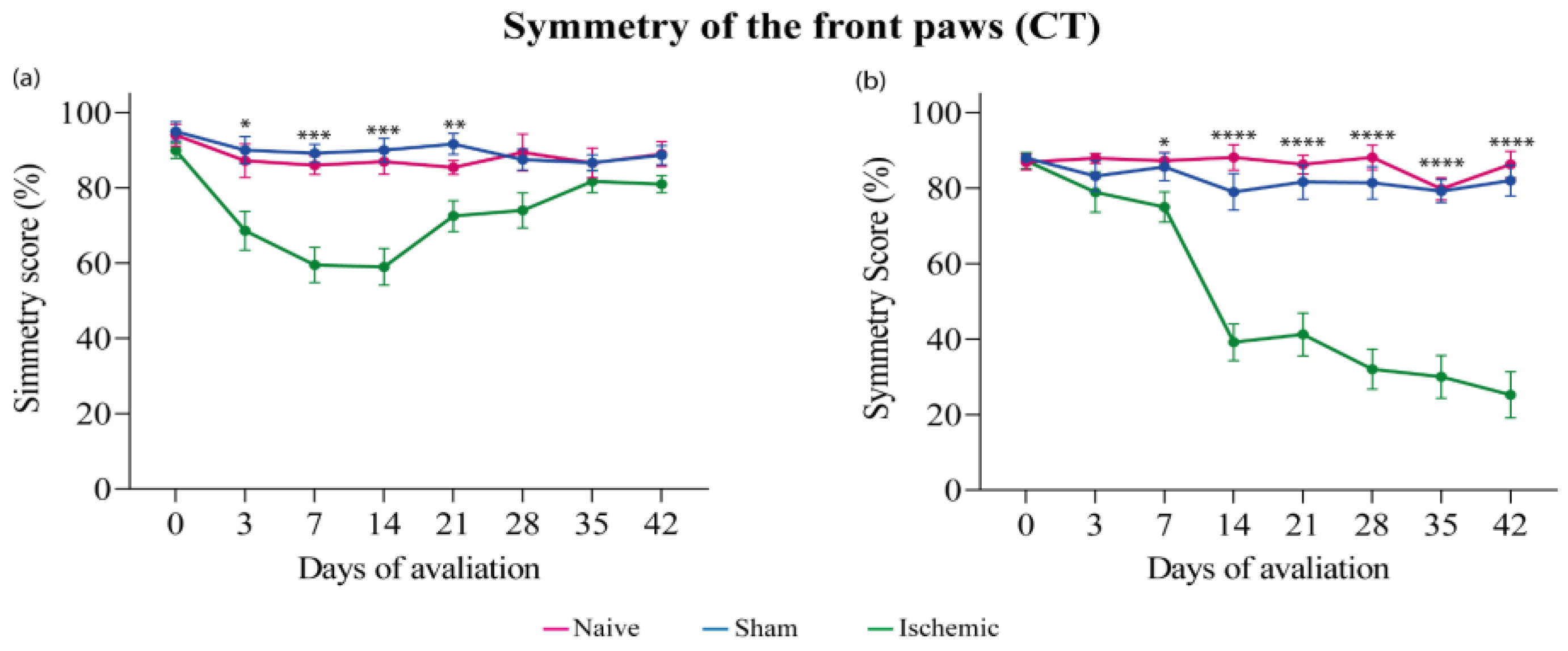
3.1. Cylinder Test (CT)
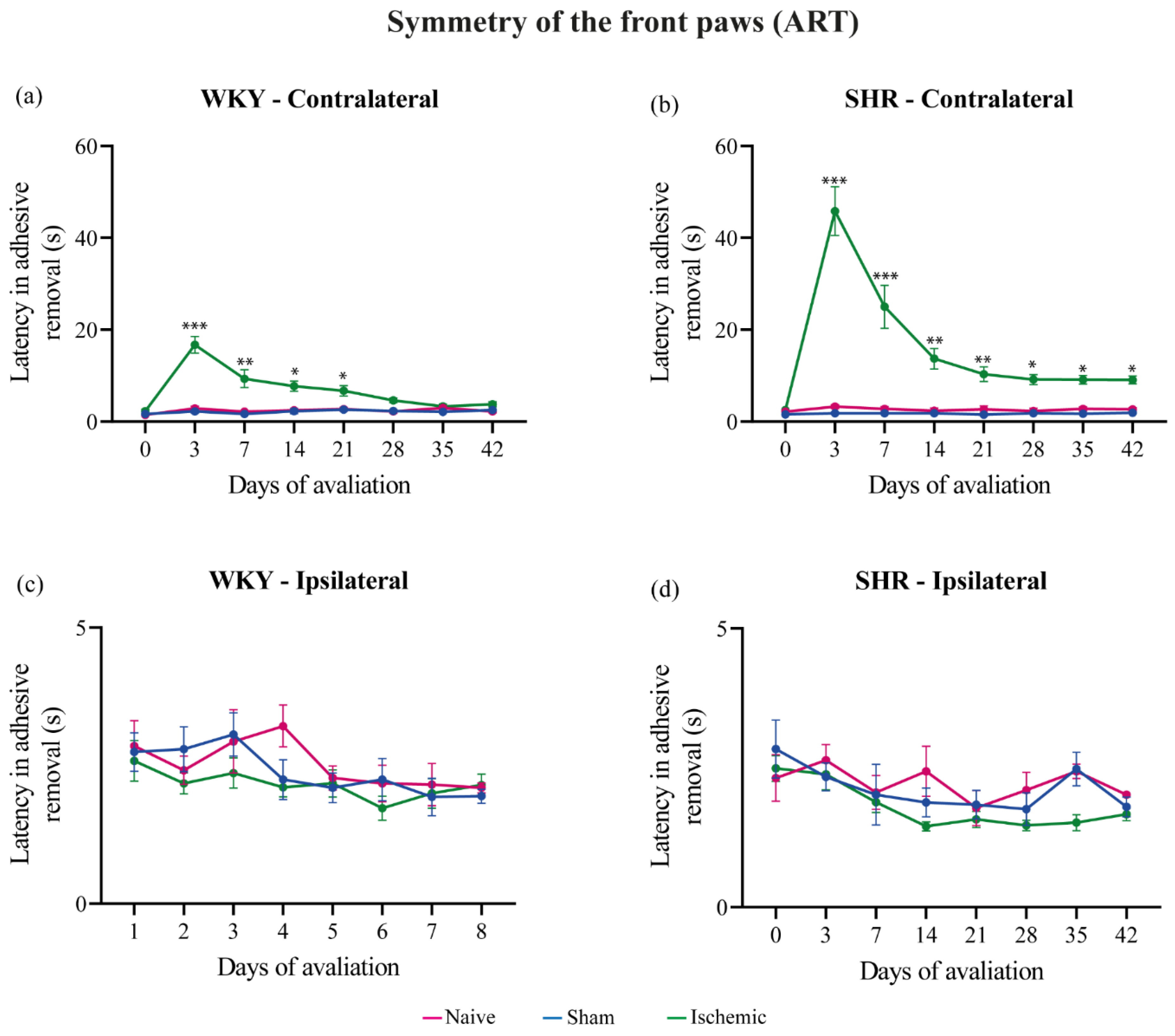
3.2. Adhesive Removal Test (ART)
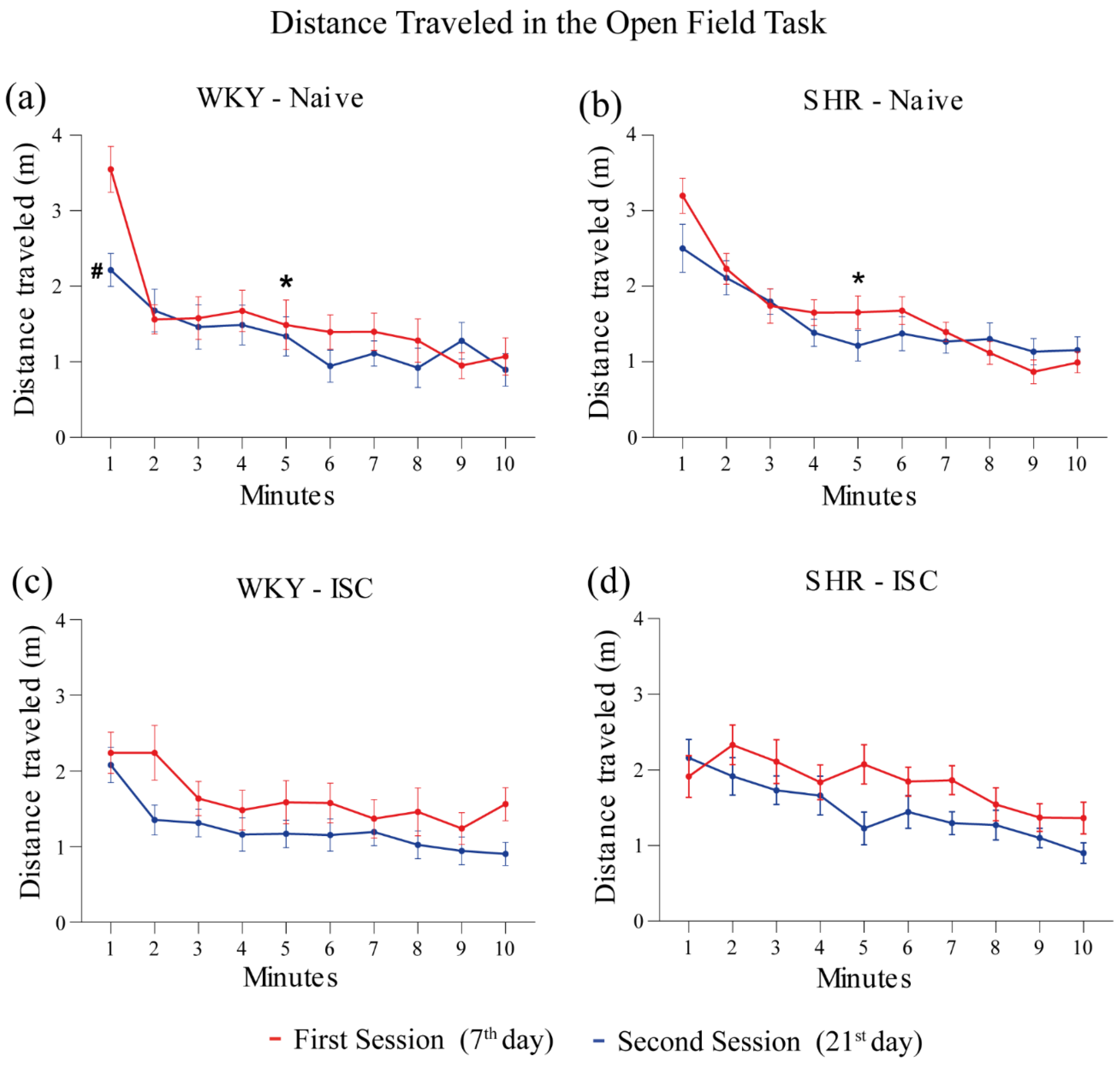
3.3. Open Field Task (OFT)
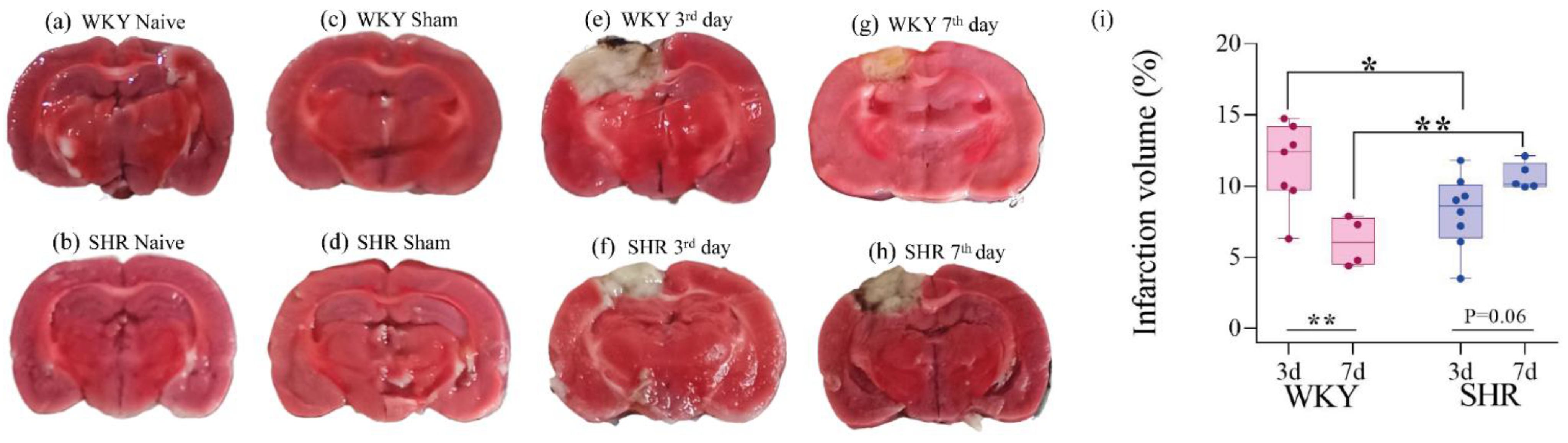
3.4. Infarct Size
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chapman:, N.; Ching, S.M.; Konradi, A.O.; Nuyt, A.M.; Khan, T.; Twumasi-Ankrah, B.; Cho, E.J.; Schutte, A.E.; Touyz, R.M.; Steckelings, U.M.; et al. Arterial Hypertension in Women: State of the Art and Knowledge Gaps. Hypertension 2023, 80, 1140–1149. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Lauder, L.; Mahfoud, F.; Azizi, M.; Bhatt, D.L.; Ewen, S.; Kario, K.; Parati, G.; Rossignol, P.; Schlaich, M.P.; Teo, K.K.; et al. Hypertension Management in Patients with Cardiovascular Comorbidities. Eur. Heart J. 2023, 44, 2066–2077. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic Stroke. Nat. Rev. Dis. Primer 2019, 5, 70. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]
- Słomka, A.; Świtońska, M.; Sinkiewicz, W.; Żekanowska, E. Haemostatic Factors Do Not Account for Worse Outcomes from Ischaemic Stroke in Patients with Higher C-Reactive Protein Concentrations. Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 54, 378–385. [Google Scholar] [CrossRef]
- Tuo, Q.; Zhang, S.; Lei, P. Mechanisms of Neuronal Cell Death in Ischemic Stroke and Their Therapeutic Implications. Med. Res. Rev. 2022, 42, 259–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jia, M.; Wang, Y.; Wang, Q.; Wu, J. Cell Death Mechanisms in Cerebral Ischemia–Reperfusion Injury. Neurochem. Res. 2022, 47, 3525–3542. [Google Scholar] [CrossRef]
- Mao, R.; Zong, N.; Hu, Y.; Chen, Y.; Xu, Y. Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke. Neurosci. Bull. 2022, 38, 1229–1247. [Google Scholar] [CrossRef]
- Kijpaisalratana, N.; Ament, Z.; Patki, A.; Bhave, V.M.; Garcia-Guarniz, A.-L.; Judd, S.E.; Cushman, M.; Long, D.L.; Irvin, M.R.; Kimberly, W.T. Association of Circulating Metabolites with Racial Disparities in Hypertension and Stroke in the REGARDS Study. Neurology 2023, 100, E2312–E2320. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.K.; Lawrence, C.B. Comorbidity and Age in the Modelling of Stroke: Are We Still Failing to Consider the Characteristics of Stroke Patients? BMJ Open Sci. 2020, 4, e100013. [Google Scholar] [CrossRef]
- Mead, G.E.; Sposato, L.A.; Sampaio Silva, G.; Yperzeele, L.; Wu, S.; Kutlubaev, M.; Cheyne, J.; Wahab, K.; Urrutia, V.C.; Sharma, V.K.; et al. A Systematic Review and Synthesis of Global Stroke Guidelines on Behalf of the World Stroke Organization. Int. J. Stroke 2023, 18, 499–531. [Google Scholar] [CrossRef]
- First WHO Report Details Devastating Impact of Hypertension and Ways to Stop It. 19 September 2023. Available online: https://www.who.int/thailand/news/detail/19-09-2023-first-who-report-details-devastating-impact-of-hypertension-and-ways-to-stop-it (accessed on 11 July 2024).
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primer 2018, 4, 18014. [Google Scholar] [CrossRef]
- Koundal, S.; Liu, X.; Sanggaard, S.; Mortensen, K.; Wardlaw, J.; Nedergaard, M.; Benveniste, H.; Lee, H. Brain Morphometry and Longitudinal Relaxation Time of Spontaneously Hypertensive Rats (SHRs) in Early and Intermediate Stages of Hypertension Investigated by 3D VFA-SPGR MRI. Neuroscience 2019, 404, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Staehr, C.; Aalkjaer, C.; Matchkov, V.V. The Vascular Na,K-ATPase: Clinical Implications in Stroke, Migraine, and Hypertension. Clin. Sci. 2023, 137, 1595–1618. [Google Scholar] [CrossRef]
- Kim, H.-L. Arterial Stiffness and Hypertension. Clin. Hypertens. 2023, 29, 31. [Google Scholar] [CrossRef]
- Majesky, M.W.; Weiser-Evans, M.C.M. The Adventitia in Arterial Development, Remodeling, and Hypertension. Biochem. Pharmacol. 2022, 205, 115259. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Iadecola, C.; Carnevale, D. Hypertension, Neurovascular Dysfunction, and Cognitive Impairment. Hypertension 2023, 80, 22–34. [Google Scholar] [CrossRef]
- Wu, H.; Fan, Y.; Zhang, M. Advanced Progress in the Role of Adipose-Derived Mesenchymal Stromal/Stem Cells in the Application of Central Nervous System Disorders. Pharmaceutics 2023, 15, 2637. [Google Scholar] [CrossRef] [PubMed]
- Rexrode, K.M.; Madsen, T.E.; Yu, A.Y.X.; Carcel, C.; Lichtman, J.H.; Miller, E.C. The Impact of Sex and Gender on Stroke. Circ. Res. 2022, 130, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Trippodo, N.C.; Frohlich, E.D. Similarities of Genetic (Spontaneous) Hypertension. Man and Rat. Circ. Res. 1981, 48, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hallbäck, M.; Weiss, L. Mechanisms of Spontaneous Hypertension in Rats. Med. Clin. N. Am. 1977, 61, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Krushovlieva, D.; Ivanova, P.; Kortenska, L. Spontaneously Hypertensive Rats vs. Wistar Kyoto and Wistar Rats: An Assessment of Anxiety, Motor Activity, Memory Performance, and Seizure Susceptibility. Physiol. Behav. 2023, 269, 114268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Zhang, Y.; Yu, Y.; Bai, F.; Zhang, H.; Chi, Y. Effects of Inverted Photoperiods on the Blood Pressure and Carotid Artery of Spontaneously Hypertensive Rats and Wistar–Kyoto Rats. J. Hypertens. 2021, 39, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Estabelecendo Procedimentos Para o Uso Científico de Animais; 2008. Available online: https://www.planalto.gov.br/ccivil_03/_ato2007-2010/2008/lei/l11794.htm (accessed on 10 July 2024).
- Conselho Nacional de Controle de Experimentação Animal—CONCEA. RESOLUÇÃO NORMATIVA N 15, DE 16 DE DEZEMBRO DE 2013. Available online: https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/concea/arquivos/pdf/legislacao/resolucao-normativa-no-15-de-16-de-dezembro-de-2013.pdf (accessed on 10 July 2024).
- Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; p. 12910. ISBN 978-0-309-15400-0.
- Guia Brasileiro de Produção, Manutenção Ou Utilização de Animais Em Atividades de Ensino Ou Pesquisa Científica. Available online: https://www.ufrgs.br/ceua/documentacao/legislacao/ (accessed on 11 July 2024).
- Guide for the Care and Use of Laboratory Animals 2024. Available online: https://arriveguidelines.org/arrive-guidelines (accessed on 11 July 2024).
- Hansel, G.; Tonon, A.C.; Guella, F.L.; Pettenuzzo, L.F.; Duarte, T.; Duarte, M.M.M.F.; Oses, J.P.; Achaval, M.; Souza, D.O. Guanosine Protects Against Cortical Focal Ischemia. Involvement of Inflammatory Response. Mol. Neurobiol. 2015, 52, 1791–1803. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Loureiro, S.O.; Pettenuzzo, L.F.; Almeida, R.F.; Ynumaru, E.Y.; Guazzelli, P.A.; Meyer, F.S.; Pasquetti, M.V.; Ganzella, M.; Calcagnotto, M.E.; et al. Effects of Intranasal Guanosine Administration on Brain Function in a Rat Model of Ischemic Stroke. Purinergic Signal. 2021, 17, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Nonose, Y.; Gewehr, P.E.; Almeida, R.F.; da Silva, J.S.; Bellaver, B.; Martins, L.A.M.; Zimmer, E.R.; Greggio, S.; Venturin, G.T.; Da Costa, J.C.; et al. Cortical Bilateral Adaptations in Rats Submitted to Focal Cerebral Ischemia: Emphasis on Glial Metabolism. Mol. Neurobiol. 2018, 55, 2025–2041. [Google Scholar] [CrossRef]
- Rohden, F.; Teixeira, L.V.; Bernardi, L.P.; Ferreira, P.C.L.; Colombo, M.; Teixeira, G.R.; De Oliveira, F.D.S.; Cirne Lima, E.O.; Guma, F.C.R.; Souza, D.O. Functional Recovery Caused by Human Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles Administered 24 h after Stroke in Rats. Int. J. Mol. Sci. 2021, 22, 12860. [Google Scholar] [CrossRef]
- Teixeira, L.V.; Almeida, R.F.; Rohden, F.; Martins, L.A.M.; Spritzer, P.M.; De Souza, D.O.G. Neuroprotective Effects of Guanosine Administration on In Vivo Cortical Focal Ischemia in Female and Male Wistar Rats. Neurochem. Res. 2018, 43, 1476–1489. [Google Scholar] [CrossRef] [PubMed]
- Magno, L.A.; Collodetti, M.; Tenza-Ferrer, H.; Romano-Silva, M. Cylinder Test to Assess Sensory-Motor Function in a Mouse Model of Parkinson’s Disease. BIO-Protocol 2019, 9, e3337. [Google Scholar] [CrossRef]
- Truong, S.H.T.; Bonnici, B.; Rupasinghe, S.; Kemp-Harper, B.K.; Samuel, C.S.; Broughton, B.R.S. Post-Stroke Administration of H2 Relaxin Reduces Functional Deficits, Neuronal Apoptosis and Immune Cell Infiltration into the Mouse Brain. Pharmacol. Res. 2023, 187, 106611. [Google Scholar] [CrossRef]
- Mehta, S.L.; Chelluboina, B.; Morris-Blanco, K.C.; Bathula, S.; Jeong, S.; Arruri, V.; Davis, C.K.; Vemuganti, R. Post-Stroke Brain Can Be Protected by Modulating the lncRNA FosDT. J. Cereb. Blood Flow Metab. 2024, 44, 239–251. [Google Scholar] [CrossRef]
- Yilmaz, U.; Tanbek, K.; Gul, S.; Koc, A.; Gul, M.; Sandal, S. Intracerebroventricular BDNF Infusion May Reduce Cerebral Ischemia/Reperfusion Injury by Promoting Autophagy and Suppressing Apoptosis. J. Cell. Mol. Med. 2024, 28, e18246. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. In Pre-Clinical Models; Guest, P.C., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1916, pp. 99–103. ISBN 978-1-4939-8993-5. [Google Scholar]
- Sayed, M.A.; Eldahshan, W.; Abdelbary, M.; Pillai, B.; Althomali, W.; Johnson, M.H.; Arbab, A.S.; Ergul, A.; Fagan, S.C. Stroke Promotes the Development of Brain Atrophy and Delayed Cell Death in Hypertensive Rats. Sci. Rep. 2020, 10, 20233. [Google Scholar] [CrossRef]
- Thakkar, P.; McGregor, A.; Barber, P.A.; Paton, J.F.R.; Barrett, C.; McBryde, F. Hypertensive Response to Ischemic Stroke in the Normotensive Wistar Rat: Mechanisms and Therapeutic Relevance. Stroke 2019, 50, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Raz, L.; Bhaskar, K.; Weaver, J.; Marini, S.; Zhang, Q.; Thompson, J.F.; Espinoza, C.; Iqbal, S.; Maphis, N.M.; Weston, L.; et al. Hypoxia Promotes Tau Hyperphosphorylation with Associated Neuropathology in Vascular Dysfunction. Neurobiol. Dis. 2019, 126, 124–136. [Google Scholar] [CrossRef]
- Heil, L.B.B.; Braga, C.L.; Magalhães, R.F.; Antunes, M.A.; Cruz, F.F.; Samary, C.S.; Battaglini, D.; Robba, C.; Pelosi, P.; Silva, P.L.; et al. Dexmedetomidine Compared to Low-Dose Ketamine Better Protected Not Only the Brain but Also the Lungs in Acute Ischemic Stroke. Int. Immunopharmacol. 2023, 124, 111004. [Google Scholar] [CrossRef]
- Mazuryk, J.; Puchalska, I.; Koziński, K.; Ślusarz, M.J.; Ruczyński, J.; Rekowski, P.; Rogujski, P.; Płatek, R.; Wiśniewska, M.B.; Piotrowski, A.; et al. PTD4 Peptide Increases Neural Viability in an In Vitro Model of Acute Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 6086. [Google Scholar] [CrossRef] [PubMed]
- Chojnowski, K.; Opielka, M.; Nazar, W.; Kowianski, P.; Smolenski, R.T. Neuroprotective Effects of Guanosine in Ischemic Stroke—Small Steps towards Effective Therapy. Int. J. Mol. Sci. 2021, 22, 6898. [Google Scholar] [CrossRef] [PubMed]
- Zuccarini, M.; Pruccoli, L.; Balducci, M.; Giuliani, P.; Caciagli, F.; Ciccarelli, R.; Di Iorio, P. Influence of Guanine-Based Purines on the Oxidoreductive Reactions Involved in Normal or Altered Brain Functions. J. Clin. Med. 2023, 12, 1172. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugimoto, N.; Hossain, S.; Islam, R.; Sumiyoshi, E.; Hashimoto, M.; Kishi, H.; Shido, O. Theobromine Improves Hyperactivity, Inattention, and Working Memory via Modulation of Dopaminergic Neural Function in the Frontal Cortex of Spontaneously Hypertensive Rats. Food Funct. 2024, 15, 5579–5595. [Google Scholar] [CrossRef]
- Sontag, T.-A.; Fuermaier, A.B.M.; Hauser, J.; Kaunzinger, I.; Tucha, O.; Lange, K.W. Spatial Memory in Spontaneously Hypertensive Rats (SHR). PLoS ONE 2013, 8, e74660. [Google Scholar] [CrossRef]
- Astrup, J.; Siesjö, B.K.; Symon, L. Thresholds in Cerebral Ischemia—The Ischemic Penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Liu, R. Four Decades of Ischemic Penumbra and Its Implication for Ischemic Stroke. Transl. Stroke Res. 2021, 12, 937–945. [Google Scholar] [CrossRef]
- Hillis, A.E.; Baron, J.-C. Editorial: The Ischemic Penumbra: Still the Target for Stroke Therapies? Front. Neurol. 2015, 6, 85. [Google Scholar] [CrossRef]
- Tzilivaki, A.; Tukker, J.J.; Maier, N.; Poirazi, P.; Sammons, R.P.; Schmitz, D. Hippocampal GABAergic Interneurons and Memory. Neuron 2023, 111, 3154–3175. [Google Scholar] [CrossRef] [PubMed]
- Kijima, C.; Inaba, T.; Hira, K.; Miyamoto, N.; Yamashiro, K.; Urabe, T.; Hattori, N.; Ueno, Y. Astrocytic Extracellular Vesicles Regulated by Microglial Inflammatory Responses Improve Stroke Recovery. Mol. Neurobiol. 2024, 61, 1002–1021. [Google Scholar] [CrossRef]
- Clain, J.; Couret, D.; Bringart, M.; Lecadieu, A.; Meilhac, O.; Lefebvre d’Hellencourt, C.; Diotel, N. Metabolic Disorders Exacerbate the Formation of Glial Scar after Stroke. Eur. J. Neurosci. 2024, 59, 3009–3029. [Google Scholar] [CrossRef]
- Constantakis, J.W.; Reed-McBain, C.A.; Famakin, B. Astrocyte Innate Immune Activation and Injury Amplification Following Experimental Focal Cerebral Ischemia. Neurochem. Int. 2023, 162, 105456. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, J.V.M.e.; Bernardi, L.P.; Teixeira, F.C.; Paniago, J.; Teixeira, L.V.; Bifi, F.; Souza, D.O.; Rohden, F. Spontaneously Hypertensive Rats Present Exacerbated Focal Stroke Behavioral Outcomes. Brain Sci. 2024, 14, 838. https://doi.org/10.3390/brainsci14080838
Moreira JVMe, Bernardi LP, Teixeira FC, Paniago J, Teixeira LV, Bifi F, Souza DO, Rohden F. Spontaneously Hypertensive Rats Present Exacerbated Focal Stroke Behavioral Outcomes. Brain Sciences. 2024; 14(8):838. https://doi.org/10.3390/brainsci14080838
Chicago/Turabian StyleMoreira, João Victor Matos e, Luis Pedro Bernardi, Fernanda Cardoso Teixeira, Jerônimo Paniago, Luciele Varaschini Teixeira, Felippo Bifi, Diogo Onofre Souza, and Francieli Rohden. 2024. "Spontaneously Hypertensive Rats Present Exacerbated Focal Stroke Behavioral Outcomes" Brain Sciences 14, no. 8: 838. https://doi.org/10.3390/brainsci14080838




